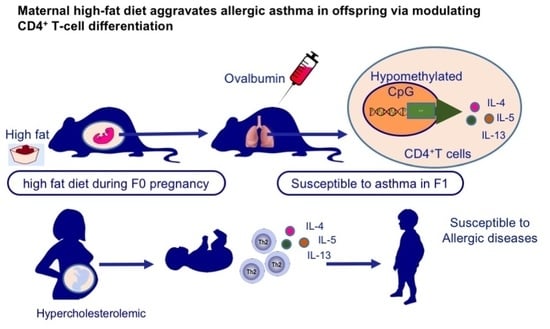
Maternal High-Fat Diet Aggravates Allergic Asthma in Offspring via Modulating CD4+ T-Cell Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Diets
2.2. OVA-Induced Experimental Asthma
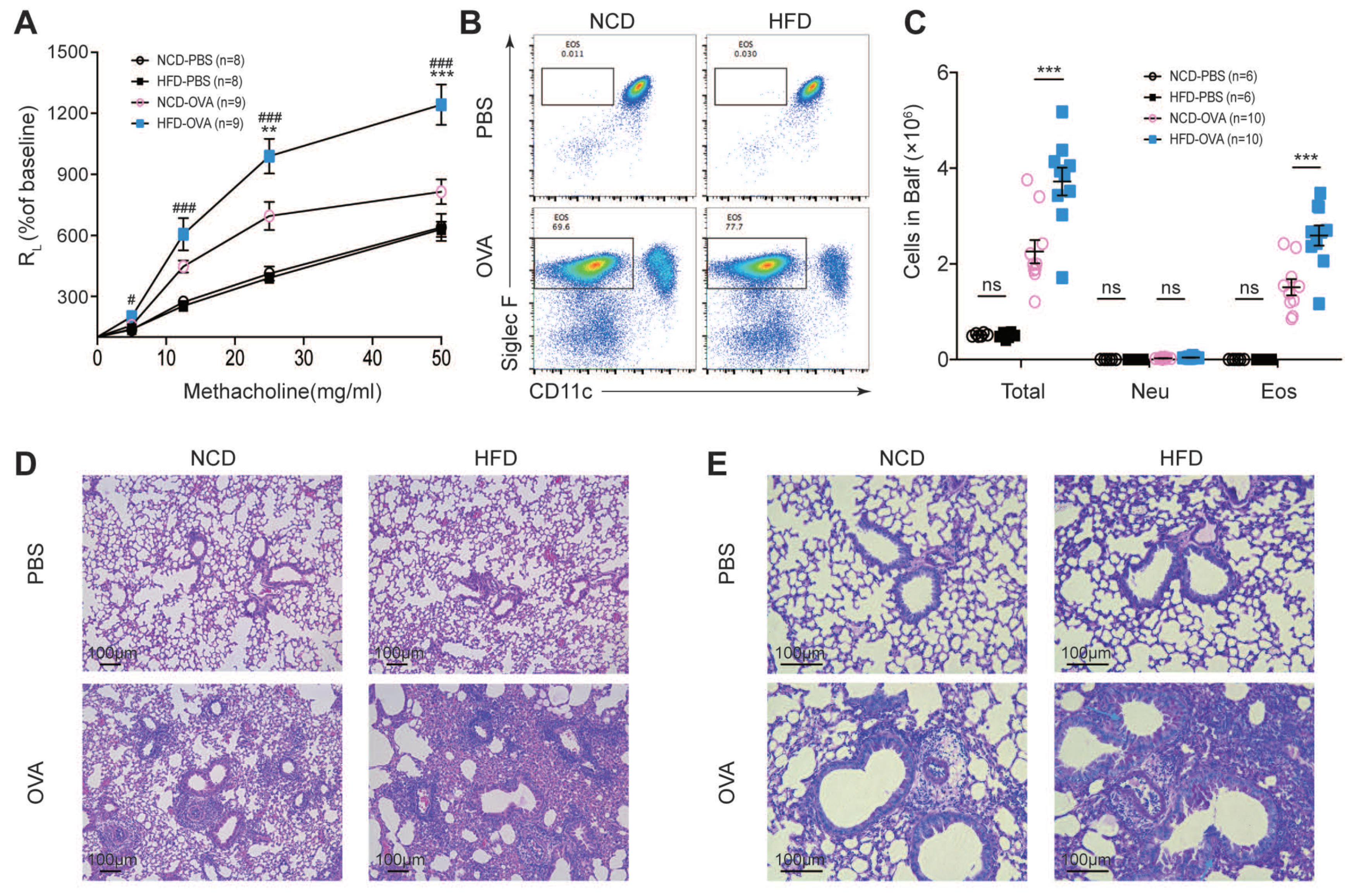
2.3. Lung Airway Hyperactivity Measurement
2.4. Bronchoalveolar Lavage
2.5. Flow Cytometry
2.6. Tissue Histologic Sections
2.7. T-Cell Isolation and Culture
2.8. Human Subjects
2.9. Relative mRNA Expression Analysis
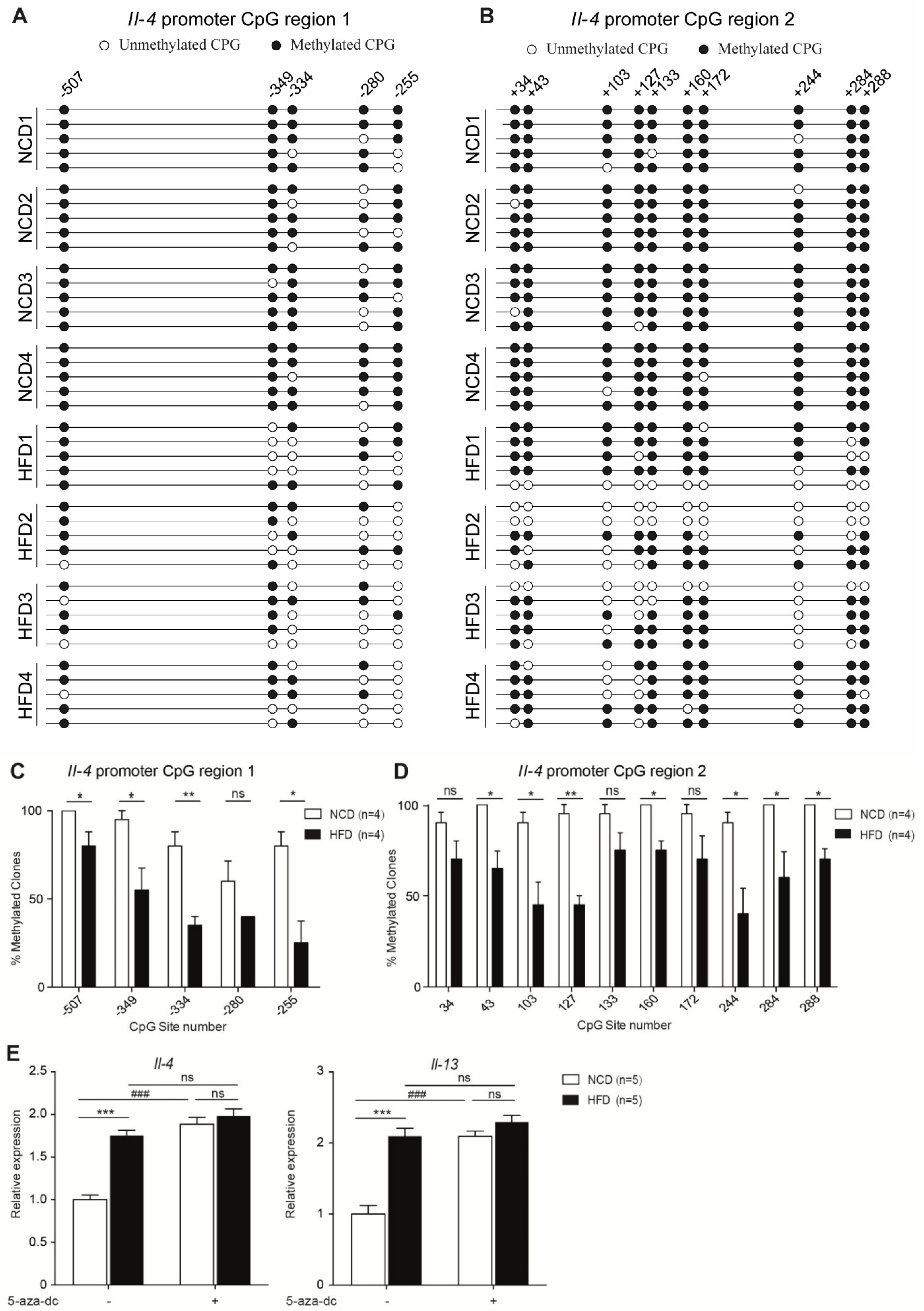
2.10. Bisulfite Sequencing
2.11. Statistical Analysis
3. Results
3.1. Maternal High-Fat Diet during Pregnancy Aggravated Experimental Asthma in Offspring
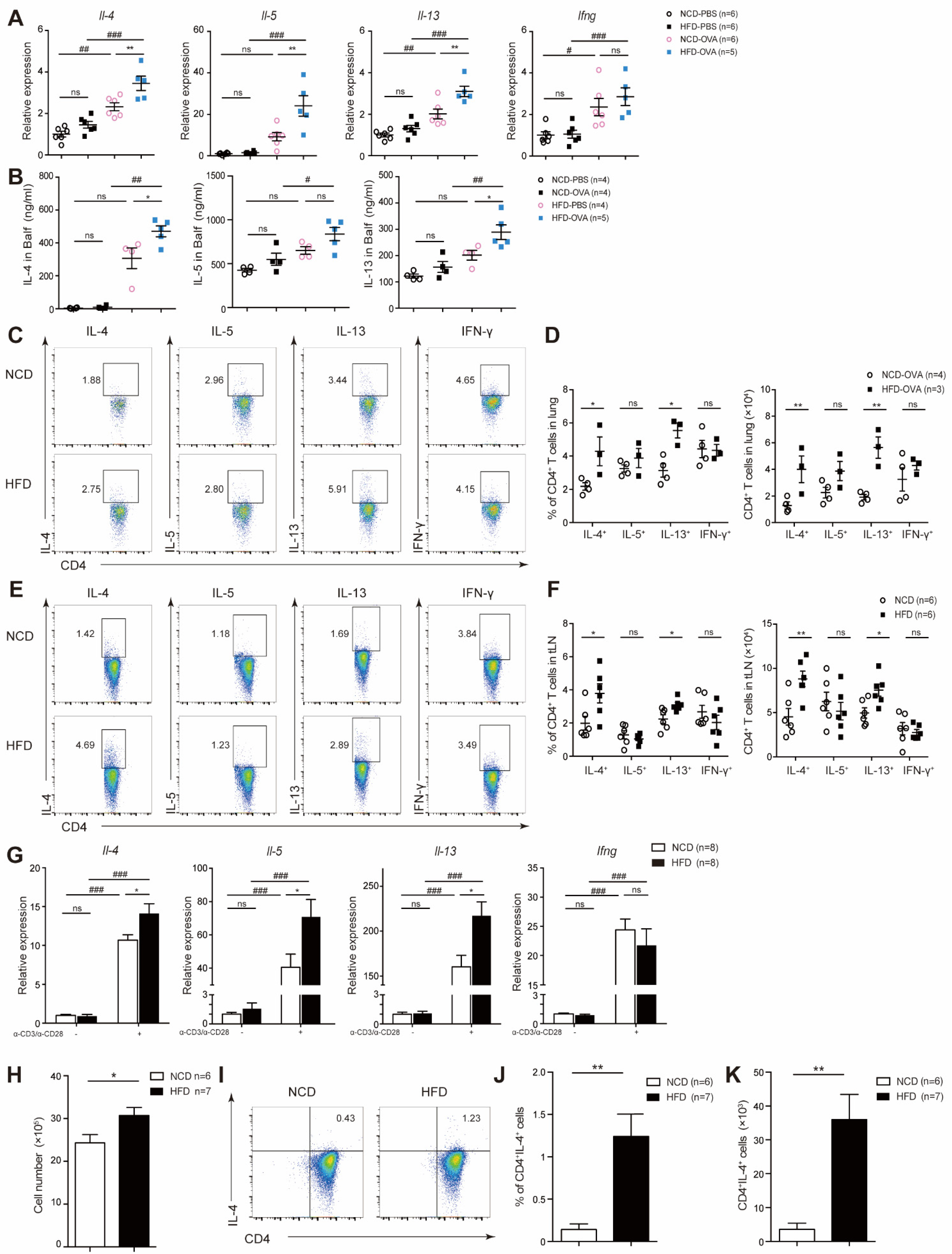
3.2. Maternal High-Fat Diet during Pregnancy Promoted CD4+ T-Cell Proliferation and Activation in Offspring
3.3. Offspring of Maternal High-Fat Diet during Pregnancy Exhibited Increased TH2 Cytokine Level
3.4. Maternal High-Fat Diet during Pregnancy Resulted in Hypomethylation of Il-4 Promoter Region
3.5. Neonates of Hypercholesterolemic Mothers Expressed Elevated Levels of TH2 Cytokines and were Prone to Allergic Diseases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; Liu, X. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. In NCHS Data Brief No. 94; United States National Center for Health Statistics: Hyattsville, MD, USA, 2012; pp. 1–8. [Google Scholar]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; Garcia-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; The ISAAC Phase III Study Group. