Abstract
Results from different clinical trials on the effects of ginseng on prediabetes and type 2 diabetes (T2DM) are still inconsistent. To fill this knowledge gap, we investigated the overall effects of ginseng supplementation on improving cardiometabolic biomarkers among these patients. A systematic literature search was conducted on PubMed/MEDLINE, Scopus, Web of Science, and Cochrane library. A random-effect model was applied to estimate the weighted mean difference and 95% CI for each outcome. Overall, 20 eligible RCTs were included. Meta-analyses revealed that ginseng supplementation significantly reduced serum concentration of FPG, TC, IL-6, and HOMA-IR values. It also increased HR and TNF-α levels. Ginseng supplementation changed HOMA-IR and HDL-C significantly based on dose and changed HOMA-IR and LDL-C significantly based on study duration in a non-linear fashion. Furthermore, meta-regression analyses indicated a linear relationship between ginseng dose and absolute changes in HDL-C. Moreover, subgroup analyses showed that ginseng supplementation changed TC and LDL-C when the supplementation dose was ≥2 g/day. Our findings suggest that ginseng supplementation may be an effective strategy for improving cardiometabolic profiles in individuals with prediabetes and T2DM.
1. Introduction
Type 2 diabetes mellitus (T2DM) is the fastest-growing metabolic disorder worldwide, imposing social, economic, and public health burdens [1,2,3]. One major diabetes comorbidity is cardiovascular disease (CVD), accounting for 32.2% of all individuals with diabetes [3]. These dysglycemia conditions are characterized by insulin resistance and β-cell dysfunction in adults [4]. Despite an increased emphasis on preemptive therapeutic options and promising new therapies, the management of T2DM remains challenging [5,6]. Meanwhile, interest in complementary and alternative medicine (CAM) remarkably continues to increase [7], becoming one of the major therapeutic approaches sought by individuals with diabetes [8].
Many herbal medications have been recommended for controlling diabetes [9]. Among them, the most popular one is ginseng, the root of plants in the Panax genus of the Araliaceae family, whose effects on complications of T2DM have been investigated extensively [10,11,12,13,14]. Panax ginseng (commonly known as Asian ginseng) and Panax quinquefolius (commonly known as American ginseng) are the most widely commercialized types [15]. Ginseng has been shown to exert various biological impacts, including anti-diabetic [6], anti-hyperlipidemic [16], anti-inflammatory [17], and hepatoprotective [18] effects. Several pathways have been proposed in this context. Major ones include reducing leptin and neuropeptide Y concentrations and appetite suppression [19], influencing inflammatory signaling pathways such as nuclear factor-kappa β (NF-κB) and activator protein 1 (AP-1) [15] and suppressing Peroxisome proliferator-activated receptor α (PPARα) [20].
Many clinical studies have investigated the potential protective effects of ginseng on cardiometabolic indices among individuals with diabetes; however, the results have been inconsistent [1,5,21,22,23,24,25]. Some studies documented improved levels of anthropometric indices [1], glucose-related markers [26,27], lipid profile components [5], blood pressure [28], and inflammatory markers [21] following ginseng consumption, whereas others indicated null effects [12,22,29,30]. Furthermore, previous systematic reviews and meta-analyses of randomized controlled trials (RCTs) on ginseng supplementation have only covered certain cardiometabolic indicators in various health conditions [19,31,32,33,34,35,36]. Besides, there was no prior systematic review and meta-analysis investigating the effects of ginseng supplementation on cardiometabolic indicators exclusively in individuals with prediabetes and T2DM. Thus, the objective of this study was to comprehensively summarize and analyze the evidence from clinical trials relating to the impact of ginseng supplements on various cardiometabolic outcomes among subjects with dysglycemia.
2. Materials and Methods
We performed and reported this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist [37].
2.1. Data Sources and Searches
Studies were systematically identified using electronic databases (PubMed/MEDLINE, Scopus, Web of Science, and Cochrane library) until 10 April 2022. The following keywords were used in the search strategy: ((Ginseng OR ginsenoside) AND (Intervention OR “controlled trial” OR randomized OR random OR randomly OR placebo OR “clinical trial” OR Trial OR “randomized clinical trial” OR RCT OR trial OR trials “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”) AND (“diabetes” OR “type 2 diabetes mellitus” OR “T2DM” OR “type 2 diabetes” OR “T2D” OR “prediabetes”)). Search terms used across the various databases are presented in Supplementary Table S1. No date restrictions were applied; however, only English-language articles were eligible for inclusion. Bibliographies of relevant studies or systematic reviews identified by the search strategy were screened for additional studies. If the data required for the meta-analysis was not reported in the literature, we contacted the corresponding author to provide the data.
2.2. Study Selection and Eligibility Criteria
All recorded articles from electronic or manual searches were imported into Endnote software for further review. Titles and abstracts of all articles found in the initial search were reviewed independently by two researchers (S.S. and K.N.). Discussion with a third reviewer (O.A.) resolved disagreement regarding full-text eligibility. To determine article eligibility, we employed the population, intervention, comparison, outcome, and study design (PICOS) framework (Table 1). Studies were excluded from this investigation if they: (1) co-administered ginseng as a part of a mixed intervention; (2) lacked a suitable control; (3) had no viable end-point data in ginseng or control groups; (4) were carried out on children, pregnant women, or animals; and (5) were performed less than 4 weeks in duration. In addition, conference abstracts, gray literature, unpublished studies, and protocols were not included.

Table 1.
PICOS criteria for inclusion of studies.
2.3. Data Extraction
The following information was extracted from each eligible clinical trial by two independent researchers: study author; year of publication; study location; study design; the number of participants; participants’ ethnicity, age, comorbidities, and body mass index; the type, dose, duration, and frequency of the intervention; and the study results (mean or median with standard deviations, standard errors, 95% CIs, or interquartile ranges) at study baseline, post-intervention, and/or changes between baseline and post-intervention. If the data for each parameter was reported in different units, we converted them to the most commonly used units.
2.4. Quality Assessment
The likelihood of bias in the included RCTs was explored through the Cochrane Risk of Bias Tool for clinical trials [38]. Two independent authors assessed each publication’s quality based on the following seven domains: (1) random sequence generation; (2) allocation concealment; (3) selective outcome reporting; (4) blinding of participants and personnel; (5) detection bias (blinding of evaluators); (6) incomplete outcome data; and (7) other probable sources of biases. Based on the Cochrane handbook recommendation, every article was assigned a label of bias (low risk (L), high risk (H), or unclear (U) risk of bias) (Supplementary Table S2).
The quality of the evidence for each result was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [39]. Two independent reviewers (S.S. and K.N.) graded each outcome based on the risk of bias, inconsistency (heterogeneity), indirectness, and imprecision, as specified in the GRADE guideline [39]. Each outcome was rated high, moderate, low, or very low.
