Effect of Dewaxed Coffee on Gastroesophageal Symptoms in Patients with GERD: A Randomized Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling
2.3. Sample Preparation
2.4. UHPLC Q-Orbitrap HRMS
2.5. Identification of Bioactive Compounds in Coffee Pods Samples through UHPLC-Q-Orbitrap HRMS
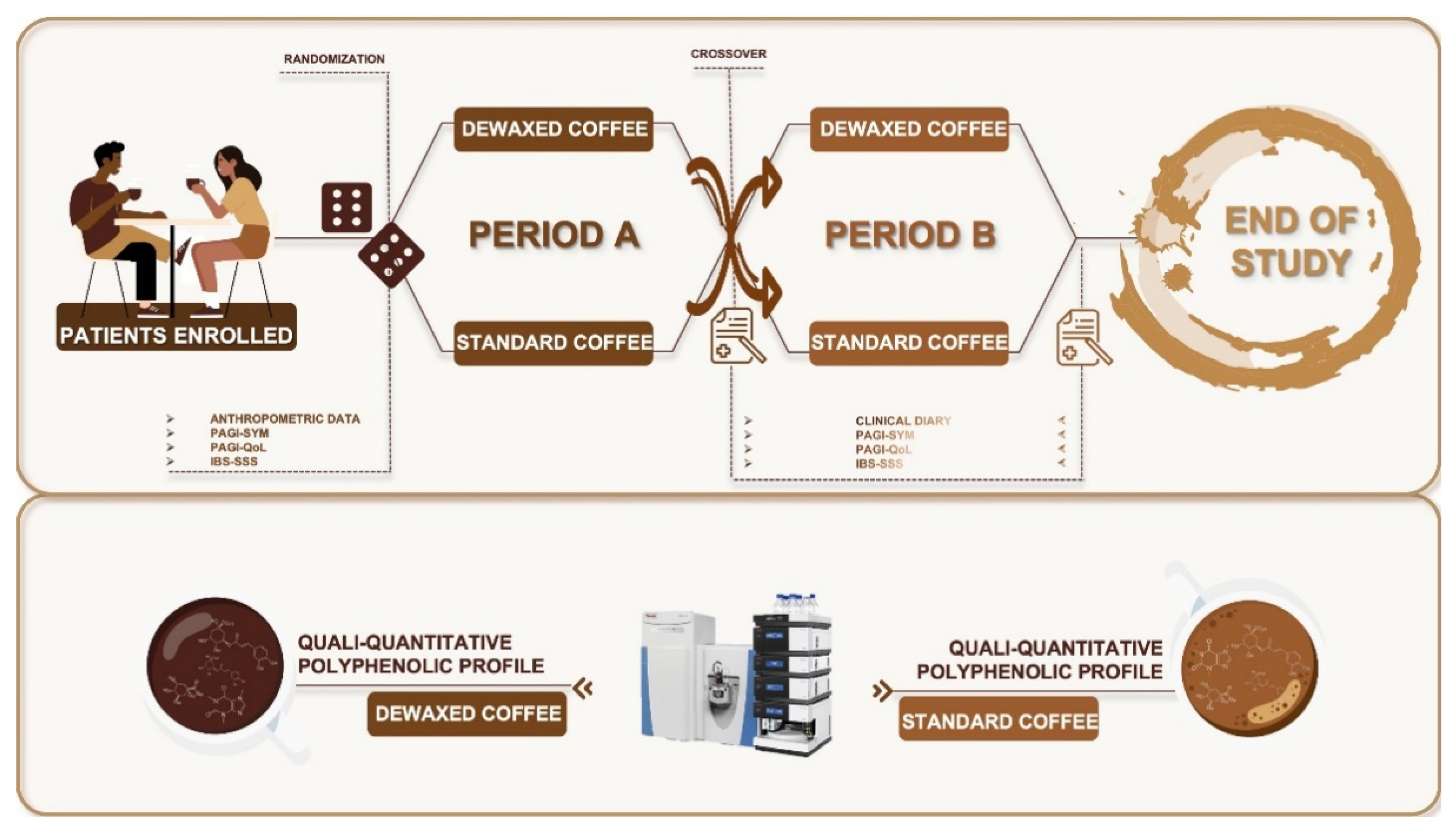
2.6. Study Design
2.7. Subjects
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Quantification of Bioactive Compounds in Coffee Pods Samples through UHPLC-Q-Orbitrap HRMS
3.2. A randomized Pilot Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- FAO. Food Balance Sheet. 2019. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 8 April 2022).
