Simultaneous Mass Spectrometry-Based Apolipoprotein Profiling and Apolipoprotein E Phenotyping in Patients with ASCVD and Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. LC-MS/MS Analyses
2.3. Laboratory Analyzes
2.4. Statistical Analysis
3. Results
3.1. Method Development and Validation
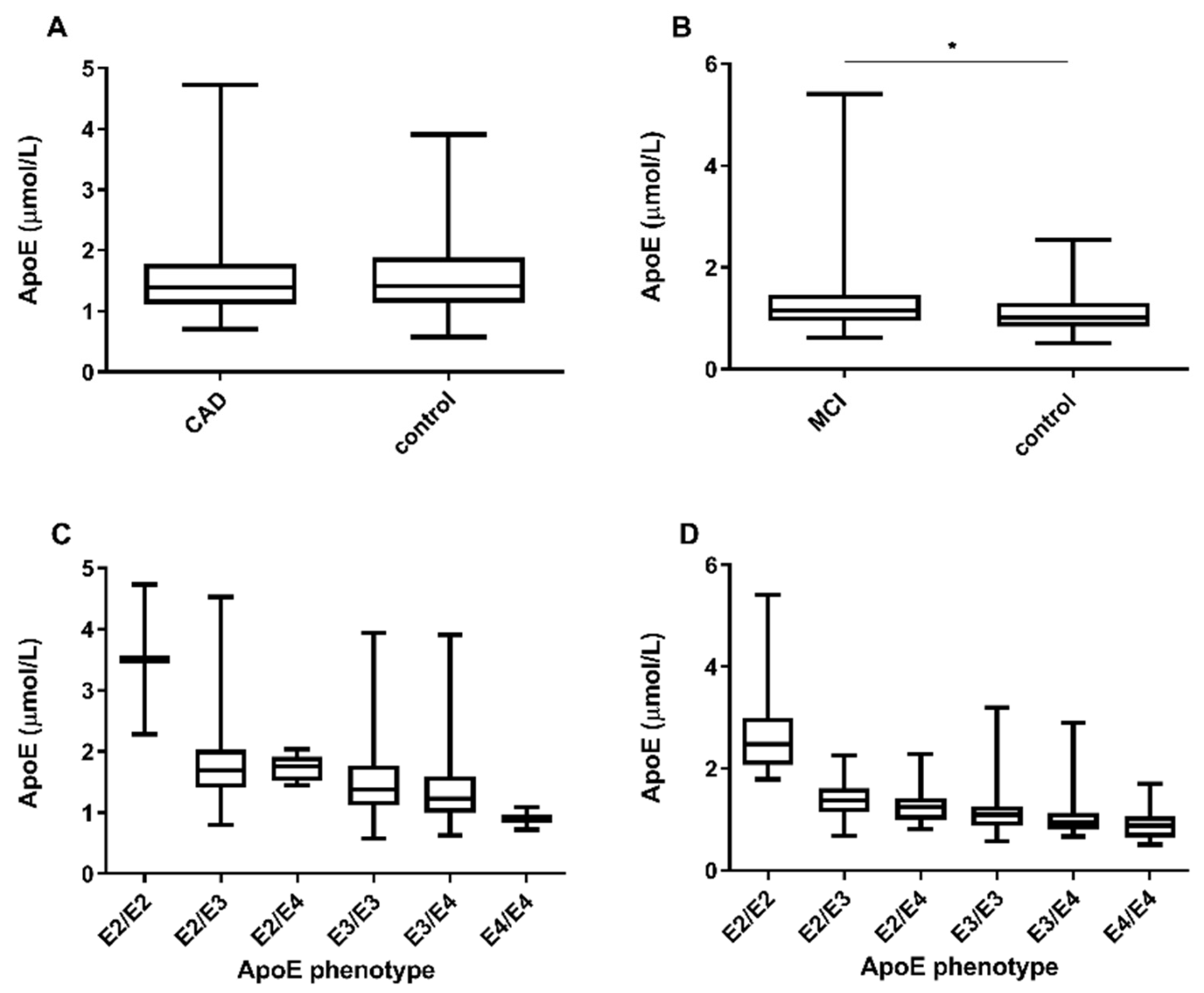
3.2. ApoE Concentration and apoE Phenotyping in Stable CAD
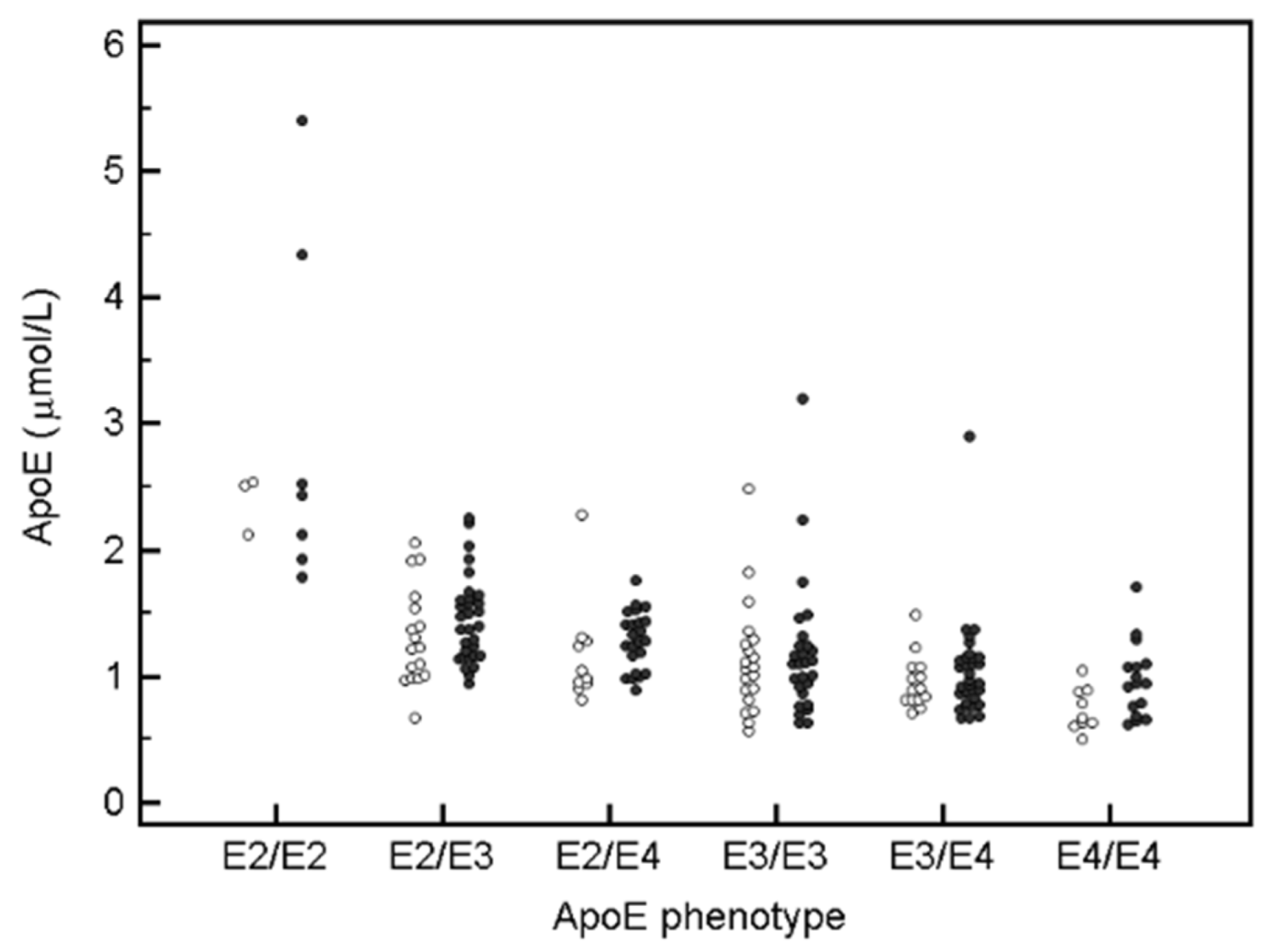
3.3. ApoE Phenotype Dependent Distribution of Apolipoprotein and Lipid Levels in Stable ASCD
3.4. ApoE Phenotyping in the MCI Sub-Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Foss, D.; Mahley, R.W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1987, 917, 148–161. [Google Scholar] [CrossRef]
- Lin, C.T.; Wu, J.Y.; Chan, L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J. Clin. Investig. 1986, 78, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Caslake, M.J.; Packard, C.J.; Shepherd, J. Concentration and distribution of human plasma apolipoprotein E. Clin. Chim. Acta 1981, 116, 35–45. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J. Clin. Investig. 1983, 72, 743–747. [Google Scholar] [CrossRef]
- Ruiz, J.; Kouiavskaia, D.; Migliorini, M.; Robinson, S.; Saenko, E.L.; Gorlatova, N.; Li, D.; Lawrence, D.; Hyman, B.T.; Weisgraber, K.H.; et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J. Lipid Res. 2005, 46, 1721–1731. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; Van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. J. Am. Med. Assoc. 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol Transport Protein with Expanding Role in Cell Biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- AlzGene—Meta-Analysis of All Published AD Association Studies (Case-Control Only) APOE_e2/3/4. Available online: http://www.alzgene.org/Meta.asp?GeneID=83 (accessed on 29 January 2020).
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- West, H.L.; William Rebeck, G.; Hyman, B.T. Frequency of the apolipoprotein E ε2 allele is diminished in sporadic Alzheimer disease. Neurosci. Lett. 1994, 175, 46–48. [Google Scholar] [CrossRef]