2.5. Data Synthesis and Meta-Analysis
We used the mean difference in the changes in the outcome variables (BW, BMI, WC, FPG, OGTT, HbA1c, fasting insulin, HOMA-IR, TG, TC, LDL-C, HDL-C, SBP, DBP, HR, CRP, IL-6, TNF-α, ALT, AST, and GGT), comparing ginseng to the control groups, to obtain the overall effect sizes. When mean changes were not reported, we computed them by considering changes in each outcome variable during the trial. We also converted standard errors (SEs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) to SDs using the relevant formulas [40]. We applied a random-effects model that considers between-study variations to obtain the overall effect sizes. Heterogeneity was determined using the I2 statistic and the Cochrane’s Q test. The Q-test’s I2 value > 50% or p < 0.05 was characterized as significant between-study heterogeneity [41,42]. Subgroup analyses were performed to find probable sources of heterogeneity, according to the predefined criteria, including ginseng dosage (≥2/<2 g/day), length of follow-up (>8/≤ 8 weeks), baseline levels of outcome variables (abnormal/normal levels) and participants’ baseline BMI level (normal/overweight or obese). Fractional polynomial modeling was executed to determine the potential non-linear effects of ginseng dosage (g/day) on each index. Due to the lack of information on ginseng dosage in some of the included studies, we decided to perform a non-linear dose-response analysis of ginseng administration. Sensitivity analysis was used to determine the extent to which inferences might depend on a particular study. The formal test of Begg assessed the possibility of publication bias. The meta-analysis was performed using the Stata version 11.2 (StataCorp). The p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Selection
Figure 1 illustrates the literature search and screening process performed for this systematic review. Initial database searches for all RCTs of ginseng supplementation yielded 1978 records, of which 540 were duplicates. Eligibility based on title and abstract was assessed for the remaining 1438 articles. Of those, 1236 RCTs were not relevant to the subject, leaving 202 records eligible for full-text review. Furthermore, after excluding 6 studies examining the impact of ginseng in combination with other ingredients due to the impossibility of determining the independent effect of ginseng [43,44,45,46,47,48], 3 eligible studies which reported in a language other than English [49,50,51], 1 study with multiple-crossover, acute dose escalation design [52], and 172 records which did not provide sufficient data and/or did not fulfill the inclusion criteria, 20 RCTs with 24 effect sizes met the inclusion criteria for qualitative synthesis.
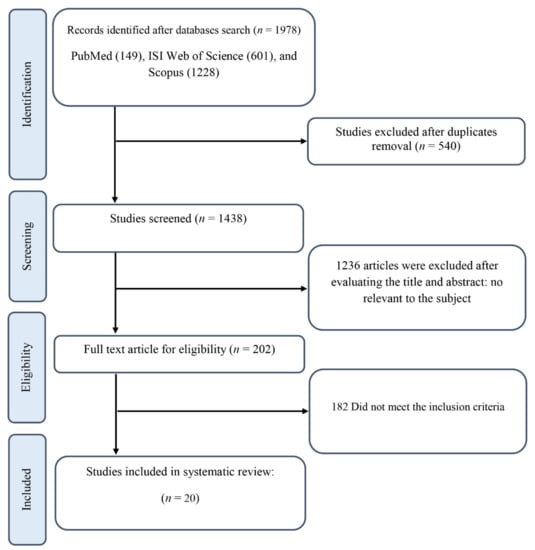
Figure 1.
The Preferred Reporting Items for Systematic Review and Meta-analysis(PRISMA) flowchart.
3.2. Study Characteristics
The detailed characteristics of all the studies included in the qualitative synthesis are described in Table 2. The selected 20 studies were all RCTs, including 17 parallel design studies [1,21,22,23,24,25,26,27,28,29,30,53,54,55,56,57,58] and 3 crossover design studies [5,12,59]. In total, 1295 participants (675 cases and 620 controls) with age range between 44.56 ± 10.48 and 64 ± 7 years old and BMI range between 23.52 ± 0.48 and 36 ± 3.46 kg/m2 were recruited. These RCTs were conducted in South Korea [24,25,26,27,30,53,56], Iran [21,29,54], Canada [5,12], Croatia [23,28], Canada and Croatia [22,55], the United States [57], Australia [1], Hong Kong [59], and Finland [58], and were published between 1995 and 2021. RCTs were performed on individuals with prediabetes [1,27,56], and T2DM [5,12,21,22,23,24,25,26,28,29,30,53,54,55,57,58,59]. One study was exclusively performed on male subjects [57], and others were conducted on both genders. Of the 20 RCTs, 5 effect sizes administered Panax quinquefolius (also known as American ginseng) [5,22,23,28,55], and 13 used Panax ginseng (Korean ginseng, Asian ginseng, and Chinese ginseng) [1,12,21,22,24,25,26,27,30,53,54,55,56,57,59] as investigational products. The remaining two studies did not report the type of ginseng [29,58]. The dosage of ginseng varied from 0.1–8 g/day, and the duration of intervention differed from 4 to 24 weeks across included RCTs.

Table 2.
Characteristics of the included studies.
3.3. Effects of Ginseng Supplementation on Cardiometabolic Parameters
3.3.1. Anthropometric Measurements
There was no significant difference between the ginseng and placebo groups at follow-up regarding BW (weighted mean difference (WMD):−0.54 kg; 95% CI: −2.54, 1.46; p = 0.598; phet = 0.998, I2 = 0.0%) [1,5,21,57], BMI (WMD: 0.05 kg/m2; 95% CI: −0.26, 0.38; p = 0.717; phet = 0.963, I2 = 0.0%) [1,21,24,25,30,57], and WC (WMD: 0.05 cm; 95% CI: −1.16, 1.27; p = 0.929; phet =0.729, I2 = 0.0%) (Supplementary Figure S1) [30]. It was impossible to conduct subgroup analyses for BW and WC as there were not enough studies (less than 2) in each group that reported on these measures. However, subgroup analyses did not reveal any differences in BMI irrespective of participants’ baseline BMI values, amount of supplemented ginseng, and length of follow-up (Table 3).

Table 3.
Subgroup analyses of ginseng supplementation on cardiovascular risk factors in patients with prediabetes and T2DM.
3.3.2. Measures of Glucose Homeostasis
Glycemic Control
FPG was significantly reduced in the ginseng group compared to the placebo group (WMD: −7.03 mg/dL; 95% CI: −10.89, −3.17; p < 0.001; phet < 0.001, I2 = 91%) (Figure 2a) [1,5,12,21,22,24,25,26,27,29,30,53,54,56,57,58,59]. However, there was no significant difference in OGTT (WMD: −6.81 mg/dL; 95% CI: −16.77, 3.14; p = 0.180; phet = 0.002, I2 = 66.3%) [12,25,26,27,30,53,56,59], and HbA1c (WMD: −0.04 %; 95% CI: −0.16, 0.07; p = 0.449; phet < 0.001, I2 = 82.9%) [1,5,12,21,22,24,25,26,30,53,54,57] between groups (Supplementary Figure S1). Subgroup analyses revealed that FPG was only reduced in those with baseline FPG ≥ 126 mg/dL. A significant decrease in FPG was also observed in participants who consumed less than 2 g/day of ginseng for a shorter time (8 weeks or less). However, no statistical difference between groups was observed based on the duration and dose of intervention in OGTT levels. In addition, there were no statistical differences observed between the two groups for the HbA1c baseline values, dose, and duration of supplementation in HbA1c percentage (Table 3).