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluckman, P.D.; Hanson, M.; Cooper, C.; Thornburg, K. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduci, E.; Martelli, A.; Miniello, V.; Landi, M.; Mariani, B.; Brambilla, M.; Diaferio, L.; Peroni, D. Nutrition in the first 1000 days and respiratory health: A descriptive review of the last five years’ literature. Allergol. Immunopathol. 2017, 45, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrenstein, O.S.; Aralis, H.; Flores, M.E.; Ritz, B. Fast food consumption in pregnancy and subsequent asthma symptoms in young children. Pediatr. Allergy Immunol. 2015, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Gold, D.; Camargo, C.A., Jr.; Sen, S.; Sordillo, J.E.; Oken, E.; et al. Associations of Prenatal Dietary Inflammatory Potential with Childhood Respiratory Outcomes in Project Viva. J. Allergy Clin. Immunol. Pract. 2020, 8, 945–952.e4. [Google Scholar] [CrossRef]
- Durham, S.R.; Till, S.J.; Corrigan, C.J. T lymphocytes in asthma: Bronchial versus peripheral responses. J. Allergy Clin. Immunol. 2000, 106 (Suppl. 5), S221–S226. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, H.; Yang, D.; Xu, W.; Liu, G.; Liu, X.; Sheng, J.; Huang, H. Early-life undernutrition reprograms CD4+ T-cell glycolysis and epigenetics to facilitate asthma. J. Allergy Clin. Immunol. 2019, 143, 2038–2051.