Decreased Acetic Acid in the Stool of Preterm Infants Is Associated with an Increased Risk of Bronchopulmonary Dysplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Selection and Study Design
2.2. Maternal and Infant Data Collection
2.3. Neonatal Clinical Outcomes
2.4. Breastmilk and Fecal Samples Collection
2.5. Short-Chain Fatty Acid Assay
2.6. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, E.A.; Edwards, E.M.; Greenberg, L.T.; Soll, R.F.; Ehret, D.E.Y.; Horbar, J.D. Severity of Bronchopulmonary Dysplasia Among Very Preterm Infants in the United States. Pediatrics 2021, 148, e2020030007. [Google Scholar] [CrossRef] [PubMed]
- Balany, J.; Bhandari, V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2015, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K. Commentary: Understanding the Impact of Infection, Inflammation and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflamm. 2017, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Mara, M.A.; Good, M.; Weitkamp, J.H. Innate and adaptive immunity in necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 394–399. [Google Scholar] [CrossRef]
- Leviton, A.; Dammann, O.; Engelke, S.; Allred, E.; Kuban, K.C.; O’Shea, T.M.; Paneth, N.; ELGAN Study Investigators. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. 2010, 99, 1795–1800. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Zheng, N.; Gao, Y.; Zhu, W.; Meng, D.; Walker, W.A. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020, 15, e0229283. [Google Scholar] [CrossRef]
- Zhang, Q.; Ran, X.; He, Y.; Ai, Q.; Shi, Y. Acetate Downregulates the Activation of NLRP3 Inflammasomes and Attenuates Lung Injury in Neonatal Mice With Bronchopulmonary Dysplasia. Front. Pediatr. 2020, 8, 595157. [Google Scholar] [CrossRef]
- Berkhout, D.J.C.; Niemarkt, H.J.; Benninga, M.A.; Budding, A.E.; van Kaam, A.H.; Kramer, B.W.; Pantophlet, C.M.; van Weissenbruch, M.M.; de Boer, N.K.H.; de Meij, T.G.J. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr. Res. 2018, 83, 412–419. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.K.; Gray, J.E.; McCormick, M.C.; Workman, K.; Goldmann, D.A. Score for Neonatal Acute Physiology: A physiologic severity index for neonatal intensive care. Pediatrics 1993, 3, 617–623. [Google Scholar] [CrossRef]
- Martin, C.R.; Cheesman, A.; Brown, J.; Makda, M.; Kutner, A.J.; DaSilva, D.; Zaman, M.; Freedman, S.D. Factors Determining Optimal Fatty Acid Absorption in Preterm Infants. J. Pediatric Gastroenterol. Nutr. 2016, 62, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Subirana, I.; Sanz, H.; Vila, J. Building Bivariate Tables: The compare Groups Package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Leviton, A.; Allred, E.N.; Yamamoto, H.; Fichorova, R.N.; Kuban, K.; O’Shea, T.M.; Dammann, O.; Investigators, E.S. Antecedents and correlates of blood concentrations of neurotrophic growth factors in very preterm newborns. Cytokine 2017, 94, 21–28. [Google Scholar] [CrossRef]
- Frau, A.; Lett, L.; Slater, R.; Young, G.R.; Stewart, C.J.; Berrington, J.; Hughes, D.M.; Embleton, N.; Probert, C. The Stool Volatile Metabolome of Pre-Term Babies. Molecules 2021, 26, 3341. [Google Scholar] [CrossRef]
- Yu, Z.T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Rao, C.; Coyte, K.Z.; Bainter, W.; Geha, R.S.; Martin, C.R.; Rakoff-Nahoum, S. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature 2021, 591, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Cabridain, C.; Aubert, H.; Kaeffer, B.; Badon, V.; Boivin, M.; Dochez, V.; Winer, N.; Faurel-Paul, E.; Planche, L.; Riochet, D.; et al. Effectiveness of an antenatal maternal supplementation with prebiotics for preventing atopic dermatitis in high-risk children (the PREGRALL study): Protocol for a randomised controlled trial. BMJ Open 2019, 9, e024974. [Google Scholar] [CrossRef]
- Prescott, S. A Randomised, Double-Blind, Placebo-Controlled Trial to Investigate the Effects of Maternal Dietary Prebiotic Fibre Supplementation, from Early Pregnancy to 6 Months Lactation, on Infant Outcomes of Immune Function and Eczema Diagnosis. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369064&isReview=true (accessed on 1 June 2022).
