Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Antibodies and Immunoblotting
2.2. Animal and Human Studies
2.2.1. Animal Studies
2.2.2. Human Liver Samples
2.3. Metabolic Measurements
2.4. Cell Culture and Transient Transfections
2.5. RNA Extraction and Real-Time PCR Analysis
2.6. Serum Cytokine Levels Measurements
2.7. Histological Analysis of Tissue Sections
2.8. Statistical Analysis
2.9. Data and Resource Availability
3. Results
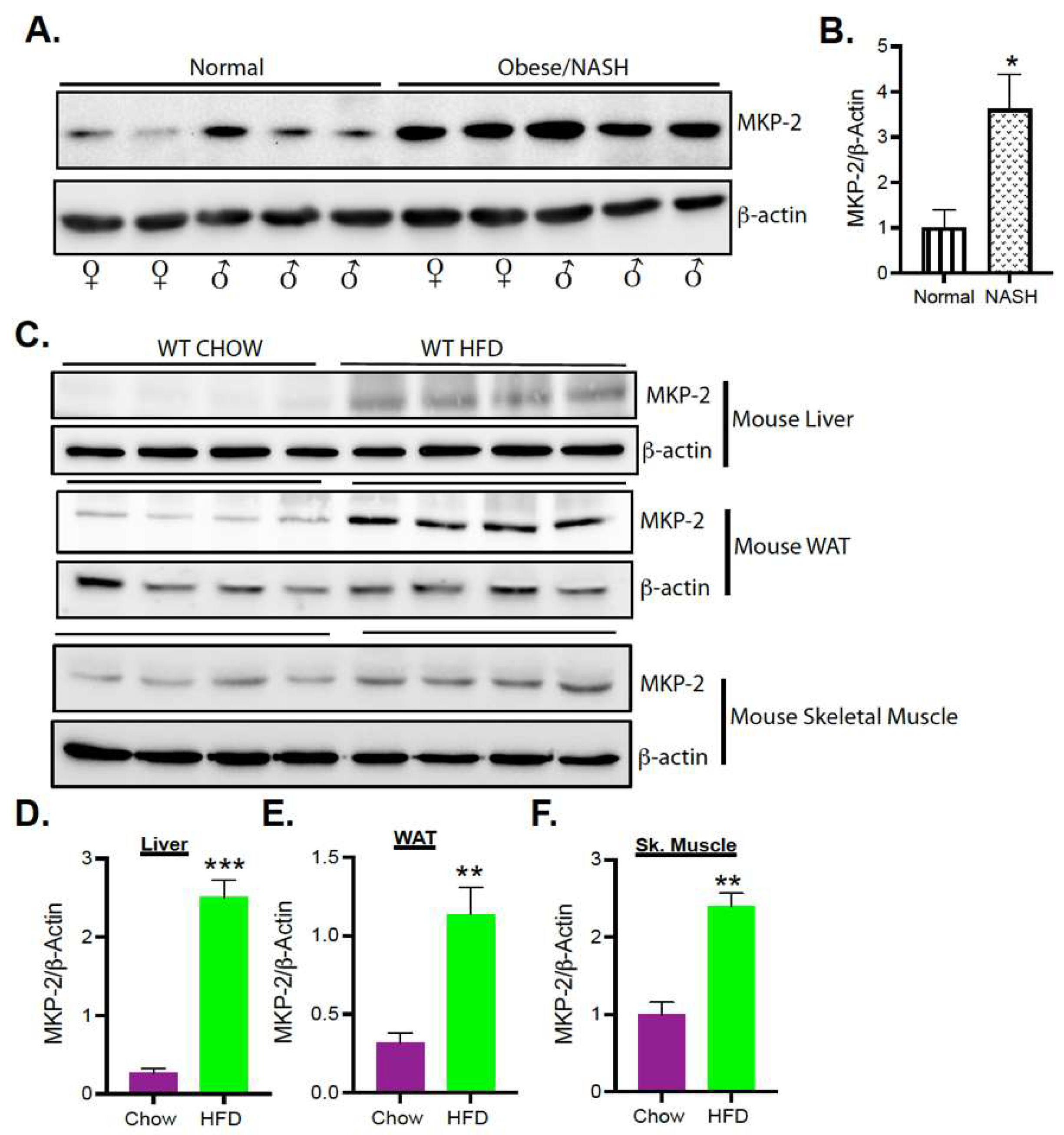
3.1. Upregulation of MKP-2 Expression in Human and Mice Livers with Obesity and Fatty Liver Disease
3.2. MAPK Phosphorylation in Human NASH Livers
3.3. Enhanced MAPK Phosphorylation in MKP-2-Deficient Mice
3.4. Resistance to Diet-Induced Obesity in MKP-2-Deficient Mice
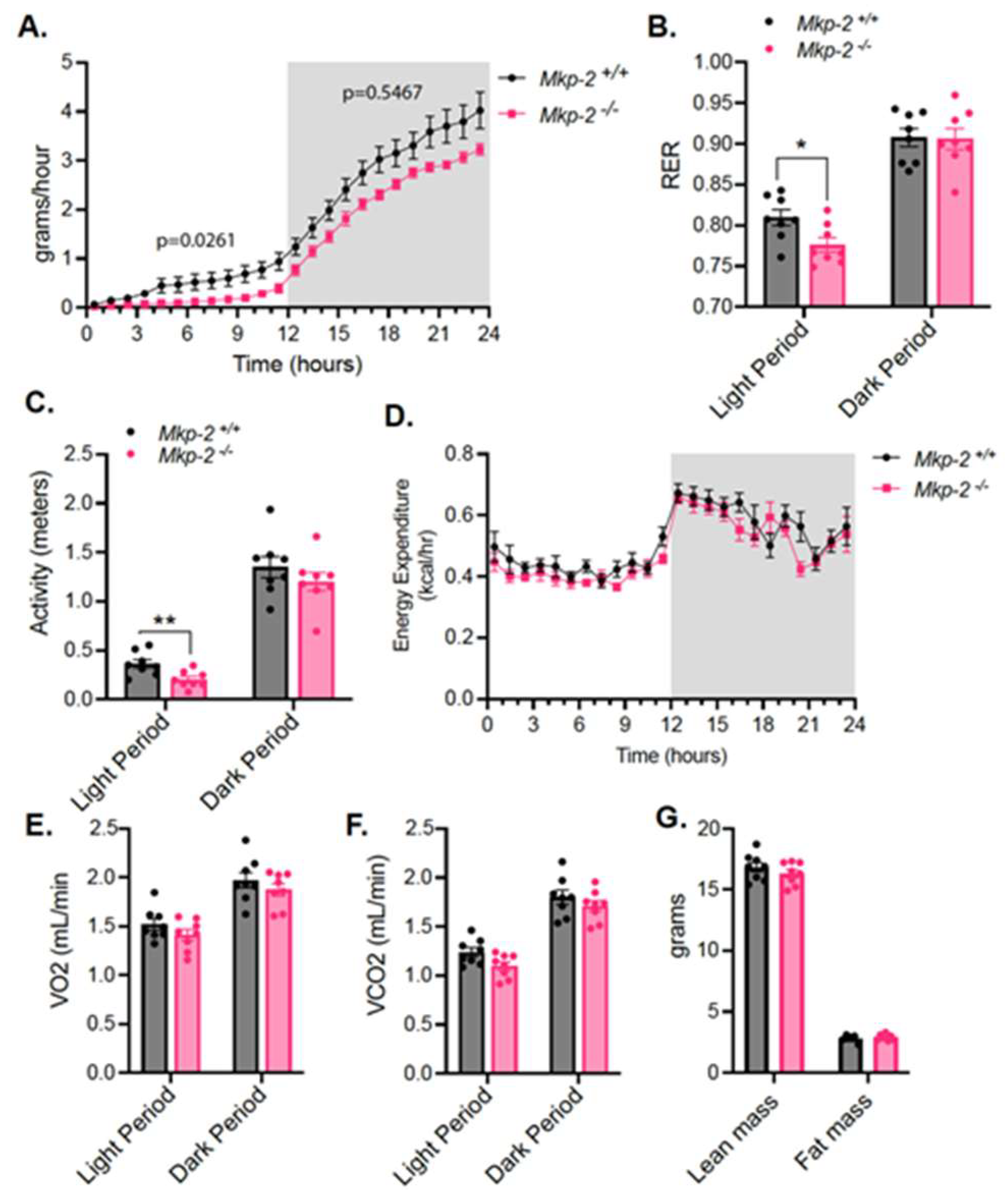
3.5. Reduced Food Intake and RER in MKP-2-Deficient Mice
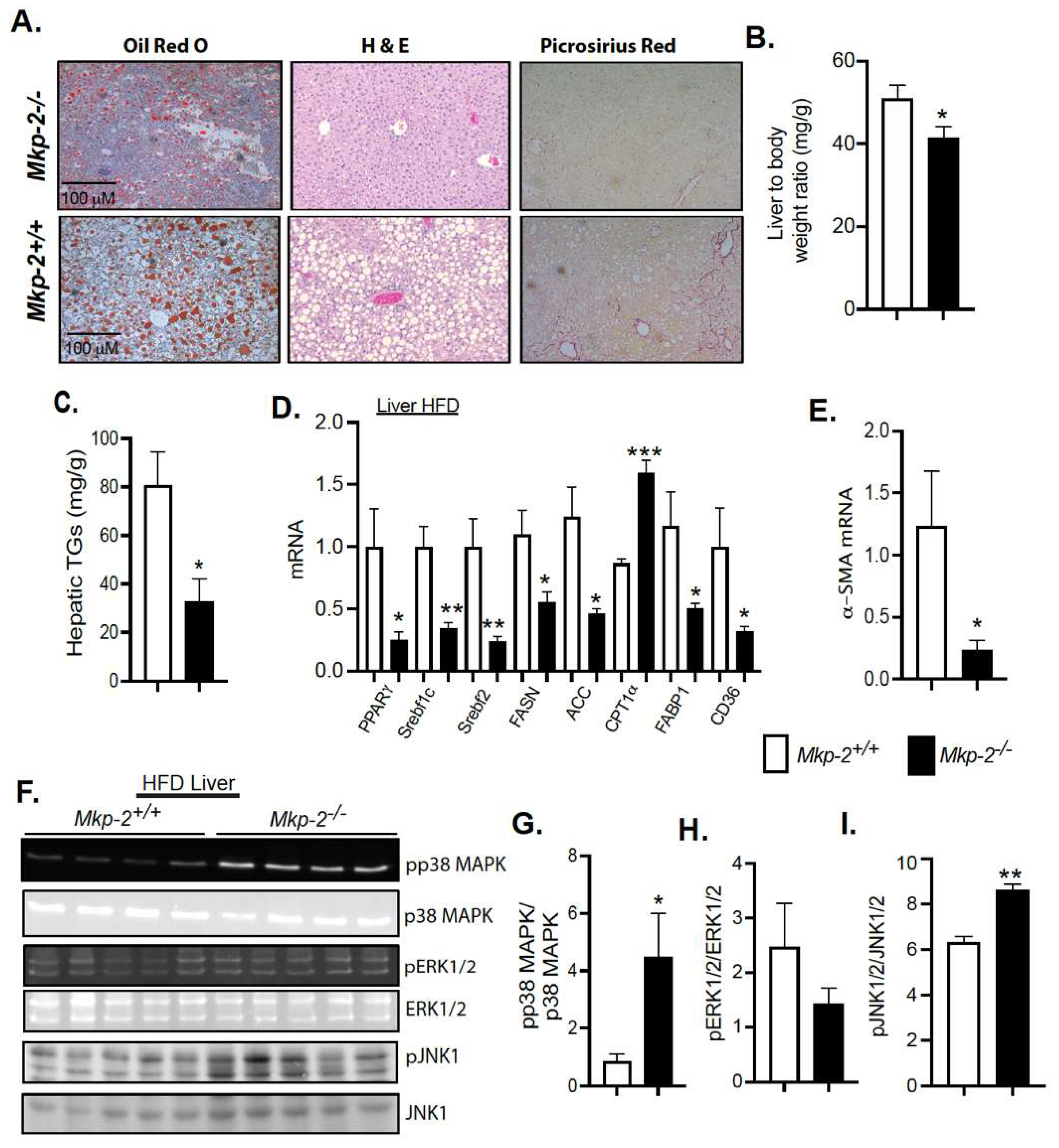
3.6. Protection from the Development of Hepatic Steatosis in MKP-2-Deficient Mice
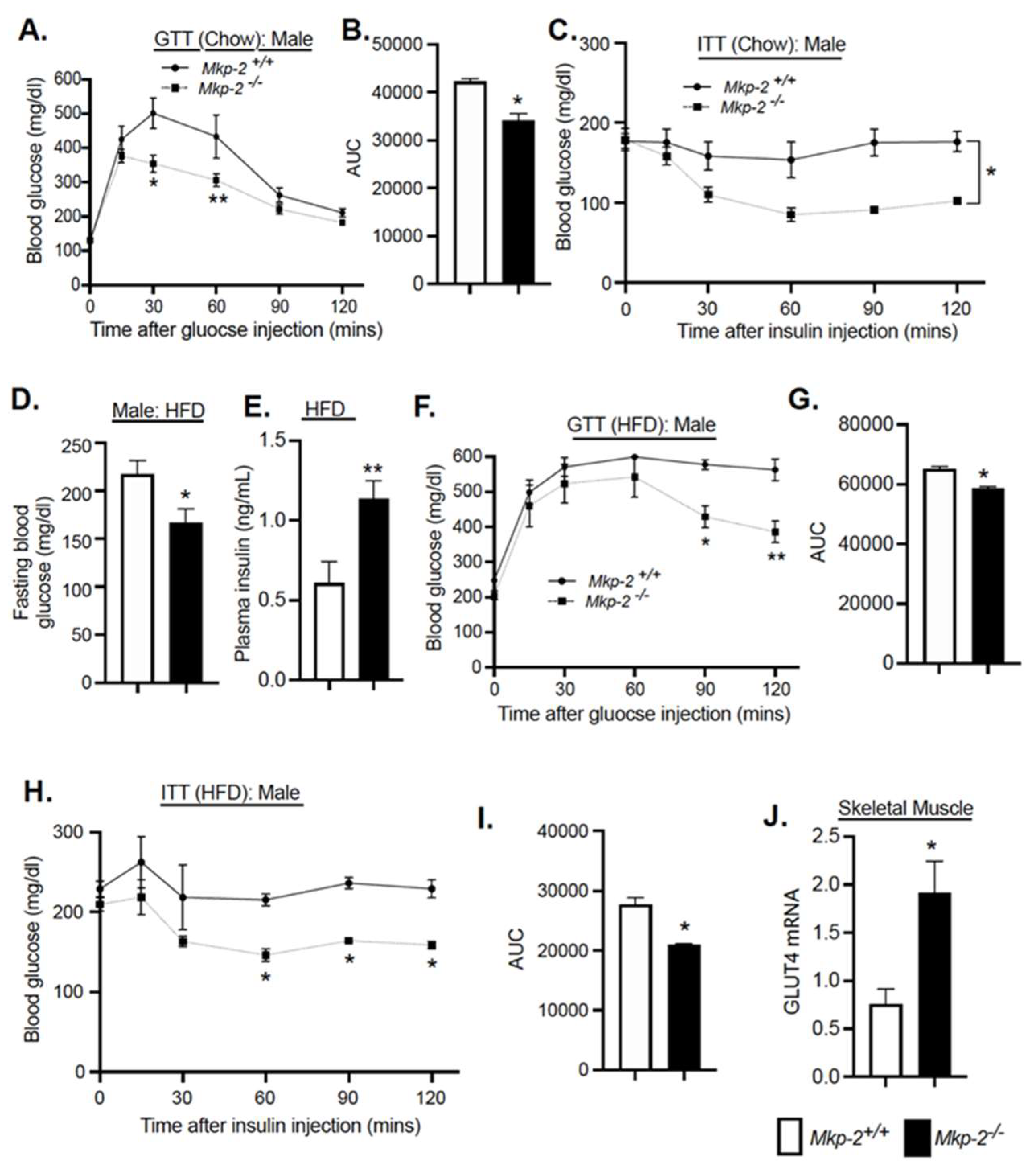
3.7. Glucose Tolerance and Insulin Sensitivity in Chow and HFD-Fed MKP-2-Deficient Mice
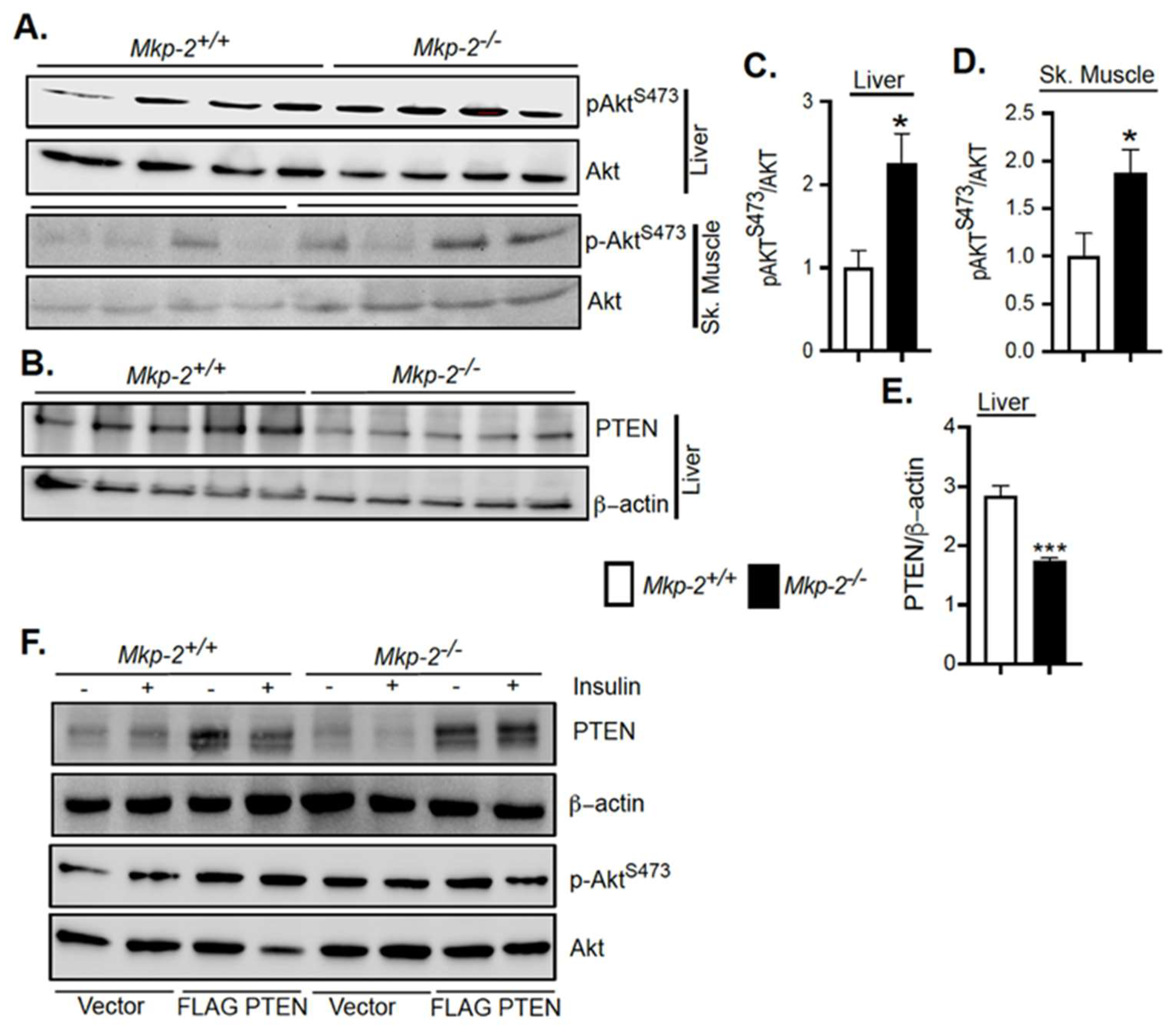
3.8. Enhanced Akt Signaling in MKP-2-Deficient Mice
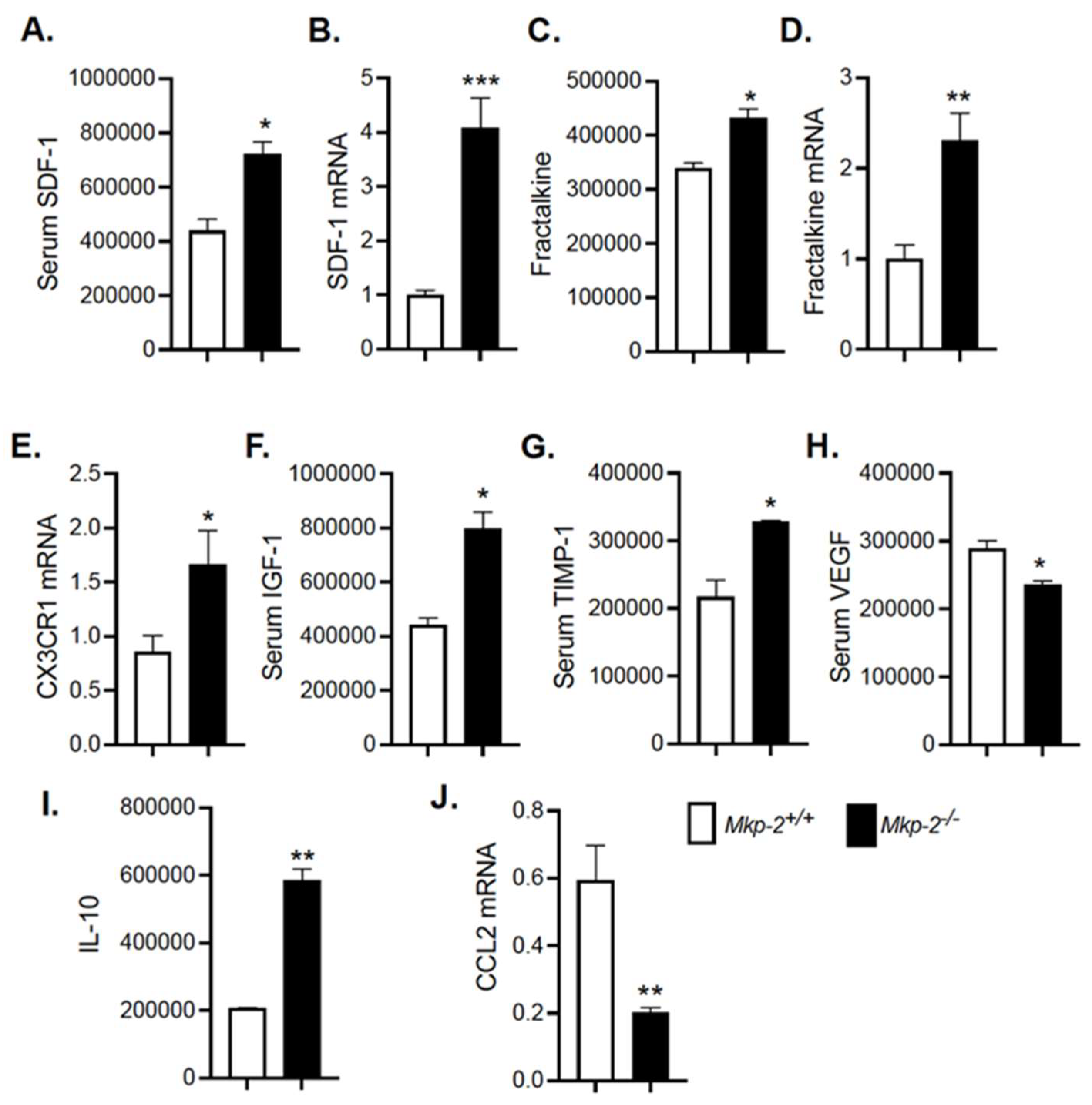
3.9. Modulation of Cytokines/Chemokines and Growth Factor Secretion in HFD-Fed MKP-2-Deficient Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo-Johnson, C.; Mincey, K. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, A.; Toschke, A.M.; Rosario, A.S.; Von Kries, R. Risk Factors for Obesity: Further Evidence for Stronger Effects on Overweight Children and Adolescents Compared to Normal-Weight Subjects. PLoS ONE 2011, 6, e15739. [Google Scholar] [CrossRef][Green Version]