- Castaldo, L.; Izzo, L.; Narváez, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Colon bioaccessibility under in vitro gastrointestinal digestion of different coffee brews chemically profiled through UHPLC-Q-Orbitrap HRMS. Foods 2021, 10, 179. [Google Scholar] [CrossRef]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Loader, T.B.; Taylor, C.G.; Zahradka, P.; Jones, P.J. Chlorogenic acid from coffee beans: Evaluating the evidence for a blood pressure–regulating health claim. Nutr. Rev. 2017, 75, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Spices, condiments, extra virgin olive oil and aromas as not only flavorings, but precious allies for our wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Scaioli, E.; Baldi, F.; Vestito, A.; Pasqui, F.; Di Biase, A.R.; Colecchia, A. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J. Gastroenterol. 2009, 15, 1690. [Google Scholar] [CrossRef]
- Filiberti, R.; Fontana, V.; De Ceglie, A.; Blanchi, S.; Grossi, E.; Della Casa, D.; Lacchin, T.; De Matthaeis, M.; Ignomirelli, O.; Cappiello, R. Association between coffee or tea drinking and Barrett’s esophagus or esophagitis: An Italian study. Eur. J. Clin. Nutr. 2017, 71, 980–986. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Oh, S.-W.; Myung, S.-K.; Kwon, H.; Lee, C.; Yun, J.; Lee, H. Association between coffee intake and gastroesophageal reflux disease: A meta-analysis. Dis. Esophagus 2014, 27, 311–317. [Google Scholar] [CrossRef]
- Kellerman, R.; Kintanar, T. Gastroesophageal reflux disease. Prim. Care Clin. Off. Pract. 2017, 44, 561–573. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef]
- Orlando, R.C. Pathophysiology of gastroesophageal reflux disease. J. Clin. Gastroenterol. 2008, 42, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, R. A quasi-experimental research to investigate the retention of basic cardiopulmonary resuscitation skills and knowledge by qualified nurses following a course in professional development. J. Adv. Nurs. 1996, 23, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ribolsi, M.; Giordano, A.; Guarino, M.P.L.; Tullio, A.; Cicala, M. New classifications of gastroesophageal reflux disease: An improvement for patient management? Expert Rev. Gastroenterol. Hepatol. 2019, 13, 761–769. [Google Scholar] [CrossRef]
- Farup, C.; Kleinman, L.; Sloan, S.; Ganoczy, D.; Chee, E.; Lee, C.; Revicki, D. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch. Intern. Med. 2001, 161, 45–52. [Google Scholar] [CrossRef]
- Ronkainen, J.; Aro, P.; Storskrubb, T.; Lind, T.; Bolling-Sternevald, E.; Junghard, O.; Talley, N.; Agreus, L. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population–the Kalixanda study. Aliment. Pharmacol. Ther. 2006, 23, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Jiang, H.-M.; Hou, X.-H.; Song, J. Anxiety and depression in patients with gastroesophageal reflux disease and their effect on quality of life. World J. Gastroenterol. 2015, 21, 4302. [Google Scholar] [CrossRef]
- Wahlqvist, P.; Reilly, M.; Barkun, A. Systematic review: The impact of gastro-oesophageal reflux disease on work productivity. Aliment. Pharmacol. Ther. 2006, 24, 259–272. [Google Scholar] [CrossRef]
- Anand, G.; Katz, P.O. Gastroesophageal reflux disease and obesity. Rev. Gastroenterol. Disord. 2008, 8, 233–239. [Google Scholar] [CrossRef]
- Chang, P.; Friedenberg, F. Obesity and GERD. Gastroenterol. Clin. 2014, 43, 161–173. [Google Scholar] [CrossRef]
- Hampel, H.; Abraham, N.S.; El-Serag, H. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005, 143, 199–211. [Google Scholar] [CrossRef]