- Phillips, M.C. Apolipoprotein e isoforms and lipoprotein metabolism. IUBMB Life 2014, 66, 616–623. [Google Scholar] [CrossRef]
- Weisgraber, K.H.; Innerarity, T.L.; Mahley, R.W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J. Biol. Chem. 1982, 257, 2518–2521. [Google Scholar] [CrossRef]
- Boerwinkle, E.; Utermann, G. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am. J. Hum. Genet. 1988, 42, 104–112. [Google Scholar] [PubMed]
- Martinez, E.; Oskar, M. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer ’ s disease patients and controls. Acta Neuropathol. 2014, 127, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Smit, N.P.M.; Suchiman, H.E.D.; Pieterse, M.M.; Romijn, F.P.H.T.M.; Beekman, M.; Cobbaert, C.M. MS-based proteomics: A metrological sound and robust alternative for apolipoprotein E phenotyping in a multiplexed test. Clin. Chem. Lab. Med. 2018, 57, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Han, B.; Bateman, R.J. Method for the Simultaneous Quantitation of Apolipoprotein E Isoforms using Tandem Mass Spectrometry. Anal. Biochem. 2009, 395, 116–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Den Broek, I.; Romijn, F.P.H.T.M.; Nouta, J.; Van Der Laarse, A.; Drijfhout, J.W.; Smit, N.P.M.; Van Der Burgt, Y.E.M.; Cobbaert, C.M. Automated multiplex LC-MS/MS assay for quantifying serum apolipoproteins A-I, B, C-I, C-II, C-III, and E with qualitative apolipoprotein E phenotyping. Clin. Chem. 2016, 62, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morillo, E.; Nielsen, H.M.; Batruch, I.; Drabovich, A.P.; Begcevic, I.; Lopez, M.F.; Minthon, L.; Bu, G.; Mattsson, N.; Portelius, E.; et al. Assessment of Peptide Chemical Modifications on the Development of an Accurate and Precise Multiplex Selected Reaction Monitoring Assay for Apolipoprotein E Isoforms. J. Proteome Res. 2014, 13, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, V.; Ramin-mangata, S.; Billon-crossouard, S.; Aguesse, A.; Durand, M.; Chemello, K.; Nativel, B.; Flet, L.; Chétiveaux, M.; Jacobi, D.; et al. Kinetics of plasma apolipoprotein E isoforms by LC-MS/MS: A pilot study. J. Lipid Res. 2018, 59, 892–900. [Google Scholar] [CrossRef]