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.; Cummings, J.; Hand, I.; Adams-Chapman, I.; Aucott, S.W.; Puopolo, K.M.; Goldsmith, J.P.; Kaufman, D.; Martin, C.; Mowitz, M. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
- Murphy, K.; Ross, R.P.; Ryan, C.A.; Dempsey, E.M.; Stanton, C. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Front. Nutr. 2021, 8, 667188. [Google Scholar] [CrossRef]
| N = 72 | Mean ± SD | Range |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (years) | 31.2 ± 5.84 | 18.0–49.0 |
| Race, White | 40 (55.6%) | - |
| Delivery, c-section | 55 (76.4%) | - |
| Gravida (≥2) | 53 (73.6%) | - |
| Multiparous | 8 (11.1%) | - |
| Preeclampsia | 9 (12.5%) | - |
| Gestational diabetes mellitus | 4 (5.6%) | - |
| Pregnancy-induced hypertension | 7 (9.7%) | - |
| Neonatal characteristics | ||
| Gestational age (weeks) | 26.3 ± 1.19 | 23.4–27.9 |
| Sex, female | 36 (50.0%) | - |
| Birthweight (g) | 839 ± 187 | 350–1160 |
| Birthweight z-score | −0.10 ± 0.90 | −2.89–1.61 |
| Birth head circumference (cm) | 24.0 ± 2.04 | 19.5–34.5 |
| Birth length (cm) | 34.3 ± 3.18 | 25.5–45.5 |
| Apgar (1min) | 5.19 ± 2.10 | 1.0–8.0 |
| Apgar (5min) | 7.18 ± 1.14 | 3.0–9.0 |
| Day 14 GV > 10 g/k/d | 11 (15.3%) | - |
| Day 28 GV > 10 g/k/d | 35 (48.6%) | - |
| Days of abx in first 14 days | 6.28 ± 4.11 | 2.0–14.0 |
| Days of abx in first 28 days | 9.12 ± 7.15 | 2.0–28.0 |
| SNAP Score | 13.9 ± 9.15 | 0.0–36.0 |
| BPD | 45 (62.5%) | - |
| ROP | 46 (63.9%) | - |
| NEC | 3 (4.2%) | - |
| Sepsis (blood culture) | 12 (16.7%) | - |
| Mortality | 4 (5.6%) | - |
| BPD | ROP | |||||
|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | |
| N | 27 | 45 | 22 | 46 | ||
| Maternal characteristics | ||||||