- Dent, J.; El-Serag, H.; Wallander, M.A.; Johansson, S. Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2005, 54, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, Z.-K.; Huang, Z.-B.; Chen, X.-L.; Liu, F.-B. Dietary and Lifestyle Factors Related to Gastroesophageal Reflux Disease: A Systematic Review. Ther. Clin. Risk Manag. 2021, 17, 305. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Crockett, S.; Gerson, L.B. Are lifestyle measures effective in patients with gastroesophageal reflux disease?: An evidence-based approach. Arch. Intern. Med. 2006, 166, 965–971. [Google Scholar] [CrossRef]
- Boekema, P.J.; Samsom, M.; Smout, A. Effect of coffee on gastro-oesophageal reflux in patients with reflux disease and healthy controls. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Boekema, P.J.; Samsom, M.; van Berge Henegouwen, G.P.; Smout, A.J.P.M. Coffee and gastrointestinal function: Facts and fiction: A review. Scand. J. Gastroenterol. 1999, 34, 35–39. [Google Scholar] [CrossRef]
- Cohen, S.; Booth, G.H., Jr. Gastric acid secretion and lower-esophageal-sphincter pressure in response to coffee and caffeine. N. Engl. J. Med. 1975, 293, 897–899. [Google Scholar] [CrossRef]
- Thomas, F.B.; Steinbaugh, J.T.; Mekhjian, H.S.; Caldwell, J.H. Inhibitory effect of coffee on lower esophageal sphincter pressure. Gastroenterology 1980, 79, 1262–1266. [Google Scholar] [CrossRef]
- Van Deventer, G.; Kamemoto, E.; Kuznicki, J.T.; Heckert, D.C.; Schulte, M.C. Lower esophageal sphincter pressure, acid secretion, and blood gastrin after coffee consumption. Dig. Dis. Sci. 1992, 37, 558–569. [Google Scholar] [CrossRef]
- Rubach, M.; Lang, R.; Seebach, E.; Somoza, M.M.; Hofmann, T.; Somoza, V. Multi-parametric approach to identify coffee components that regulate mechanisms of gastric acid secretion. Mol. Nutr. Food Res. Int. 2012, 56, 325–335. [Google Scholar] [CrossRef]
- Van der Stegen, G. The effect of dewaxing of green coffee on the coffee brew. Food Chem. 1979, 4, 23–29. [Google Scholar] [CrossRef]
- Rentz, A.; Kahrilas, P.; Stanghellini, V.; Tack, J.; Talley, N.; Trudeau, E.; Dubois, D.; Revicki, D. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual. Life Res. 2004, 13, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Spent Coffee Grounds-Enriched Cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon bioaccessibility under in vitro gastrointestinal digestion of a red cabbage extract chemically profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. Clin. Risk Manag. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, E.; Marquis, P.; Kahrilas, P.; Stanghellini, V.; Talley, N.J.; Tack, J.; Revicki, D.A.; Rentz, A.M.; Dubois, D. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: The PAGI-QOL©. Qual. Life Res. 2004, 13, 1751–1762. [Google Scholar] [CrossRef]
- Revicki, D.A.; Rentz, A.M.; Tack, J.; Stanghellini, V.; Talley, N.J.; Kahrilas, P.; De La Loge, C.; Trudeau, E.; Dubois, D. Responsiveness and interpretation of a symptom severity index specific to upper gastrointestinal disorders. Clin. Gastroenterol. Hepatol. 2004, 2, 769–777. [Google Scholar] [CrossRef]
- Fraser, A.; Delaney, B.; Moayyedi, P. Symptom-based outcome measures for dyspepsia and GERD trials: A systematic review. Off. J. Am. Coll. Gastroenterol. 2005, 100, 442–452. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Kechribari, I.; Sotirakoglou, Κ.; Tarantilis, P.; Gourdomichali, T.; Michas, G.; Kravvariti, V.; Voumvourakis, K.; Zampelas, A. Acute effects of coffee consumption on self-reported gastrointestinal symptoms, blood pressure and stress indices in healthy individuals. Nutr. J. 2015, 15, 26. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ahuja, V. Questionnaire based gastroesophageal reflux disease (GERD) assessment scales. Indian J. Gastroenterol. 2011, 30, 108–117. [Google Scholar] [CrossRef]