- Lawan, A.; Zhang, L.; Gatzke, F.; Min, K.; Jurczak, M.J.; Al-Mutairi, M.; Richter, P.; Camporez, J.P.G.; Couvillon, A.; Pesta, D.; et al. Hepatic Mitogen-Activated Protein Kinase Phosphatase 1 Selectively Regulates Glucose Metabolism and Energy Homeostasis. Mol. Cell. Biol. 2015, 35, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Manieri, E.; Sabio, G. Stress kinases in the modulation of metabolism and energy balance. J. Mol. Endocrinol. 2015, 55, R11–R22. [Google Scholar] [CrossRef]
- Xiong, Y.; Collins, Q.F.; An, J.; Lupo, E.; Liu, H.-Y.; Liu, D.; Robidoux, J.; Liu, Z.; Cao, W. p38 Mitogen-activated Protein Kinase Plays an Inhibitory Role in Hepatic Lipogenesis. J. Biol. Chem. 2007, 282, 4975–4982. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, W. p38 mitogen-activated protein kinase: A critical node linking insulin resistance and cardiovascular diseases in type 2 diabetes mellitus. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 38–46. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Lin, J.; Wu, Z.; Yoon, J.C.; Zhang, C.Y.; Krauss, S.; Mootha, V.K.; Lowell, B.B.; Spiegelman, B.M. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol. Cell 2001, 8, 971–982. [Google Scholar] [CrossRef]
- Vernia, S.; Cavanagh-Kyros, J.; Garcia-Haro, L.; Sabio, G.; Barrett, T.; Jung, D.Y.; Kim, J.K.; Xu, J.; Shulha, H.P.; Garber, M.; et al. The PPARα-FGF21 Hormone Axis Contributes to Metabolic Regulation by the Hepatic JNK Signaling Pathway. Cell Metab. 2014, 20, 512–525. [Google Scholar] [CrossRef]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK Expression by Macrophages Promotes Obesity-Induced Insulin Resistance and Inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Kennedy, N.J.; Cavanagh-Kyros, J.; Jung, D.Y.; Ko, H.J.; Ong, H.; Barrett, T.; Kim, J.K.; Davis, R.J. Role