- Blanchard, V.; Garçon, D.; Jaunet, C.; Chemello, K.; Billon-Crossouard, S.; Aguesse, A.; Garfa, A.; Famchon, G.; Torres, A.; Le May, C.; et al. A high-throughput mass spectrometry–based assay for large-scale profiling of circulating human apolipoproteins. J. Lipid Res. 2020, 61, 1128–1139. [Google Scholar] [CrossRef]
- Dittrich, J.; Beutner, F.; Teren, A.; Thiery, J.; Burkhardt, R.; Scholz, M.; Ceglarek, U. Plasma levels of apolipoproteins C-III, A-IV, and E are independently associated with stable atherosclerotic cardiovascular disease. Atherosclerosis 2019, 281, 17–24. [Google Scholar] [CrossRef]
- Scholz, M.; Henger, S.; Beutner, F.; Teren, A.; Baber, R.; Willenberg, A.; Ceglarek, U.; Pott, J.; Burkhardt, R.; Thiery, J. Cohort Profile: The Leipzig Research Center for Civilization Diseases-Heart Study (LIFE-Heart). Int. J. Epidemiol. 2020, 49, 1439–1440h. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Engel, C.; Ahnert, P.; Alfermann, D.; Arelin, K.; Baber, R.; Beutner, F.; Binder, H.; Brähler, E.; Burkhardt, R.; et al. The LIFE-Adult-Study: Objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015, 15, 691. [Google Scholar] [CrossRef] [PubMed]
- Zaudig, M. Leichte kognitive Beeinträchtigung im Alter. In Demenzen in Theorie und Praxis; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783642197956. [Google Scholar]
- Ceglarek, U.; Dittrich, J.; Becker, S.; Baumann, F.; Kortz, L.; Thiery, J. Quantification of seven apolipoproteins in human plasma by proteotypic peptides using fast LC-MS/MS. Proteom.—Clin. Appl. 2013, 7, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, J.; Adam, M.; Maas, H.; Hecht, M.; Reinicke, M.; Ruhaak, L.R.; Cobbaert, C.; Engel, C.; Wirkner, K.; Löffler, M.; et al. Targeted On-line SPE-LC-MS/MS Assay for the Quantitation of 12 Apolipoproteins from Human Blood. Proteomics 2018, 18, 1700279. [Google Scholar] [CrossRef]