- Moayyedi, P.; Forman, D.; Braunholtz, D.; Feltbower, R.; Crocombe, W.; Liptrott, M.; Axon, A.; Leeds HELP Study Group. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Am. J. Gastroenterol. 2000, 95, 1448–1455. [Google Scholar] [CrossRef]
- Rubach, M.; Lang, R.; Bytof, G.; Stiebitz, H.; Lantz, I.; Hofmann, T.; Somoza, V. A dark brown roast coffee blend is less effective at stimulating gastric acid secretion in healthy volunteers compared to a medium roast market blend. Mol. Nutr. Food Res. 2014, 58, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Brazer, S.R.; Onken, J.E.; Dalton, C.B.; Smith, J.W.; Schiffman, S.S. Effect of different coffees on esophageal acid contact time and symptoms in coffee-sensitive subjects. Physiol. Behav. 1995, 57, 563–567. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Staller, K.; Chan, A.T. Association between beverage intake and incidence of gastroesophageal reflux symptoms. Clin. Gastroenterol. Hepatol. 2020, 18, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Rubach, M.; Lang, R.; Skupin, C.; Hofmann, T.; Somoza, V. Activity-guided fractionation to characterize a coffee beverage that effectively down-regulates mechanisms of gastric acid secretion as compared to regular coffee. J. Agric. Food Chem. 2010, 58, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Nwokediuko, S. Gastroesophageal reflux disease: A population based study. Gastroenterol. Res. 2009, 2, 152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Chen, S.-H. Effect of coffee on gastroesophageal reflux disease. Food Sci. Technol. Res. 2013, 19, 1–6. [Google Scholar] [CrossRef][Green Version]
- Feldman, M.; Barnett, C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gastroenterology 1995, 108, 125–131. [Google Scholar] [CrossRef]
- Folstar, P.; Schols, H.A.; Van der Plas, H.C.; Pilnik, W.; Landheer, C.A.; Van Veldhuizen, A. New tryptamine derivatives isolated from wax of green coffee beans. J. Agric. Food Chem. 1980, 28, 872–874. [Google Scholar] [CrossRef]
- Song, J.L.; Asare, T.S.; Kang, M.Y.; Lee, S.C. Changes in bioactive compounds and antioxidant capacity of coffee under different roasting conditions. Korean J. Plant Resour. 2018, 31, 704–713. [Google Scholar]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A.; Severini, C. How grinding level and brewing method (Espresso, American, Turkish) could affect the antioxidant activity and bioactive compounds in a coffee cup. J. Sci. Food Agric. 2018, 98, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- DiBaise, J.K. A randomized, double-blind comparison of two different coffee-roasting processes on development of heartburn and dyspepsia in coffee-sensitive individuals. Dig. Dis. Sci. 2003, 48, 652–656. [Google Scholar] [CrossRef] [PubMed]



| Compound * | Chemical | Adduct | RT * | Measured | Theoretical | Accuracy |
|---|---|---|---|---|---|---|
| Formula | Ion | (min) | Mass (m/z) | Mass (m/z) | (Δ mg/kg) | |
| Quinic Acid | C7H12O6 | [M−H]− | 1.12 | 191.05531 | 191.05611 | 4.18 |
| 3-pCoQA | C16H18O8 | [M−H]− | 2.84 | 337.09232 | 337.09289 | −1.69 |
| 3-FQA | C17H20O9 | [M−H]− | 3.03 | 367.10367 | 367.10346 | −0.57 |
| Caffeic Acid | C9H8O4 | [M−H]− | 3.07 | 179.03426 | 179.03498 | 4.02 |