of Muscle c-Jun NH2-Terminal Kinase 1 in Obesity-Induced Insulin Resistance. Mol. Cell. Biol. 2010, 30, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bengal, E.; Aviram, S.; Hayek, T. p38 MAPK in Glucose Metabolism of Skeletal Muscle: Beneficial or Harmful? Int. J. Mol. Sci. 2020, 21, 6480. [Google Scholar] [CrossRef] [PubMed]
- Somwar, R.; Koterski, S.; Sweeney, G.; Sciotti, R.; Djuric, S.; Berg, C.; Trevillyan, J.; Scherer, P.E.; Rondinone, C.M.; Klip, A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J. Biol. Chem. 2002, 277, 50386–50395. [Google Scholar] [CrossRef]
- Seternes, O.-M.; Kidger, A.M.; Keyse, S.M. Dual-specificity MAP kinase phosphatases in health and disease. Biochim. Biophys. Acta 2019, 1866, 124–143. [Google Scholar] [CrossRef]
- Caunt, J.C.; Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FEBS J. 2013, 280, 489–504. [Google Scholar] [CrossRef]
- Wu, J.J.; Roth, R.J.; Anderson, E.J.; Hong, E.-G.; Lee, M.-K.; Choi, C.S.; Neufer, P.D.; Shulman, G.; Kim, J.; Bennett, A.M. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006, 4, 61–73. [Google Scholar] [CrossRef]
- Lawan, A.; Min, K.; Zhang, L.; Canfran-Duque, A.; Jurczak, M.J.; Camporez, J.P.G.; Nie, Y.; Gavin, T.P.; Shulman, G.I.; Fernandez-Hernando, C.; et al. Skeletal Muscle–Specific Deletion of MKP-1 Reveals a p38 MAPK/JNK/Akt Signaling Node That Regulates Obesity-Induced Insulin Resistance. Diabetes 2018, 67, 624–635. [Google Scholar] [CrossRef]
- Wu, Z.; Jiao, P.; Huang, X.; Feng, B.; Feng, Y.; Yang, S.; Hwang, P.; Du, J.; Nie, Y.; Xiao, G.; et al. MAPK phosphatase–3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice. J. Clin. Investig. 2010, 120, 3901–3911. [Google Scholar] [CrossRef]