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litonjua, A.A.; Weiss, S.T. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007, 120, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Lee, D.U.; Rao, A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 2003, 4, 616–623. [Google Scholar] [CrossRef]
- Grogan, J.L.; Mohrs, M.; Harmon, B.; Lacy, D.A.; Sedat, J.W.; Locksley, R.M. Early Transcription and Silencing of Cytokine Genes Underlie Polarization of T Helper Cell Subsets. Immunity 2001, 14, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.; Yan, W.; Ji, X.; Gao, R.; Ma, J.; Rao, Z.; Li, G.; Sang, N. Maternal Exposure of BALB/c Mice to Indoor NO2 and Allergic Asthma Syndrome in Offspring at Adulthood with Evaluation of DNA Methylation Associated Th2 Polarization. Environ. Health Perspect. 2017, 125, 097011. [Google Scholar] [CrossRef] [Green Version]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Bi, Y.; Fu, Z.; Gong, P.; Li, Y.; Yu, Q.; Jia, A.; Wang, J.; Xue, L.; et al. mTOR signaling disruption from myeloid-derived suppressive cells protects against immune-mediated hepatic injury through the HIF1α-dependent glycolytic pathway. J. Leukoc. Biol. 2016, 100, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zhang, J.; Jiang, P.; Gong, D.; Wang, J.-W.; Xia, Y.; Østergaard, M.V.; Sangild, P.T. Marked methylation changes in intestinal genes during the perinatal period of preterm neonates. BMC Genom. 2014, 15, 716. [Google Scholar] [CrossRef] [Green Version]