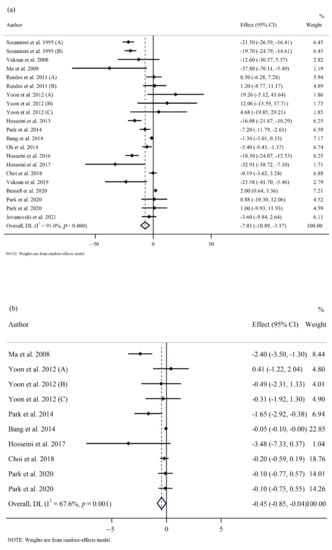
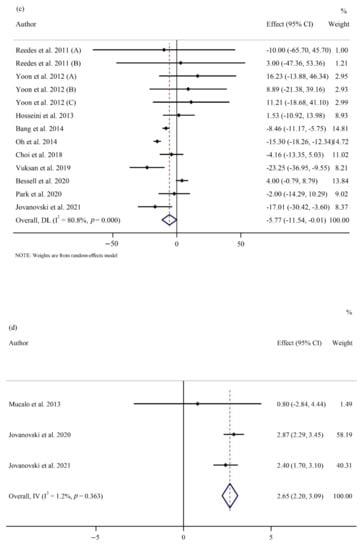
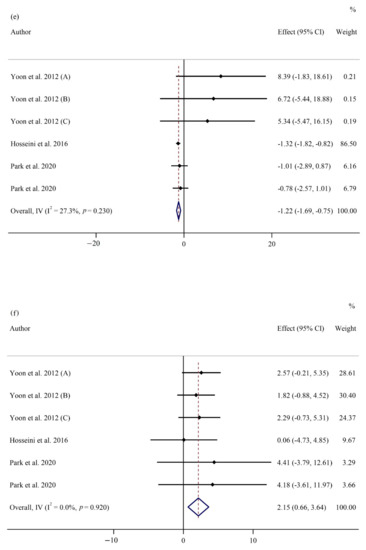
Figure 2.
Forest plots of randomized controlled trials illustrating weighted mean difference (WMD) in biomarkers between the intervention and placebo groups for all eligible studies in overall analysis. (a) Fasting plasma glucose (FPG) [1,5,12,21,22,24,25,26,27,29,30,53,54,56,57,58,59]; (b) homeostatic model assessment for insulin resistance (HOMA-IR) [24,25,26,29,30,53,56,59]; (c) total cholesterol (TC) [1,5,22,25,26,27,30,53,54,57]; (d) heart rate (HR) [22,28,55]; (e) interleukin 6 (IL-6) [21,24,25,30]; (f) tumor necrosis factor-α (TNF- α) [21,24,25,30].
Insulin Resistance and Secretion
Our results showed a significant decrease in HOMA-IR (WMD: −0.44; 95% CI: −0.84, −0.04; p = 0.03; phet = 0.001, I2 = 67.6%) (Figure 2b) [24,25,26,29,30,53,56,59]. However, our results indicated no difference in fasting insulin levels (WMD: −0.13 µU/mL; 95% CI: −1.18, 0.90; p = 0.796; phet < 0.001, I2 = 89.8%) [5,12,22,24,25,26,27,29,30,53,56,57,59] between the groups (Supplementary Figure S1). The subgroup analyses showed that ginseng consumption was associated with a significant reduction in HOMA-IR irrespective of the follow-up length. Additionally, subgroup analyses did not reveal any differences in fasting insulin irrespective of ginseng interventional dosage and length of follow-up (Table 3).
3.3.3. Cardiovascular Risk Factors
Lipid Profile
Pooling of 13 effect sizes showed a significant reduction in TC concentrations between ginseng and placebo groups at follow-up (WMD: −5.77 mg/dL; 95% CI: −11.53, −0.01; p = 0.04; phet < 0.001, I2 = 80.8%) (Figure 2c) [1,5,22,25,26,27,30,53,54,57]. However, ginseng supplementation had no effect on TG (WMD: 6.05 mg/dL; 95% CI: −4.37, 16.48; p = 0.255; phet < 0.001, I2 = 73.5%) [1,22,25,26,27,30,53,54,57], LDL-C (WMD: −4.16 mg/dL; 95% CI: −8.98, 0.65; p = 0.090; phet < 0.001, I2 = 78.2%) [1,5,22,25,26,27,30,53,54,57], and HDL-C (WMD: −1.28 mg/dL; 95% CI: −5.58, 3.01; p = 0.557; phet < 0.001, I2 = 96.9%) [1,5,22,25,26,27,30,53,54,57] in a pooled analysis of 12, 13, and 13, respectively (Supplementary Figure S1). On subgroup analyses, we found that ginseng supplementation significantly reduced serum TC levels in studies that included participants with baseline levels of TC below 200 mg/dL and studies with supplementation dose of ginseng at 2 g/day or above. Also, studies with 2 g/day or a higher dose of ginseng supplementation reported a reduction in serum LDL-C levels. Interestingly, TG concentration significantly elevated following ginseng supplementation in studies involving individuals with baseline TG levels below 150 mg/dL. However, there was no significant reduction in HDL-C levels in the ginseng group compared to the control group, irrespective of baseline levels of HDL-C, supplementation dose, and follow-up duration (Table 3).
Blood Pressure and Heart Rate
In a meta-analysis of three studies, HR significantly increased in the intervention group compared to the control group (WMD: 2.65 bpm; 95% CI: 2.20, 3.09; p < 0.001; phet = 0.363, I2 = 1.2%) (Figure 2d) [22,28,55]. However, there were no significant differences in SBP (WMD: −2.78 mmHg; 95% CI: −6.97, 1.40; p = 0.193; phet < 0.001, I2 = 87.4%) [5,12,22,24,25,28,30,53] and DBP values (WMD: −0.24 mmHg; 95% CI: −1.88, 1.39; p = 0.770; phet = 0.003, I2 = 63.4%) [5,12,22,24,25,28,30,53] between the ginseng and control groups (Supplementary Figure S1). Subgroup analyses did not reveal any differences in SBP and DBP regardless of baseline values, dose, and duration of intervention. Furthermore, subgroup analysis for HR was not possible due to the limited number of studies (n = 3) (Table 3).
3.3.4. Inflammatory Markers and Adipocytokines
Ginseng administration significantly reduced serum IL-6 levels (WMD: −1.22 pg/mL; 95% CI: −1.68, −0.75; p < 0.001; phet = 0.230, I2 = 27.3%) (Figure 2e) [21,24,25,30] while increasing TNF-α concentration (WMD: 2.15 pg/mL; 95% CI: 0.66, 3.63; p = 0.005; phet = 0.920, I2 = 0.0%) (Figure 2f) [21,24,25,30] at follow-up. However, there was no significant between-group difference regarding CRP (WMD: −0.10 µg/mL; 95% CI: −0.61, 0.41; p = 0.696; phet = 0.046, I2 = 55.8%) [21,24,25,30], adiponectin (WMD: −0.27µg/mL; 95% CI: −1.41, 0.86; p = 0.639; phet = 0.906, I2 = 0.0%) [30], and leptin (WMD: −0.67 pg/mL; 95% CI: −2.01, 0.65; p = 320; phet = 0.847, I2 = 0.0%) (Supplementary Figure S1) [30]. Subgroup analysis was not conducted for CRP, IL-6, TNF-α, adiponectin, or leptin, as there were not enough studies reported on these parameters (Table 3).