- Emanuelli, B.; Eberlé, D.; Suzuki, R.; Kahn, C.R. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 3545–3550. [Google Scholar] [CrossRef]
- Parsons, R.; Simpson, L. PTEN and cancer. Methods Mol. Biol. 2003, 222, 147–166. [Google Scholar] [PubMed]
- Al-Mutairi, M.S.; Cadalbert, L.C.; McGachy, H.A.; Shweash, M.; Schroeder, J.; Kurnik, M.; Sloss, C.M.; Bryant, C.E.; Alexander, J.; Plevin, R. MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by Leishmania mexicana. PLoS Pathog. 2010, 6, e1001192. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, M.; Al-Harthi, S.; Cadalbert, L.; Plevin, R. Over-expression of mitogen-activated protein kinase phosphatase-2 enhances adhesion molecule expression and protects against apoptosis in human endothelial cells. Br. J. Pharmacol. 2010, 161, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Lawan, A.; Al-Harthi, S.; Cadalbert, L.; McCluskey, A.G.; Shweash, M.; Grassia, G.; Grant, A.; Boyd, M.; Currie, S.; Plevin, R. Deletion of the Dual Specific Phosphatase-4 (DUSP-4) Gene Reveals an Essential Non-redundant Role for MAP Kinase Phosphatase-2 (MKP-2) in Proliferation and Cell Survival. J. Biol. Chem. 2011, 286, 12933–12943. [Google Scholar] [CrossRef]
- Lawan, A.; Torrance, E.; Shweash, M.; Alnasser, S.; Neamatallah, T.; Schroeder, J.; Plevin, R.; Al-Harthi, S. MKP-2: Out of the DUSP-bin and back into the limelight. Biochem. Soc. Trans. 2012, 40, 235–239. [Google Scholar] [CrossRef]
- Jiao, H.; Tang, P.; Zhang, Y. MAP Kinase Phosphatase 2 Regulates Macrophage-Adipocyte Interaction. PLoS ONE 2015, 10, e0120755. [Google Scholar] [CrossRef]
- Mina, A.; LeClair, R.A.; LeClair, K.B.; Cohen, D.E.; Lantier, L.; Banks, A.S. CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 2018, 28, 656–666.e1. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Ren, J.M.; A Marshall, B.; Mueckler, M.M.; McCaleb, M.; Amatruda, J.M.; I Shulman, G. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J. Clin. Investig. 1995, 95, 429–432. [Google Scholar] [CrossRef]