| Maternal age (years) | 32.3 ± 6.61 | 30.7 ± 5.68 | 0.3 | 32.0 ± 5.80 | 31.1 ± 6.33 | 0.6 |
| Race, White | 17 (63.0%) | 22 (48.9%) | 0.4 | 16 (72.7%) | 21 (45.7%) | 0.07 |
| Delivery | 0.3 | 0.09 | ||||
| Vaginal | 10 (37.0%) | 10 (22.2%) | 10 (45.5%) | 10 (21.7%) | ||
| C-section | 17 (63.0%) | 35 (77.8%) | 12 (54.5%) | 36 (78.3%) | ||
| Gravida (≥2) | 17 (63.0%) | 32 (71.1%) | 0.7 | 14 (63.6%) | 32 (69.6%) | 0.8 |
| Multiparous | 2 (7.41%) | 5 (11.1%) | 0.7 | 0 (0.00%) | 6 (13.0%) | 0.2 |
| Preeclampsia | 1 (3.70%) | 7 (15.6%) | 0.2 | 1 (4.55%) | 7 (15.2%) | 0.3 |
| Gestational diabetes mellitus | 3 (11.1%) | 1 (2.22%) | 0.2 | 3 (13.6%) | 1 (2.17%) | 0.1 |
| Pregnancy-induced hypertension | 3 (11.1%) | 3 (6.67%) | 0.7 | 2 (9.09%) | 4 (8.70%) | 1.0 |
| Neonatal characteristics | ||||||
| Gestational age (weeks) | 26.9 ± 0.81 | 26.0 ± 1.24 | <0.001 | 26.9 ± 0.96 | 26.0 ± 1.18 | 0.001 |
| Sex, female | 9 (33.3%) | 24 (53.3%) | 0.2 | 6 (27.3%) | 26 (56.5%) | 0.05 |
| Birthweight (g) | 966 ± 134 | 776 ± 168 | <0.001 | 932 ± 140 | 793 ± 180 | 0.001 |
| Birthweight z-score | 0.30 ± 0.62 | −0.30 ± 0.92 | 0.002 | 0.09 ± 0.56 | −0.18 ± 0.98 | 0.2 |
| Birth head circumference (cm) | 25.4 ± 2.23 | 23.3 ± 1.33 | <0.001 | 24.8 ± 1.44 | 23.7 ± 2.21 | 0.02 |
| Birth length (cm) | 35.8 ± 1.81 | 33.4 ± 2.72 | <0.001 | 36.3 ± 2.05 | 33.3 ± 2.57 | <0.001 |
| Apgar (1 min) | 5.96 ± 1.81 | 4.91 ± 2.18 | 0.03 | 5.73 ± 2.14 | 5.07 ± 2.13 | 0.2 |
| Apgar (5 min) | 7.70 ± 0.87 | 6.93 ± 1.19 | 0.002 | 7.14 ± 1.46 | 7.20 ± 0.98 | 0.9 |
| Day 14 GV > 10 g/k/d | 2 (7.69%) | 7 (16.7%) | 0.5 | 3 (14.3%) | 6 (14.0%) | 1.0 |
| Day 14 BM volume (ml/kg) | 551 ± 364 | 320 ± 273 | 0.007 | 454 ± 331 | 369 ± 329 | 0.3 |
| Days of abx use in first 14 days | 4.70 ± 3.17 | 6.93 ± 4.23 | 0.01 | 5.41 ± 4.12 | 6.78 ± 3.88 | 0.2 |