3.3.5. Liver Function Tests
Ginseng supplementation did not affect ALT (WMD: 0.62 U/L; 95% CI: −1.90, 3.15; p = 0.630; phet = 0.085, I2 = 46.0%) [5,22,23,30], AST (WMD: −0.28 U/L; 95% CI: −1.77, 1.19; p = 0.704; phet = 0.272, I2 = 22.3%) [12,23,30], and GGT (WMD: 2.03 U/L; 95% CI: −6.22, 10.28; p = 0.630; phet = 0.285, I2 = 20.3%) (Supplementary Figure S1) [30]. However, subgroup analyses were not performed for ALT, AST, or GGT due to the limited number of studies that reported liver function tests.
3.4. Sensitivity Analysis
Sensitivity analysis for HOMA-IR showed that the overall estimates were affected by the exclusion of the studies conducted by Ma et al. [59] (WMD: −0.15, 95% CI: −0.37, 0.07) and Park et al. [56] (WMD: −0.33, 95% CI: −0.71, 0.04). Exclusion of studies carried out by Bang et al. [53] (WMD: −4.70 mg/dL, 95% CI: −12.58, 3.17), Oh et al. [27] (WMD: −4.17 mg/dL, 95% CI: −10.15, 1.79), Choi et al. [26] (WMD: −5.91 mg/dL, 95% CI: −12.16, 0.33), Vuksan et al. [5] (WMD: −4.25 mg/dL, 95% CI: −10.18, 1.67), and Jovanovski et al. [22] (WMD: −4.72 mg/dL, 95% CI: −10.79, 1.34) changed the overall effect size for TC. Furthermore, the results of the sensitivity analysis for TG showed that removing the Jovanovski et al. [22] study changed the overall effect size (WMD: 9.99 mg/dL, 95% CI: 0.32, 19.66). Furthermore, overall estimates for LDL-C were influenced by the exclusion of a study performed by Yoon et al. (A) [30] (WMD: −4.88 mg/dL, 95% CI: −9.73, −0.04). Additionally, the exclusion of Hosseini et al. [21] study also changed the overall effect size for IL-6 (WMD: −0.57 pg/mL, 95% CI: −1.84, 0.69). Finally, sensitivity analysis for BW, BMI, WC, FPG, HbA1c, OGTT, fasting insulin, HDL-C, SBP, DBP, HR, CRP, TNF-α, adiponectin, leptin, ALT, AST, and GGT did not indicate any evidence of sensitivity.
3.5. Publication Bias
Based on visual inspection of funnel plots (Supplementary Figure S2), as well as Egger’s [60] and Begg’s [61] statistical tests (Supplementary Table S3), we found no evidence of publication bias for BW, BMI, WC, HbA1c, OGTT, fasting insulin, HOMA-IR, TG, TC, LDL-C, HDL-C, SBP, HR, CRP, TNF-α, adiponectin, leptin, ALT, AST, and GGT. The Funnel plot and Egger’s test manifested that there was publication bias for FPG (p = 0.03), DBP (p = 0.05), and IL-6 (p = 0.006).
3.6. Non-Linear Dose-Response between Dose and Duration of Ginseng Supplementation on Cardiometabolic Indicators
Dose-response analysis showed that ginseng supplementation significantly altered HOMA-IR based on dose (r = −0.26, p-nonlinearity = 0.02) and study duration (r = 7.18, p-nonlinearity = 0.04) in a non-linear fashion. Furthermore, the dose of ginseng affected HDL-C (r = −0.31, p-nonlinearity = 0.009) and duration of intervention affected LDL-C (r = −16.61, p-nonlinearity = 0.04) in a non-linear fashion. No significant associations were observed for other outcomes in non-linear dose-responses (Table 4), (Supplementary Figures S3 and S4).

Table 4.
Linear and non-linear dose-responses between dose and duration of Panax supplementation and cardiometabolic biomarkers.
3.7. Meta-Regression Analysis
Meta-regression using the random-effects model was undertaken to investigate the potential association between a change in cardiometabolic indicators and the dose of ginseng (g/day) and the duration of the trial. Meta-regression analysis indicated a linear relationship between dose absolute changes in HDL-C (p = 0.02) but not for other studied outcomes (Table 4), (Supplementary Figures S5 and S6).
3.8. GRADE Assessment
An evaluation of the quality of evidence using the GRADE approach is presented in Table 5. For HR and TNF-α, the quality of evidence was high since most studies had a low to moderate risk of bias with low statistical and clinical heterogeneity and narrow CIs. Moreover, the quality of evidence for TC and HOMA-IR was deemed moderate due to inconsistency (I2 = 80.8% and I2 = 67.6% for heterogeneity, respectively). In addition, the evidence regarding IL-6 was determined to be of moderate quality, owing to serious publication bias (p = 0.006). Moreover, there was moderate evidence on BW, BMI, WC, adiponectin, leptin, ALT, AST, and GGT, owing to serious imprecision (wide CI). For HbA1c, OGTT, fasting insulin, TG, LDL-C, HDL-C, SBP, DBP, and CRP, the evidence was deemed low quality due to serious inconsistency (I2 = 82.9% (HbA1c), I2 = 66.3% (OGTT), I2 = 89.8% (fasting insulin), I2 = 73.5% (TG), I2 = 78.2% (LDL-C), I2 = 96.9% (HDL-C), I2 = 87.4% (SBP), I2 = 63.4% (DBP), and I2 = 55.8% (CRP) for heterogeneity) and imprecision (Wide CI). Finally, for FPG, the quality of evidence was also low due to serious inconsistency (I2 = 91.0% for heterogeneity) and publication bias (p = 0.03).

Table 5.
GRADE profile of ginseng supplementation for cardiovascular biomarkers.
4. Discussion
This meta-analysis evaluated the effects of ginseng supplementation on cardiovascular biomarkers, including anthropometric indices, glycemic and lipid profiles, blood pressure (BP), inflammatory biomarkers, adipocytokines, and liver function indicators among subjects with prediabetes and T2DM. According to the findings of this study, ginseng consumption was associated with a reduction in FPG, HOMA-IR, TC, and IL-6, and escalations in HR and TNF-α, without any significant alterations in anthropometric measurements (BW, BMI, and WC), glycemic responses (OGTT, HbA1c, and fasting insulin), lipid profile (TG, LDL-C, and HDL-C), BP (SBP and DBP), inflammatory markers and adipocytokines (CRP, adiponectin, and leptin), and liver enzymes (ALT, AST, and GGT) when compared with a control group.