- Kandel, E.S.; Hay, N. The Regulation and Activities of the Multifunctional Serine/Threonine Kinase Akt/PKB. Exp. Cell Res. 1999, 253, 210–229. [Google Scholar] [CrossRef]
- Lee, Y.S.; Morinaga, H.; Kim, J.J.; Lagakos, W.; Taylor, S.; Keshwani, M.; Perkins, G.; Dong, H.; Kayali, A.G.; Sweet, I.R.; et al. The Fractalkine/CX3CR1 System Regulates β Cell Function and Insulin Secretion. Cell 2013, 153, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Ishikawa, K.; Takeda, K.; Fujimoto, M.; Ono, H.; Kumagai, J.; Inoue, H.; Yokoh, H.; Yokote, K. CXCL12-CXCR4 pathway activates brown adipocytes and induces insulin resistance in CXCR4-deficient mice under high-fat diet. Sci. Rep. 2019, 9, 6165. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Adamek, A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis (review). Int. J. Oncol. 2012, 41, 1919–1931. [Google Scholar] [CrossRef]
- Ramezani-Moghadam, M.; Wang, J.; Ho, V.; Iseli, T.J.; Alzahrani, B.; Xu, A.; Van der Poorten, D.; Qiao, L.; George, J.; Hebbard, L. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J. Biol. Chem. 2015, 290, 5533–5542. [Google Scholar] [CrossRef]
- Elias, I.; Franckhauser, S.; Ferré, T.; Vilà, L.; Tafuro, S.; Muñoz, S.; Roca, C.; Ramos, D.; Pujol, A.; Riu, E.; et al. Adipose Tissue Overexpression of Vascular Endothelial Growth Factor Protects Against Diet-Induced Obesity and Insulin Resistance. Diabetes 2012, 61, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. cJun NH2-terminal kinase 1 (JNK1): Roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 2010, 35, 490–496. [Google Scholar] [CrossRef]