| 5-CQA | C16H18O9 | [M−H]− | 3.09 | 353.08813 | 353.08780 | −0.93 |
| 4-CQA | C16H18O9 | [M−H]− | 3.10 | 353.08901 | 353.08780 | −3.42 |
| 3-CQA | C16H18O9 | [M−H]− | 3.12 | 353.08852 | 353.08780 | −2.03 |
| Caffeine | C8H10N4O2 | [M + H]+ | 3.20 | 195.08751 | 195.08765 | 0.72 |
| p-Coumaric acid | C9H8O3 | [M−H]− | 3.25 | 163.03926 | 163.04006 | 4.91 |
| 5-pCoQA | C16H18O8 | [M−H]− | 3.27 | 337.09389 | 337.09289 | −2.97 |
| 3,4-diCQA | C25H24O12 | [M−H]− | 3.28 | 515.12036 | 515.11950 | −1.67 |
| 4 + 5-FQA | C17H20O9 | [M−H]− | 3.34 | 367.10303 | 367.10346 | 4.72 |
| Ferulic Acid | C10H10O4 | [M−H]− | 3.38 | 193.05017 | 193.05063 | −2.38 |
| 3,5-diCQA | C25H24O12 | [M−H]− | 3.45 | 515.12036 | 515.11950 | −1.67 |
| Compound * | Dewaxed Coffee | Standard Coffee | ||
|---|---|---|---|---|
| mg/g | ±SD | mg/g | ±SD | |
| Quinic Acid | 0.672 | 0.049 | 0.684 | 0.033 |
| 3-pCoQA | 0.509 a | 0.001 | 0.404 b | 0.002 |
| 3-FQA | 0.094 | 0.003 | 0.103 | 0.005 |
| Caffeic Acid | 0.022 a | 0.001 | 0.015 b | 0.002 |
| 5-CQA | 3.132 a | 0.016 | 2.928 b | 0.017 |
| 4-CQA | 1.034 a | 0.012 | 0.928 b | 0.013 |
| 3-CQA | 0.932 a | 0.005 | 0.728 b | 0.013 |
| Caffeine | 5.691 a | 0.07 | 11.091 b | 0.11 |
| p-Coumaric acid | NF | NF * | ||
| 5-pCoQA | 0.071 a | 0.007 | 0.053 b | 0.003 |
| 3,4-diCQA | 0.083 | 0.001 | 0.086 | 0.002 |
| 4 + 5-FQA | 0.303 a | 0.024 | 0.344 b | 0.008 |
| Ferulic Acid | 0.440 a | 0.094 | 0.420 b | 0.033 |
| 3,5-diCQA | 0.025 a | 0.000 | 0.028 b | 0.001 |
| Patients | T0 |
|---|---|
| Age (years) | 41.5 ± 12 |
| Sex n (%) | F 16 (40) |
| Weight (kg) | 75.3 ± 15.9 |
| Height (m) | 1.7 ± 0.1 |
| BMI (kg/m2) | 25.5 ± 4 |
| Smoke n (%) | 13 (32.5) |
| Physical Activity n (%) | 19 (47.5) |
| SC | DC | p-Value | |
|---|---|---|---|
| Heartburn-free days, % | 50.18 ± 17.46 | 79.82 ± 10.84 | p < 0.05 |
| Regurgitation-free days, % | 64.46 ± 14.87 | 82.68 ± 12.83 | p < 0.05 |
| Antacid-free days, % | 62.5 ± 22.22 | 87.5 ± 11.29 | p < 0.05 |
| B | SC * | DC * | p-Value * | |
|---|---|---|---|---|
| PAGI-SYM | 1.6 ± 0.75 | 1.7 ± 0.72 | 0.9 ± 0.48 | p < 0.01 |
| PAGI-QoL | 1.3 ± 0.73 | 1.2 ± 0.81 | 0.8 ± 0.64 | p < 0.01 |
| IBS-SSS | 196.9 ± 71.61 | 215.65 ± 68.51 | 149.75 ± 56.97 | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polese, B.; Izzo, L.; Mancino, N.; Pesce, M.; Rurgo, S.; Tricarico, M.C.; Lombardi, S.; De Conno, B.; Sarnelli, G.; Ritieni, A. Effect of Dewaxed Coffee on Gastroesophageal Symptoms in Patients with GERD: A Randomized Pilot Study. Nutrients 2022, 14, 2510. https://doi.org/10.3390/nu14122510
Polese B, Izzo L, Mancino N, Pesce M, Rurgo S, Tricarico MC, Lombardi S, De Conno B, Sarnelli G, Ritieni A. Effect of Dewaxed Coffee on Gastroesophageal Symptoms in Patients with GERD: A Randomized Pilot Study. Nutrients. 2022; 14(12):2510. https://doi.org/10.3390/nu14122510
Chicago/Turabian StylePolese, Barbara, Luana Izzo, Nicola Mancino, Marcella Pesce, Sara Rurgo, Maria Cristina Tricarico, Sonia Lombardi, Barbara De Conno, Giovanni Sarnelli, and Alberto Ritieni. 2022. "Effect of Dewaxed Coffee on Gastroesophageal Symptoms in Patients with GERD: A Randomized Pilot Study" Nutrients 14, no. 12: 2510. https://doi.org/10.3390/nu14122510
APA StylePolese, B., Izzo, L., Mancino, N., Pesce, M., Rurgo, S., Tricarico, M. C., Lombardi, S., De Conno, B., Sarnelli, G., & Ritieni, A. (2022). Effect of Dewaxed Coffee on Gastroesophageal Symptoms in Patients with GERD: A Randomized Pilot Study. Nutrients, 14(12), 2510. https://doi.org/10.3390/nu14122510