A comprehensive review of human trials and in vitro and in vivo studies suggests that ginseng modulates insulin secretion, glucose uptake, and glucose metabolism through inhibition of β-cell apoptosis and raising the production of glucagon-like peptide-1 (GLP-1) to exert anti-diabetic effects [11]. However, in 2011, a systematic review and meta-analysis of four RCTs showed that ginseng intake did not change blood glucose-related indices in patients with T2DM [13]. Furthermore, data from two meta-analyses on the general population suggested conflicting reports regarding ginseng supplementation effects on FPG levels. One study reported significantly reduced levels of FPG following ginseng intake [6], and the other indicated no effect [34]; both confirmed that there was no significant effect of ginseng consumption on fasting plasma insulin and HbA1c. Similar to our results, a recent experimental study revealed that Panax quinquefolius decreased FPG levels and improved insulin resistance (IR) in T2DM [62]. In addition, Gui et al. [63], based on a meta-analysis of eight RCTs, reported that FPG and HOMA-IR were improved by ginseng consumption with no change in OGTT, HbA1c, and fasting insulin in patients with T2DM. Likewise, we extended these findings by pooling the results of 24 effect sizes (n = 1295 participants) and showed that ginseng supplementation significantly improved FPG stronger when the consumption lasted for 8 weeks or more and significantly reduced HOMA-IR values regardless of the length of follow-up. Moreover, as seen in our subgroup analyses, ginseng supplementation had a beneficial effect on the concentration of FPG for either patient with a baseline level of FPG ≥ 126 mg/dL or when the supplementation dosage of ginseng was less than two g/day. Therefore, it seems that the effect of ginseng intake on FPG depends on baseline levels of FPG, dose, and duration of intervention. In dose-response analyses, lower duration and dose of ginseng consumption had a greater lower effect on HOMA-IR values. It is noteworthy that the discrepancies between meta-analyses could be due to different numbers of included studies and various studied populations.
Insulin resistance is the driving factor that leads to the development of T2DM [64]. Long-term IR in adipocytes leads to elevated free fatty acids (FFAs) and an accelerated TG formation, which contributes to dyslipidemia in patients with T2DM [65,66]. A series of pharmacological investigations suggested hypolipidemic effects of ginseng administration, mainly through activating AMP-activated protein kinase (AMPK) among individuals with prediabetes and T2DM [5,22,62,67]. In this study, we have shown that consumption of ginseng reduced TC levels stronger among those with baseline TC < 200 mg/dL and intake of ≥ 2 g/day. Additionally, we found that patients with prediabetes and T2DM had a significantly lower level of LDL-C after consuming ≥2 g/day of ginseng. The subgroup analysis in the current study also suggests that ginseng supplementation reduces serum levels of TG in individuals with baseline TG values < 150 mg/dL. The underlying mechanisms of the lipid-lowering effects of ginseng are still unclear. However, we assumed a possible reason for the relationship between ginseng intake and levels of lipid profile components in our meta-analysis. The steroidal structure of triterpene saponins may alter gene transcription, protein synthesis, and cholesterol production in the liver through inhibition of β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase [68,69], which was also reported as the possible anti-diabetic mechanism of some citrus flavonoids [70]. The meta-regression and dose-response analyses also revealed that the longer the study duration, the greater the effect of ginseng supplementation on lowering LDL-C. The higher the dose, the greater the effect of ginseng supplementation on lowering HDL-C. We found a disparity in findings obtained from previous meta-analyses investigating the efficacy of ginseng administration on lipid profile parameters. One suggested no lipid-lowering effects of ginseng consumption from three previous meta-analyses conducted on healthy and unhealthy individuals [36], while others indicated significant exerted effects of ginseng intake [32,34]. Additionally, a previous meta-analysis that investigated ginseng supplementation in patients with T2DM demonstrated a significant effect of ginseng supplementation on serum TC, TG, and LDL-C levels [63]. The disagreements may be due to different types of ginseng supplements, different target populations, and a limited number of studies included in previous reviews.
Another aspect of the pathogenesis of T2DM is low-grade chronic inflammation. The inflammatory process contributes to IR and, consequently, to T2DM-associated cardiovascular complications [71]. Elevated levels of TNFα have been shown to directly affect insulin receptor signaling and decrease insulin sensitivity [71,72]. Several molecular pathways could be influenced by ginsenosides, manifesting anti-inflammatory effects. The most notable anti-inflammatory mechanisms are inhibition of toll-like receptor four signaling pathway, inhibition of NF-κB signaling pathway, activation of AMPK, and increased nuclear factor erythroid-2-related factor 2 (Nrf2) expression and translocation [73]. A growing body of evidence proposed that ginseng treatment significantly inhibits the expression of inflammatory factors and exerts a protective effect in patients with prediabetes and T2DM [74,75,76]. However, the recent meta-analyses in the general population did not show significant overall effects of ginseng consumption on CRP levels [33,35]. The second study also demonstrated significant reductions in IL-6 and TNF-α following ginseng supplementation [33]. Notably, the current study is the first systematic review and meta-analysis investigating the effectiveness of ginseng supplementation on inflammatory biomarkers in subjects with prediabetes and T2DM. Our meta-analysis was in line with the previous ones concerning CRP and IL-6 changes. However, we observed significantly elevated levels of TNF-α after ginseng consumption. The results of this study should be interpreted cautiously, and more research should be conducted on the effects of ginseng supplementation on inflammatory markers. Future long-term dose-escalation studies are necessary since the TC, HOMA-IR, and IL-6 findings were not robust due to their sensitivity.
Obesity is believed to be a promoter of T2DM [77]. Increased BMI is associated with higher leptin levels, one of the major adipokines released by adipocytes [72]. It has already been known that ginsenosides inhibit adipogenesis and lipid accumulation in adipocytes [78]. The current study is the first report investigating the effects of ginseng administration on anthropometric indices and adipocytokines among subjects with prediabetes and T2DM. Our meta-analysis showed that ginseng consumption did not affect anthropometric measurements (BW, BMI, and WC) and adipocytokines (adiponectin and leptin) in individuals with prediabetes and T2DM. The findings are consistent with previous studies [19,34], suggesting that ginseng intake did not significantly differ in anthropometric measurements between the intervention and placebo groups in the general population. The findings from the present study were concluded in a relatively small sample size (n = 648 participants). Therefore, if well-designed clinical studies establishing appropriate inclusion criteria and larger sample sizes are documented, significant results are not far from expectation on these markers.
We have also demonstrated that consumption of ginseng significantly accelerated the HR. However, its effects on SBP and DBP among individuals with prediabetes and T2DM were insignificant. Also noteworthy is that the present study represents the first meta-analysis to investigate the effects of ginseng on BP and HR in people with prediabetes and type 2 diabetes. In preclinical evidence, ginseng supplementation decreased the BP through the activation of endothelial nitric oxide synthase and the release of nitric oxide and sped up the HR [79,80]. A systematic review and meta-analysis study in subjects with hypertension revealed significantly reduced BP levels following ginseng intake. However, the total sample size was insufficient to draw conclusions [81]. Similarly, another review record on healthy and unhealthy individuals showed the same results [34]. However, non-significant levels of SBP and DBP were observed after ginseng supplementation in the general population with a larger sample size than the previous ones in a meta-analysis setting [82]. Likewise, the findings do align with those of our meta-analysis. Although we can consider the HR acceleration as a side effect of ginseng consumption, the limited number of included studies necessitates more direct investigations.