- Amin, K. The Role of the T lymphocytes and Remodeling in Asthma. Inflammation 2016, 39, 1475–1482. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Saglani, S. T cells in asthma: Influences of genetics, environment, and T-cell plasticity. J. Allergy Clin. Immunol. 2013, 131, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Brunkow, M.E.; Jeffery, E.W.; Hjerrild, K.A.; Paeper, B.; Clark, L.B.; Yasayko, S.-A.; Wilkinson, J.E.; Galas, D.; Ziegler, S.F.; Ramsdell, F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001, 27, 68–73. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Skon, C.N.; Lee, Y.J.; Oh, S.; Taylor, J.J.; Malhotra, D.; Jenkins, M.K.; Rosenfeld, M.G.; Hogquist, K.A.; Jameson, S.C. The Transcription Factor KLF2 Restrains CD4+ T Follicular Helper Cell Differentiation. Immunity 2015, 42, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Boonen, G.J.J.C.; van Oirschot, B.A.; van Diepen, A.; Mackus, W.J.M.; Verdonck, L.F.; Rijksen, G.; Medema, R.H. Cyclin D3 Regulates Proliferation and Apoptosis of Leukemic T Cell Lines. J. Biol. Chem. 1999, 274, 34676–34682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabbisetty, S.K.; Rabacal, W.; Maseda, D.; Cendron, D.; Collins, P.L.; Hoek, K.L.; Parekh, V.V.; Aune, T.M.; Sebzda, E. KLF2 is a rate-limiting transcription factor that can be targeted to enhance regulatory T-cell production. Proc. Natl. Acad. Sci. USA 2014, 111, 9579–9584. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, O.; Bahmer, T.; Rabe, K.F.; von Mutius, E. Asthma transition from childhood into adulthood. Lancet Respir. Med. 2017, 5, 224–234. [Google Scholar] [CrossRef]