| Days of abx use in first 28 days | 6.37 ± 6.35 | 9.98 ± 6.67 | 0.03 | 7.64 ± 7.59 | 9.63 ± 6.31 | 0.3 |
| SNAP Score | 8.78 ± 7.80 | 15.7 ± 8.45 | 0.001 | 11.1 ± 8.01 | 14.9 ± 8.74 | 0.08 |
| PDA closure (indomethacin) | 17 (63.0%) | 33 (73.3%) | 0.5 | 13 (59.1%) | 34 (73.9%) | 0.3 |
| NEC | 0 (0.00%) | 3 (6.98%) | 0.3 | 1 (4.76%) | 2 (4.55%) | 1.0 |
| Sepsis (blood culture) | 3 (11.5%) | 8 (18.2%) | 0.5 | 3 (14.3%) | 8 (17.8%) | 1.0 |
| Mortality | 0 (0.00%) | 2 (4.76%) | 0.5 | 1 (4.76%) | 1 (2.33%) | 1.0 |
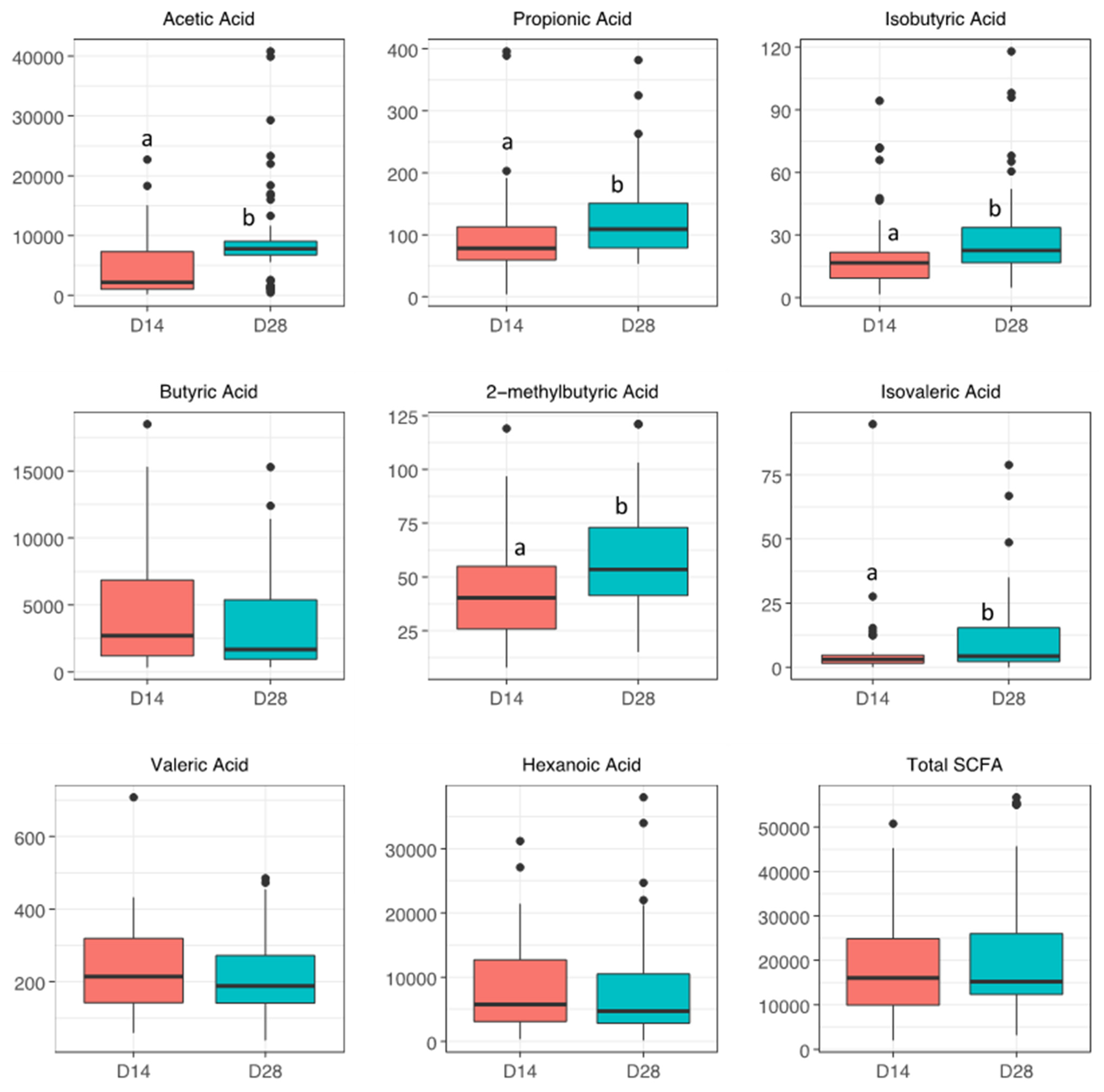
| SCFA (ng/mL) | No BPD | BPD | p | p (Adj) | No ROP | ROP | p | p (Adj) |
|---|---|---|---|---|---|---|---|---|
| BM Day 14 | ||||||||
| N | 26 | 42 | 21 | 43 | ||||
| Acetic | 3590 [1080, 7185] | 1730 [810, 7615] | 0.7 | 0.8 | 5020 [891, 8280] | 1710 [1010, 7055] | 0.2 | 0.4 |
| Propionic | 73.8 [57.5, 110] | 87.3 [63.6, 120] | 0.4 | 0.8 | 82.8 [58.8, 156] | 76.1 [58.1, 108] | 0.7 | 0.8 |
| Isobutyric | 18.0 [11.6, 21.8] | 12.6 [8.14, 23.1] | 0.2 | 0.8 | 16.8 [9.25, 23.9] | 16.6 [8.67, 23.0] | 0.9 | 0.9 |
| Butyric | 2390 [1198, 5615] | 3345 [1272, 7012] | 0.7 | 0.8 | 2130 [982, 3970] | 4390 [1240, 7290] | 0.1 | 0.4 |
| 2-methylbutyric | 47.9 [39.3, 61.6] | 32.8 [22.8, 49.7] | 0.01 | 0.1 | 43.2 [35.3, 61.9] | 32.9 [23.9, 51.8] | 0.2 | 0.4 |
| Isovaleric | 2.88 [1.52, 3.81] | 3.10 [1.76, 4.96] | 0.6 | 0.8 | 1.98 [1.39, 3.78] | 3.22 [1.47, 4.70] | 0.6 | 0.7 |
| Valeric | 180 [140, 313] | 238 [172, 328] | 0.4 | 0.8 | 183 [108, 260] | 232 [158, 336] | 0.05 | 0.4 |
| Hexanoic | 6430 [3500, 13400] | 7595 [2640, 12175] | 0.5 | 0.8 | 4660 [1820, 8680] | 9020 [3300, 14100] | 0.1 | 0.4 |
| Total SCFA | 14857 [9948, 25013] | 16797 [10451, 24277] | 0.9 | 0.9 | 12617 [9566, 22302] | 17456 [10823, 26175] | 0.4 | 0.5 |