Based on our findings, ginseng supplementation did not change measures of hepatic function in patients with prediabetes and T2DM. Nevertheless, some evidence has suggested that ginseng has favorable impacts on hepatocellular function through its anti-inflammatory, anti-oxidative, and anti-apoptotic properties [83]. In addition, a recent clinical trial study on individuals with hepatic dysfunction reported that ginseng supplementation significantly changes liver function enzymes level [84]. Our study confirmed the earlier meta-analysis study, which found that ginseng did not appear to have hepatoprotective effects in the general population [31]. Finally, it is noteworthy that we could not perform a subgroup analysis for any outcome based on the different forms of ginseng supplementation (extract vs. powder) since most of the included studies intervened extract form of ginseng. A study of 4-week supplementation of fermented red ginseng showed that only glucose values following oral glucose tolerance test were lowered, without any significant changes in FPG following ginseng supplementation [27]. In contrast, in another study with the administration of ginseng extract, FPG concentrations decreased [5]. As the extract form of any supplement has higher bioavalibity than the powder form, we can hypothesize that studies with extraction form have more promising findings.
5. Strengths and Limitations
In this paper, we included multiple endpoints to provide a comprehensive overview of the effects of ginseng on cardiometabolic parameters in individuals with prediabetes and T2DM. Both parallel and crossover randomized trials written in the English language were included. Additionally, we conducted dose-response and meta-regression analyses to assess the association between pooled effect size, dosage, and duration of ginseng supplementation. Subgroup analyses were also conducted to further explore each listed outcome’s results. Finally, we graded the overall certainty of evidence across the studies according to the GRADE guidelines. Despite the above strengths, the present study is not without limitations. First, the sample sizes of the included studies were also relatively small, with only one study including more than 100 participants. Second, relatively half of the studies were conducted in Asia, limiting generalizability. Third, some factors, such as duration of diabetes and smoking status, may influence the cardiovascular risk and should also be considered confounders, but were not included in the analysis due to poor reporting of these variables. Fourth, statistical heterogeneity is apparent in our analysis. This may be attributed to the poor methodological quality and/or differences in treatment regimens (doses/durations) or the ginseng type used.
6. Conclusions
This meta-analysis suggests that ginseng can improve cardiometabolic outcomes in individuals with prediabetes and type 2 diabetes. These results may provide important information to health agencies in formulating future guidelines for the use of ginseng in managing diabetes and the associated risk factors and preventing the progression of prediabetes. However, large-scale, well-designed RCTs should be performed to further verify these findings in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14122401/s1, Figure S1: Forrest plots presenting mean difference (MD) and 95% confidence intervals for the impact of ginseng supplementation; Figure S2: Funnel plots demonstrating publication bias in the trials reporting the effect of ginseng supplementation; Figure S3: Dose-response relations between ginseng dosage (mg/day) and mean difference in each outcomes; Figure S4: Dose-response relations between duration of ginseng (week) and mean difference in each outcomes; Figure S5: Random-effects meta-regression plots of the association between dose of ginseng (mg/day) and weighted mean difference of each outcome; Figure S6: Random-effects meta-regression plots of the association between duration of ginseng (week) and weighted mean difference of each outcome; Table S1: Search terms used across the various databases; Table S2: Risk of bias assessment; Table S3: Publication bias assessment.
Author Contributions
Conceptualization, K.N., S.S. and R.-Y.G.; methodology, K.N., S.S., F.G. and A.S.; software, K.N., S.S. and O.A.; validation, O.A., F.G. and H.-B.L.; formal analysis, K.N., S.S. and O.A.; investigation, K.N., S.S., F.Z. and H.-B.L.; resources, K.N., S.S. and H.-B.L.; data curation, K.N. and S.S. and A.S.; writing—original draft preparation, K.N. and S.S.; writing—review and editing, F.Z., R.-Y.G. and H.-B.L.; visualization, K.N., S.S. and A.S.; supervision, R.-Y.G.; project administration, R.-Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Lau, N.S.; Burk, J.; Hendy, C.; Picone, T.; Li, A.; Caterson, I.D. Effects of α-cyclodextrin on cholesterol control and hydrolyzed ginseng extract on glycemic control in people with prediabetes: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2023491. [Google Scholar] [CrossRef] [PubMed]
- Woldeamlak, B.; Yirdaw, K.; Biadgo, B. Role of gut microbiota in type 2 diabetes mellitus and its complications: Novel insights and potential intervention strategies. Korean J. Gastroenterol. 2019, 74, 314–320. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018, 41, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Mark Stavro, P.; Zurbau, A.; Duvnjak, L.; Li, M.Z. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2019, 58, 1237–1245. [Google Scholar] [CrossRef]
- Shishtar, E.; Sievenpiper, J.L.; Djedovic, V.; Cozma, A.I.; Ha, V.; Jayalath, V.H.; Jenkins, D.J.; Meija, S.B.; de Souza, R.J.; Jovanovski, E. The effect of ginseng (the genus Panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 2014, 9, e107391. [Google Scholar] [CrossRef] [Green Version]
- Tackett, K.L.; Jones, M.C. Complementary and alternative medicines for the treatment of diabetes. J. Pharm. Pract. 2009, 22, 546–552. [Google Scholar] [CrossRef]
- Manya, K.; Champion, B.; Dunning, T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement. Altern. Med. 2012, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.U.; Sreenivasulu, M.; Chengaiah, B.; Reddy, K.J.; Chetty, C.M. Herbal medicines for diabetes mellitus: A review. Int J PharmTech Res 2010, 2, 1883–1892. [Google Scholar]
- Xie, J.-T.; Mehendale, S.; Yuan, C.-S. Ginseng and diabetes. Am. J. Chin. Med. 2005, 33, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.-D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuksan, V.; Sung, M.-K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.-S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, B.-C.; Lee, M.S.; Lee, H.; Ernst, E. Red ginseng for type 2 diabetes mellitus: A systematic review of randomized controlled trials. Chin. J. Integr. Med. 2011, 17, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Arnason, J.T.; Vidgen, E.; Leiter, L.A.; Vuksan, V. a systematic quantitative analysis of the literature of the high variability in ginseng (Panax spp.) should ginseng be trusted in diabetes? Diabetes Care 2004, 27, 839–840. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Parlakpınar, H.; Özhan, O.; Ermiş, N.; Acet, A. Cardiovascular effects of Panax ginseng. J. Turgut Ozal Med. Cent. 2016, 23, 482–487. [Google Scholar] [CrossRef]