- MacDonald, K.D.; Moran, A.R.; Scherman, A.J.; McEvoy, C.T.; Platteau, A.S. Maternal high-fat diet in mice leads to innate airway hyperresponsiveness in the adult offspring. Physiol. Rep. 2017, 5, e13082. [Google Scholar] [CrossRef]
- Raemdonck, K.; Baker, K.; Dale, N.; Dubuis, E.; Shala, F.; Belvisi, M.G.; Birrell, M.A. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir. Res. 2016, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; DeKruyff, R.H.; Umetsu, D.T. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010, 11, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Gundavarapu, S.; Peña-Philippides, J.C.; Rir-Sima-Ah, J.; Mishra, N.C.; Wilder, J.A.; Langley, R.J.; Smith, K.R.; Sopori, M.L. Prenatal Secondhand Cigarette Smoke Promotes Th2 Polarization and Impairs Goblet Cell Differentiation and Airway Mucus Formation. J. Immunol. 2011, 187, 4542–4552. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Romero, R.; Tarca, A.L.; Bhatti, G.; Kim, C.J.; Lee, J.; Elsey, A.; Than, N.G.; Chaiworapongsa, T.; Hassan, S.S.; et al. Methylome of Fetal and Maternal Monocytes and Macrophages at the Feto-Maternal Interface. Am. J. Reprod. Immunol. 2012, 68, 8–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]

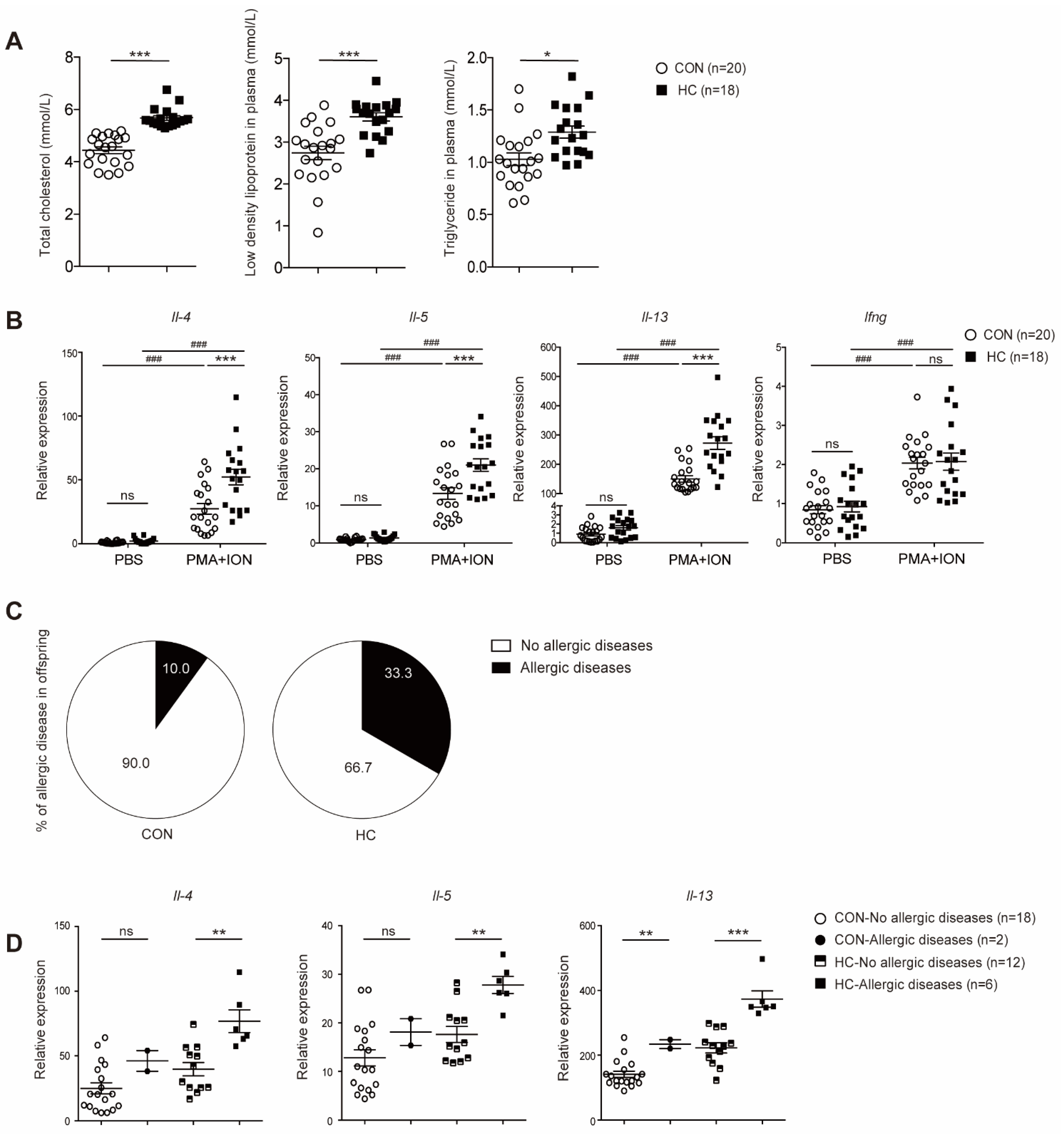