| BM Day 28 | ||||||||
| N | 23 | 41 | 18 | 43 | ||||
| Acetic | 7850 [6605, 9530] | 7670 [6900, 9150] | 1.0 | 1.0 | 8415 [7168, 18050] | 7500 [6215, 8830] | 0.03 | 0.1 |
| Propionic | 111 [74.7, 158] | 108 [84.8, 139] | 1.0 | 1.0 | 126 [87.6, 166] | 103 [76.0, 126] | 0.08 | 0.2 |
| Isobutyric | 20.5 [14.3, 33.6] | 22.7 [17.5, 33.7] | 0.6 | 1.0 | 25.8 [18.7, 36.8] | 21.2 [14.4, 32.9] | 0.2 | 0.3 |
| Butyric | 1790 [1125, 5970] | 1670 [892, 3590] | 0.6 | 1.0 | 1275 [930, 5450] | 1890 [887, 5540] | 0.8 | 0.9 |
| 2-methylbutyric | 54.1 [42.8, 73.5] | 51.8 [40.7, 71.2] | 0.7 | 1.0 | 64.2 [46.6, 73.2] | 44.3 [38.1, 65.9] | 0.07 | 0.2 |
| Isovaleric | 3.96 [2.27, 19.4] | 4.27 [1.41, 9.68] | 0.8 | 1.0 | 18.9 [4.13, 25.7] | 3.49 [1.32, 7.94] | 0.02 | 0.1 |
| Valeric | 214 [140, 242] | 185 [155, 298] | 0.4 | 1.0 | 202 [156, 229] | 184 [140, 294] | 0.9 | 0.9 |
| Hexanoic | 4890 [2645, 12450] | 4590 [3310, 9760] | 1.0 | 1.0 | 4210 [2435, 11590] | 4890 [3475, 10500] | 0.4 | 0.6 |
| Total SCFA | 15597 [12498, 29200] | 15220 [12091, 25028] | 0.7 | 1.0 | 19381 [12416, 39166] | 14948 [11843, 25907] | 0.3 | 0.5 |
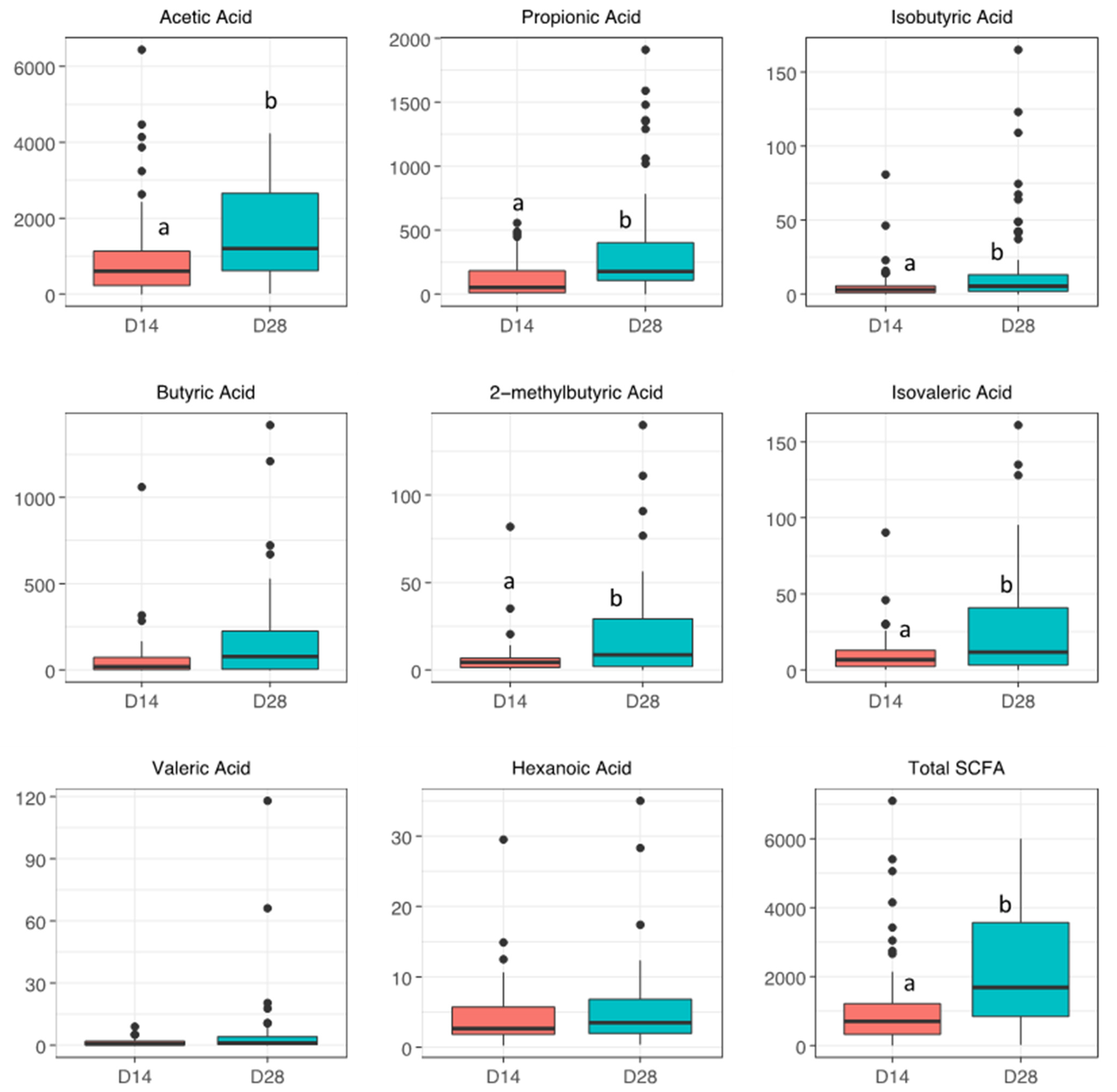
| SCFA (ng/mL) | No BPD | BPD | p | p (Adj) | No ROP | ROP | p | p (Adj) |
|---|---|---|---|---|---|---|---|---|
| Stool Day 14 | ||||||||
| N | 26 | 37 | 20 | 39 | ||||
| Acetic Acid | 1000 [656, 1368] | 446 [215, 779] | 0.01 | 0.05 | 918 [415, 1512] | 595 [212, 1025] | 0.09 | 0.4 |
| Propionic Acid | 72.2 [11.2, 167] | 22.9 [12.0, 99.1] | 0.5 | 0.5 | 41.1 [14.4, 120] | 52.2 [11.0, 121] | 0.9 | 0.9 |
| Isobutyric Acid | 3.03 [1.44, 12.1] | 3.29 [1.03, 5.39] | 0.2 | 0.4 | 3.38 [1.59, 5.78] | 3.29 [1.10, 5.65] | 0.7 | 0.9 |