- Aslanidis, C.; Schmitz, G. High-speed apolipoprotein E genotyping and apolipoprotein B3500 mutation detection using real-time fluorescence PCR and melting curves. Clin. Chem. 1999, 45, 1094–1097. [Google Scholar] [CrossRef]
- Cobbaert, C.M.; Althaus, H.; Begcevic Brkovic, I.; Ceglarek, U.; Coassin, S.; Delatour, V.; Deprez, L.; Dikaios, I.; Dittrich, J.; Hoofnagle, A.N.; et al. Towards an SI-Traceable Reference Measurement System for Seven Serum Apolipoproteins Using Bottom-Up Quantitative Proteomics: Conceptual Approach Enabled by Cross-Disciplinary/Cross-Sector Collaboration. Clin. Chem. 2021, 67, 478–489. [Google Scholar] [CrossRef]
- Kowal, R.C.; Herz, J.; Weisgraber, K.H.; Mahley, R.W.; Brown, M.S.; Goldstein, J.L. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990, 265, 10771–10779. [Google Scholar] [CrossRef]
- Sehayek, E.; Eisenberg, S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 1991, 266, 18259–18267. [Google Scholar] [CrossRef]
- Weisgraber, K.H.; Mahley, R.W.; Kowal, R.C.; Herz, J.; Goldstein, J.L.; Brown, M.S. Apolipoprotein C-I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990, 265, 22453–22459. [Google Scholar] [CrossRef]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J. Lipid Res. 2009, 50, S183–S188. [Google Scholar] [CrossRef]
- Cohn, J.S.; Tremblay, M.; Boulet, L.; Jacques, H.; Davignon, J.; Roy, M.; Bernier, L. Plasma concentration and lipoprotein distribution of ApoC-I is dependent on ApoE genotype rather than the Hpa I ApoC-I promoter polymorphism. Atherosclerosis 2003, 169, 63–70. [Google Scholar] [CrossRef]
- Mooijaart, S.P.; van Vliet, P.; van Heemst, D.; Rensen, P.C.N.; Berbée, J.F.P.; Jolles, J.; de Craen, A.J.M.; Westendorp, R.G.J. Plasma levels of apolipoprotein E and cognitive function in old age. Ann. N. Y. Acad. Sci. 2007, 1100, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.L. Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: A review. Atherosclerosis 2016, 255, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Shah, T.; Prieto, D.; Zhang, W.; Price, J.; Fowkes, G.R.; Cooper, J.; Talmud, P.J.; Humphries, S.E.; Sundstrom, J.; et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: Systematic review and meta-analysis of 14 015 stroke cases and pooled analysis of primary biomarker data from up to 60 883 individuals. Int. J. Epidemiol. 2013, 42, 475–492. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begcevic Brkovic, I.; Zöhrer, B.; Scholz, M.; Reinicke, M.; Dittrich, J.; Kamalsada, S.; Baber, R.; Beutner, F.; Teren, A.; Engel, C.; et al. Simultaneous Mass Spectrometry-Based Apolipoprotein Profiling and Apolipoprotein E Phenotyping in Patients with ASCVD and Mild Cognitive Impairment. Nutrients 2022, 14, 2474. https://doi.org/10.3390/nu14122474
Begcevic Brkovic I, Zöhrer B, Scholz M, Reinicke M, Dittrich J, Kamalsada S, Baber R, Beutner F, Teren A, Engel C, et al. Simultaneous Mass Spectrometry-Based Apolipoprotein Profiling and Apolipoprotein E Phenotyping in Patients with ASCVD and Mild Cognitive Impairment. Nutrients. 2022; 14(12):2474. https://doi.org/10.3390/nu14122474
Chicago/Turabian StyleBegcevic Brkovic, Ilijana, Benedikt Zöhrer, Markus Scholz, Madlen Reinicke, Julia Dittrich, Surab Kamalsada, Ronny Baber, Frank Beutner, Andrej Teren, Christoph Engel, and et al. 2022. "Simultaneous Mass Spectrometry-Based Apolipoprotein Profiling and Apolipoprotein E Phenotyping in Patients with ASCVD and Mild Cognitive Impairment" Nutrients 14, no. 12: 2474. https://doi.org/10.3390/nu14122474
APA StyleBegcevic Brkovic, I., Zöhrer, B., Scholz, M., Reinicke, M., Dittrich, J., Kamalsada, S., Baber, R., Beutner, F., Teren, A., Engel, C., Wirkner, K., Thiele, H., Löffler, M., Riedel-Heller, S. G., & Ceglarek, U. (2022). Simultaneous Mass Spectrometry-Based Apolipoprotein Profiling and Apolipoprotein E Phenotyping in Patients with ASCVD and Mild Cognitive Impairment. Nutrients, 14(12), 2474. https://doi.org/10.3390/nu14122474