| CON n (%) | HC n (%) | p Value | |
|---|---|---|---|
| Allergy diseases | 0.09 | ||
| No | 18(0.90) | 12(66.67) | |
| Yes | 2(0.10) | 6(33.33) | |
| Asthma | 0.47 | ||
| No | 20(100) | 17(94.44) | |
| Yes | 0 | 1(5.56) | |
| Atopic dermatitis | 0.40 | ||
| No | 18(0.90) | 14(77.78) | |
| Yes | 2(0.10) | 4(22.22) | |
| Food allergy | 0.17 | ||
| No | 19(0.95) | 14(77.78) | |
| Yes | 1(0.05) | 4(22.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhao, Y.; Zhu, Y.; Li, C.; Xu, W.; Chen, X.; Huang, H.; Jin, L. Maternal High-Fat Diet Aggravates Allergic Asthma in Offspring via Modulating CD4+ T-Cell Differentiation. Nutrients 2022, 14, 2508. https://doi.org/10.3390/nu14122508
Lin H, Zhao Y, Zhu Y, Li C, Xu W, Chen X, Huang H, Jin L. Maternal High-Fat Diet Aggravates Allergic Asthma in Offspring via Modulating CD4+ T-Cell Differentiation. Nutrients. 2022; 14(12):2508. https://doi.org/10.3390/nu14122508
Chicago/Turabian StyleLin, Hui, Yiran Zhao, Yajie Zhu, Cheng Li, Wei Xu, Xi Chen, Hefeng Huang, and Li Jin. 2022. "Maternal High-Fat Diet Aggravates Allergic Asthma in Offspring via Modulating CD4+ T-Cell Differentiation" Nutrients 14, no. 12: 2508. https://doi.org/10.3390/nu14122508
APA StyleLin, H., Zhao, Y., Zhu, Y., Li, C., Xu, W., Chen, X., Huang, H., & Jin, L. (2022). Maternal High-Fat Diet Aggravates Allergic Asthma in Offspring via Modulating CD4+ T-Cell Differentiation. Nutrients, 14(12), 2508. https://doi.org/10.3390/nu14122508