- Baek, K.S.; Yi, Y.S.; Son, Y.J.; Yoo, S.; Sung, N.Y.; Kim, Y.; Hong, S.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J. Ginseng Res. 2016, 40, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Lee, Y.H.; Kim, S.; Suk, K.T.; Bang, C.S.; Yoon, J.H.; Baik, G.H.; Kim, D.J.; Kim, M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J. Ginseng Res. 2016, 40, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Miraghajani, M.; Hadi, A.; Hajishafiee, M.; Arab, A.; Ghaedi, E.; Moodi, V. The effects of ginseng supplementation on anthropometric indices and body composition: A systematic review and meta-analysis. J. Herb. Med. 2020, 23, 100379. [Google Scholar] [CrossRef]
- Yoon, M.; Lee, H.; Jeong, S.; Kim, J.J.; Nicol, C.J.; Nam, K.W.; Kim, M.; Cho, B.G.; Oh, G.T. Peroxisome proliferator-activated receptor alpha is involved in the regulation of lipid metabolism by ginseng. Br. J. Pharmacol. 2003, 138, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.A.; Alipour, M.; Ghadiry, A.; Zakerkish, M. The effects of standardized extract of ginseng (G115) on blood sugar control and inflammatory factors in patients with type 2 diabetes: A double-blind clinical trial. Int. J. Pharm. Res. Allied Sci. 2016, 5, 55–59. [Google Scholar]
- Jovanovski, E.; Smircic-Duvnjak, L.; Komishon, A.; Au-Yeung, F.R.; Sievenpiper, J.L.; Zurbau, A.; Jenkins, A.L.; Sung, M.K.; Josse, R.; Li, D.; et al. Effect of coadministration of enriched Korean Red Ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) on cardiometabolic outcomes in type-2 diabetes: A randomized controlled trial. J. Ginseng Res. 2021, 45, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, I.; Jovanovski, E.; Vuksan, V.; Božikov, V.; Romić, Z.; Rahelić, D. American ginseng extract (Panax quinquefolius L.) is safe in long-term use in type 2 diabetic patients. Evid.-Based Complement. Altern. Med. Ecam 2014, 2014, 969168. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Ahn, C.W.; Kim, Y.; Nam, J.S. The effect of Korean Red Ginseng on sarcopenia biomarkers in type 2 diabetes patients. Arch. Gerontol. Geriatr. 2020, 90, 104108. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, Y.; Kim, J.; Kang, S.; Park, J.S.; Ahn, C.W.; Nam, J.S. Supplementation with Korean red ginseng improves current perception threshold in Korean type 2 diabetes patients: A randomized, double-blind, placebo-controlled trial. J. Diabetes Res. 2020, 2020, 5295328. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Kim, S.; Kim, M.J.; Kim, M.S.; Kim, J.; Park, C.W.; Seo, D.; Shin, S.S.; Oh, S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: A 12-wk randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2018, 42, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.R.; Park, S.H.; Kim, S.Y.; Back, H.I.; Kim, M.G.; Jeon, J.Y.; Ha, K.C.; Na, W.T.; Cha, Y.S.; Park, B.H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2014, 14, 237. [Google Scholar] [CrossRef] [Green Version]
- Mucalo, I.; Jovanovski, E.; Rahelić, D.; Božikov, V.; Romić, Z.; Vuksan, V. Effect of American ginseng (Panax quinquefolius L.) on arterial stiffness in subjects with type-2 diabetes and concomitant hypertension. J. Ethnopharmacol. 2013, 150, 148–153. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Ghaedi, E.; Zakerkish, M.; Ghadiri, A.; Ashtary-larky, D.; Safari, M.; Parsanahad, M.; Alipour, M. Effects of ginseng extract on chemerin, apelin and glycemic biomarkers in type 2 diabetic patients. Indian J. Physiol. Pharm. 2017, 61, 152–158. [Google Scholar]
- Yoon, J.W.; Kang, S.M.; Vassy, J.L.; Shin, H.; Lee, Y.H.; Ahn, H.Y.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: Results of a double-blind, placebo-controlled study. J. Diabetes Investig. 2012, 3, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Ghavami, A.; Ziaei, R.; Foshati, S.; Hojati Kermani, M.A.; Zare, M.; Amani, R. Benefits and harms of ginseng supplementation on liver function? a systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2020, 39, 101173. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, D.; Granado-Serrano, A.B.; Martín-Gari, M.; Naudí, A.; Serrano, J.C. Efficacy of Panax ginseng supplementation on blood lipid profile: A meta-analysis and systematic review of clinical randomized trials. J. Ethnopharmacol. 2019, 243, 112090. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Hadi, A.; Kord-Varkaneh, H.; Arab, A.; Afshari, M.; Ferguson, A.J.R.; Ghaedi, E. Effects of ginseng supplementation on selected markers of inflammation: A systematic review and meta-analysis. Phytother. Res. PTR 2019, 33, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Chung, S.; Chung, M.-Y.; Choi, H.-K.; Hwang, J.-T.; Park, J.H. Effects of Panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng Res. 2022, 46, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Saboori, S.; Falahi, E.; Yousefi Rad, E.; Asbaghi, O.; Khosroshahi, M.Z. Effects of ginseng on C-reactive protein level: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2019, 45, 98–103. [Google Scholar] [CrossRef]
- Ziaei, R.; Ghavami, A.; Ghaedi, E.; Hadi, A.; Javadian, P.; Clark, C.C.T. The efficacy of ginseng supplementation on plasma lipid concentration in adults: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 48, 102239. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Brondani, L.A.; Assmann, T.S.; de Souza, B.M.; Bouças, A.P.; Canani, L.H.; Crispim, D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1-3 genes with body mass index variability. PLoS ONE 2014, 9, e96411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, K.; Marquina, C.; Hoj, P.; Liew, D.; Mousa, A.; de Courten, B. Carnosine and histidine-containing dipeptides improve dyslipidemia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Morgan, L.M.; Bishop, J.; Jovanovski, E.; Jenkins, D.J.A.; Vuksan, V. Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: A randomized controlled, cross-over clinical trial. Eur. J. Nutr. 2018, 57, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoon, K.H.; Kang, M.J.; Yim, H.W.; Lee, K.S.; Vuksan, V.; Sung, M.K. A six-month supplementation of mulberry, korean red ginseng, and banaba decreases biomarkers of systemic low-grade inflammation in subjects with impaired glucose tolerance and type 2 diabetes. Evid.-Based Complement. Altern. Med. 2012, 2012, 735191. [Google Scholar] [CrossRef]
- Mohammad-Saeed, B.; Mohammad-Ali, A.; Mahmoud, R.-k. Effect of diabetan on blood glucose, glycosylated hemoglobin, lipid profile, liver and kidney function tests of diabetic patients: A clinical, double blind, randomized trial. Afr. J. Pharm. Pharmacol. 2013, 7, 50–53. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.J.; Ha, K.C.; Baek, H.I.; Yang, H.J.; Kim, M.J.; Park, S.J. Efficacy and safety of aronia, red ginseng, shiitake mushroom, and nattokinase mixture on insulin resistance in prediabetic adults: A randomized, double-blinded, placebo-controlled trial. Foods 2021, 10, 1558. [Google Scholar] [CrossRef]