| Butyric Acid | 27.2 [18.4, 151] | 4.43 [1.82, 65.5] | 0.4 | 0.5 | 37.7 [20.3, 202] | 18.4 [2.14, 92.3] | 0.3 | 0.6 |
| 2-methylbutyric Acid | 4.78 [2.14, 7.97] | 2.91 [1.27, 5.82] | 0.2 | 0.4 | 3.68 [1.85, 5.04] | 4.78 [1.22, 7.73] | 0.9 | 0.9 |
| Isovaleric Acid | 9.69 [2.96, 13.0] | 7.43 [2.44, 13.0] | 0.5 | 0.5 | 6.96 [3.38, 13.0] | 7.15 [2.54, 13.1] | 0.8 | 0.9 |
| Valeric Acid | 0.87 [0.19, 2.80] | 0.54 [0.13, 0.91] | 0.2 | 0.4 | 0.19 [0.14, 2.30] | 0.62 [0.19, 1.38] | 0.7 | 0.9 |
| Hexanoic Acid | 2.15 [1.71, 5.20] | 2.86 [1.95, 5.72] | 0.5 | 0.5 | 2.02 [1.54, 2.22] | 3.84 [2.09, 6.28] | 0.01 | 0.05 |
| Total SCFA | 1051 [726, 1622] | 482 [235, 1047] | 0.01 | 0.05 | 977 [498, 1791] | 658 [271, 1095] | 0.1 | 0.4 |
| Stool Day 28 | ||||||||
| 24 | 41 | 21 | 40 | |||||
| Acetic Acid | 1790 [1115, 3105] | 905 [614, 1910] | 0.03 | 0.1 | 1820 [809, 3280] | 1055 [618, 1932] | 0.1 | 0.3 |
| Propionic Acid | 395 [126, 594] | 142 [47.1, 318] | 0.02 | 0.08 | 307 [131, 471] | 136 [45.3, 376] | 0.1 | 0.3 |
| Isobutyric Acid | 6.29 [2.96, 19.3] | 5.15 [1.59, 13.8] | 0.38 | 0.4 | 6.08 [2.96, 29.4] | 5.15 [1.92, 11.1] | 0.5 | 0.6 |
| Butyric Acid | 114 [45.2, 264] | 11.8 [1.96, 160] | 0.06 | 0.1 | 114 [18.1, 255] | 57.8 [2.68, 177] | 0.2 | 0.4 |
| 2-methylbutyric Acid | 13.8 [4.04, 34.5] | 5.93 [1.33, 16.4] | 0.09 | 0.1 | 10.8 [3.94, 24.3] | 5.93 [1.73, 20.3] | 0.3 | 0.4 |
| Isovaleric Acid | 29.1 [5.66, 58.0] | 10.6 [2.90, 36.2] | 0.10 | 0.1 | 19.8 [5.66, 40.1] | 10.6 [2.37, 35.5] | 0.3 | 0.4 |
| Valeric Acid | 1.07 [0.36, 5.11] | 0.65 [0.18, 3.16] | 0.29 | 0.4 | 1.16 [0.20, 8.71] | 0.94 [0.30, 3.17] | 0.5 | 0.6 |
| Hexanoic Acid | 3.41 [2.12, 7.17] | 3.81 [2.40, 7.04] | 0.58 | 0.6 | 3.50 [2.25, 5.60] | 3.96 [2.42, 7.27] | 0.6 | 0.6 |
| Total SCFA | 2749 [1594, 3906] | 1252 [756, 2145] | 0.01 | 0.06 | 2567 [1165, 4245] | 1334 [789, 2320] | 0.08 | 0.3 |