- Lawan, A.; Bennett, A.M. Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism. Trends Endocrinol. Metab. 2017, 28, 868–878. [Google Scholar] [CrossRef]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The physiological control of eating: Signals, neurons, and networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef]
- Pytka, K.; Dawson, N.; Tossell, K.; Ungless, M.A.; Plevin, R.; Brett, R.R.; Bushell, T.J. Mitogen-activated protein kinase phosphatase-2 deletion modifies ventral tegmental area function and connectivity and alters reward processing. Eur. J. Neurosci. 2020, 52, 2838–2852. [Google Scholar] [CrossRef]
- Clemmons, D.R. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J. Clin. Investig. 2004, 113, 25–27. [Google Scholar] [CrossRef]
- Yano, T.; Liu, Z.; Donovan, J.; Thomas, M.K.; Habener, J.F. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes 2007, 56, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Cavanagh-Kyros, J.; Ko, H.J.; Jung, D.Y.; Gray, S.; Jun, J.Y.; Barrett, T.; Mora, A.; Kim, J.; Davis, R.J. Prevention of Steatosis by Hepatic JNK1. Cell Metab. 2009, 10, 491–498. [Google Scholar] [CrossRef]
- Kujiraoka, T.; Satoh, Y.; Ayaori, M.; Shiraishi, Y.; Arai-Nakaya, Y.; Hakuno, D.; Yada, H.; Kuwada, N.; Endo, S.; Isoda, K.; et al. Hepatic Extracellular Signal–Regulated Kinase 2 Suppresses Endoplasmic Reticulum Stress and Protects from Oxidative Stress and Endothelial Dysfunction. J. Am. Heart Assoc. 2013, 2, e000361. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Lehr, S.; Hartwig, S.; Haas, J.; Kaber, G.; Dicken, H.-D.; Susanto, F.; Bohne, L.; Jacob, S.; Nitzgen, U.; et al. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism and the secretome of human liver cell line HepG2. Arch. Physiol. Biochem. 2014, 120, 216–227. [Google Scholar] [CrossRef]
- Engelking, L.J.; Cantoria, M.J.; Xu, Y.; Liang, G. Developmental and extrahepatic physiological functions of SREBP pathway genes in mice. Semin. Cell Dev. Biol. 2018, 81, 98–109. [Google Scholar] [CrossRef]
- Rong, S.; Cortés, V.A.; Rashid, S.; Anderson, N.N.; McDonald, J.G.; Liang, G.; Moon, Y.A.; Hammer, R.E.; Horton, J.D. Expression of SREBF1c Requires SREBF-2-mediated Generation of a Sterol Ligand for LXR in Livers of Mice. eLife 2017, 6, e25015. [Google Scholar] [CrossRef]
- Kotzka, J.; Knebel, B.; Haas, J.; Kremer, L.; Jacob, S.; Hartwig, S.; Nitzgen, U.; Muller–Wieland, D. Preventing Phosphorylation of Sterol Regulatory Element-Binding Protein 1a by MAP-Kinases Protects Mice from Fatty Liver and Visceral Obesity. PLoS ONE 2012, 7, e32609. [Google Scholar] [CrossRef]
- Barbour, M.; Plevin, R.; Jiang, H.R. MAP kinase phosphatase 2 deficient mice develop attenuated experimental autoimmune encephalomyelitis through regulating dendritic cells and T cells. Sci. Rep. 2016, 6, 38999. [Google Scholar] [CrossRef]


| Ingredient | kcal./g | g/kg | kcal./kg |
|---|---|---|---|
| Casein | 3.58 | 200 | 716.00 |
| Constarch | 3.6 | 0 | 0.00 |
| Dyetrose | 3.8 | 125.00 | 475.00 |
| Sucrose | 4 | 68.8 | 275.20 |
| Cellulose | 0 | 50 | 0.00 |
| Soybean Oil | 9 | 25 | 225.00 |
| TBHQ | 0 | 0.005 | 0.00 |
| Lard | 9 | 245 | 2205.00 |
| Salt Mix #210088 | 1.6 | 10 | 16.00 |
| Dicalcium Phosphate | 0 | 13 | 0.00 |
| Calcium Carbpnate | 0 | 5.5 | 0.00 |
| Patassium Citrate H2O | 0 | 16.5 | 0.00 |
| Vitamin Mix #300050 | 3.92 | 10 | 39.20 |
| L-Cystine | 4 | 3 | 12.00 |
| Choline Bitartrate | 0 | 2 | 0.00 |
| 773.805 | 3963.400 |
| Ingredient | kcal./g | g/kg | kcal./kg |