| BPD 1 OR (95% CI) | p-Value | ROP 1 OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Day 14 | ||||
| Acetic acid (per quartile increase) | 0.41 [0.18, 0.83] | 0.02 | 0.66 [0.35, 1.17] | 0.2 |
| Q2 vs. Q1 | 1.51 [0.15, 1.74] | 0.7 | 0.42 [0.06, 2.67] | 0.4 |
| Q3 vs. Q1 | 0.18 [0.02, 1.45] | 0.1 | 1.62 [0.21, 13.4] | 0.6 |
| Q4 vs. Q1 | 0.11 [0.008, 0.98] | 0.06 | 0.18 [0.02, 1.17] | 0.08 |
| Day 28 | ||||
| Acetic acid (per quartile increase) | 0.28 [0.09, 0.64] | 0.009 | 0.62 [0.32, 1.11] | 0.1 |
| Q2 vs. Q1 | 0.28 [0.01,3.86] | 0.4 | 0.61 [0.08, 4.51] | 0.6 |
| Q3 vs. Q1 | 0.06 [0.003, 0.78] | 0.05 | 0.93 [0.11, 7.21] | 0.9 |
| Q4 vs. Q1 | 0.02 [0.001, 0.31] | 0.01 | 0.20 [0.03, 1.28] | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazer, L.C.; Yakah, W.; Martin, C.R. Decreased Acetic Acid in the Stool of Preterm Infants Is Associated with an Increased Risk of Bronchopulmonary Dysplasia. Nutrients 2022, 14, 2412. https://doi.org/10.3390/nu14122412
Frazer LC, Yakah W, Martin CR. Decreased Acetic Acid in the Stool of Preterm Infants Is Associated with an Increased Risk of Bronchopulmonary Dysplasia. Nutrients. 2022; 14(12):2412. https://doi.org/10.3390/nu14122412
Chicago/Turabian StyleFrazer, Lauren C., William Yakah, and Camilia R. Martin. 2022. "Decreased Acetic Acid in the Stool of Preterm Infants Is Associated with an Increased Risk of Bronchopulmonary Dysplasia" Nutrients 14, no. 12: 2412. https://doi.org/10.3390/nu14122412
APA StyleFrazer, L. C., Yakah, W., & Martin, C. R. (2022). Decreased Acetic Acid in the Stool of Preterm Infants Is Associated with an Increased Risk of Bronchopulmonary Dysplasia. Nutrients, 14(12), 2412. https://doi.org/10.3390/nu14122412