|---|---|---|---|
| Casein | 3.58 | 200 | 716.00 |
| L-Cystine | 4 | 3 | 12.00 |
| Sucrose | 4 | 350 | 1400.00 |
| Constarch | 3.6 | 315 | 1134.00 |
| Dyetrose | 3.8 | 35 | 133.00 |
| Soybean Oil | 9 | 25 | 225.00 |
| t-Butylhy droquinone | 0 | 0.005 | 0.00 |
| Lard | 9 | 20 | 180.00 |
| Cellulose | 0 | 50 | 0.00 |
| Salt Mix #210088 | 1.6 | 10 | 16.00 |
| Dicalcium Phosphate | 0 | 13 | 0.00 |
| Calcium Carbpnate | 0 | 5.5 | 0.00 |
| Patassium Citrate H2O | 0 | 16.5 | 0.00 |
| Vitamin Mix #300050 | 3.92 | 10 | 39.20 |
| Choline Bitartrate | 0 | 2 | 0.00 |
| 1055.005 | 3855.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, S.; Sellers, J.; Smith, S.; Bhogoju, S.; Junkins, S.; Welch, M.; Willoughby, O.; Ghimire, N.; Secunda, C.; Barmanova, M.; et al. Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease. Nutrients 2022, 14, 2475. https://doi.org/10.3390/nu14122475
Fernando S, Sellers J, Smith S, Bhogoju S, Junkins S, Welch M, Willoughby O, Ghimire N, Secunda C, Barmanova M, et al. Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease. Nutrients. 2022; 14(12):2475. https://doi.org/10.3390/nu14122475
Chicago/Turabian StyleFernando, Savanie, Jacob Sellers, Shauri Smith, Sarayu Bhogoju, Sadie Junkins, Morgan Welch, Orion Willoughby, Nabin Ghimire, Cassandra Secunda, Marina Barmanova, and et al. 2022. "Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease" Nutrients 14, no. 12: 2475. https://doi.org/10.3390/nu14122475
APA StyleFernando, S., Sellers, J., Smith, S., Bhogoju, S., Junkins, S., Welch, M., Willoughby, O., Ghimire, N., Secunda, C., Barmanova, M., Kumer, S. C., Min, K., & Lawan, A. (2022). Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease. Nutrients, 14(12), 2475. https://doi.org/10.3390/nu14122475