- Shin, S.K.; Kwon, J.H.; Jeong, Y.J.; Jeon, S.M.; Choi, J.Y.; Choi, M.S. Supplementation of cheonggukjang and red ginseng cheonggukjang can improve plasma lipid profile and fasting blood glucose concentration in subjects with impaired fasting glucose. J. Med. Food 2011, 14, 108–113. [Google Scholar] [CrossRef]
- Zurbau, A.; Smircic Duvnjak, L.; Magas, S.; Jovanovski, E.; Miocic, J.; Jenkins, A.L.; Jenkins, D.J.A.; Josse, R.G.; Leiter, L.A.; Sievenpiper, J.L.; et al. Co-administration of viscous fiber, Salba-chia and ginseng on glycemic management in type 2 diabetes: A double-blind randomized controlled trial. Eur. J. Nutr. 2021, 60, 3071–3083. [Google Scholar] [CrossRef]
- Choi, K.M.; Lee, E.J.; Kim, Y.H.; Baik, S.H.; Kim, Y.K.; Choi, D.S. Erythrocyte and antioxidant superoxide dismutase (SOI) activity in NIDDM patients. Korean J. Ginseng Sci. 1997, 21, 153–159. [Google Scholar]
- Kim, H.-O.; Park, M.-J.; Han, J.-S. Effects of fermented red ginseng supplementation on blood glucose and insulin resistance in type 2 diabetic patients. J. Korean Soc. Food Sci. Nutr. 2011, 40, 696–703. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Alipour, M.; Zakerish, M.; Haghighizadeh, M.H. Effects of standardized extract of ginseng [G115] on biomarkers of systemic low-grade inflammation in patients with type 2 diabetes: A double-blind clinical trial. Iran. J. Endocrinol. Metab. 2014, 16, 175–182. [Google Scholar]
- Jovanovski, E.; Jenkins, A.; Vuksan, V. Effects of Korean White Ginseng (Panax ginseng CA Meyer) on vascular and glycemic health in type 2 diabetes: Results of a randomized, double blind, placebo-controlled, multiple-crossover, acute dose escalation trial. Clin. Nutr. Res. 2014, 3, 89–97. [Google Scholar]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.A.; Ehsanpour, A.; Asgari, M.; Malihi, R. Evaluation of standardized ginseng extract Panax (G115®) effect on fasting blood glucose levels, glycated hemoglobin and lipid profile in patients with diabetes type 2. Jundishapur J. Chronic Dis. Care 2013, 2, 26–32. [Google Scholar]
- Jovanovski, E.; Lea Duvnjak, S.; Komishon, A.; Au-Yeung, F.; Zurbau, A.; Jenkins, A.L.; Sung, M.K.; Josse, R.; Vuksan, V. Vascular effects of combined enriched Korean Red ginseng (Panax ginseng) and American ginseng (Panax quinquefolius) administration in individuals with hypertension and type 2 diabetes: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102338. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, M.R.; Choi, E.K.; Kim, M.G.; Ha, K.C.; Lee, S.K.; Kim, Y.G.; Park, B.H.; Kim, D.S.; Chae, S.W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J. Ginseng Res. 2014, 38, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Reeds, D.N.; Patterson, B.W.; Okunade, A.; Holloszy, J.O.; Polonsky, K.S.; Klein, S. Ginseng and ginsenoside Re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care 2011, 34, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Sotaniemi, E.A.; Haapakoski, E.; Rautio, A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995, 18, 1373–1375. [Google Scholar] [CrossRef]
- Ma, S.W.; Benzie, I.F.; Chu, T.T.; Fok, B.S.; Tomlinson, B.; Critchley, L.A. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: Results of a placebo-controlled human intervention trial. Diabetes Obes. Metab. 2008, 10, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qu, C.-Y.; Li, J.-X.; Wang, Y.-F.; Li, W.; Wang, C.-Z.; Wang, D.-S.; Song, J.; Sun, G.-Z.; Yuan, C.-S. Hypoglycemic and hypolipidemic effects of malonyl ginsenoides from American ginseng (Panax quinquefolius L.) on type 2 diabetic mice. ACS Omega 2021, 6, 33652–33664. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.-f.; Xu, Z.-r.; Xu, K.-y.; Yang, Y.-m. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Medicine 2016, 95, e2584. [Google Scholar] [CrossRef]
- Ma, C.; Yu, H.; Xiao, Y.; Wang, H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm. Biol. 2017, 55, 2170–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, T.; Nguyen, T.T.; Zimmerman, B.R. Hyperlipidemia and diabetes mellitus. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1998; pp. 969–976. [Google Scholar]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar] [CrossRef]
- Wang, D.-S.; Wang, J.-M.; Zhang, F.-R.; Lei, F.-J.; Wen, X.; Song, J.; Sun, G.-Z.; Liu, Z. Ameliorative effects of malonyl ginsenoide from Panax ginseng on glucose-lipid metabolism and insulin resistance via IRS1/PI3K/Akt and AMPK signaling pathways in type 2 diabetic mice. Am. J. Chin. Med. 2022, 50, 863–882. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, K.-S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Song, Y.B.; An, Y.R.; Kim, S.J.; Park, H.W.; Jung, J.W.; Kyung, J.S.; Hwang, S.Y.; Kim, Y.S. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2012, 92, 388–396. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martinez de Maranon, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Alzamil, H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alolga, R.N.; Nuer-Allornuvor, G.F.; Kuugbee, E.D.; Yin, X.; Ma, G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020, 152, 104630. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.N.; Ji, M.G.; Kang, T.H. The efficacy of red ginseng in type 1 and type 2 diabetes in animals. Evid.-Based Complement. Altern. Med. 2013, 2013, 593181. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Ding, C.; Zhao, Y.; Chen, X.; Khatoon, S.; Zheng, Y.; Cheng, Z.; Xi, G. Antidiabetic effects of arginyl-fructosyl-glucose, a nanosaponin fraction from ginseng processing in streptozotocin-induced type 2 diabetic mice through regulating the PI3K/AKT/GSK-3β and Bcl-2/Bax Signaling pathways. Evid.-Based Complement. Altern. Med. 2020, 2020, 3707904. [Google Scholar] [CrossRef]
- Park, S.J.; Nam, J.; Ahn, C.W.; Kim, Y. Anti-diabetic properties of different fractions of Korean red ginseng. J. Ethnopharmacol. 2019, 236, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.S.; Yoon, M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J. Ethnopharmacol. 2018, 210, 80–87. [Google Scholar] [CrossRef]
- Nagar, H.; Kang, S.K.; Choi, S.W.; Song, H.-J.; Choi, S.-J.; Piao, S.; Kim, S.; Lee, I.; Kim, C.-S. Antihypertensive effects of Rg3-enriched Korean vitamin ginseng in spontaneously hypertensive rats. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ran, X.; Dou, D.; Chen, H.; Ren, G. The correlations of adverse effect and tonifying effect of ginseng medicines. J. Ethnopharmacol. 2022, 291, 115113. [Google Scholar] [CrossRef] [PubMed]
- Hur, M.-H.; Lee, M.-S.; Yang, H.-J.; Kim, C.; Bae, I.-L.; Ernst, E. Ginseng for reducing the blood pressure in patients with hypertension: A systematic review and meta-analysis. J. Ginseng Res. 2010, 34, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Komishon, A.; Shishtar, E.; Ha, V.; Sievenpiper, J.; de Souza, R.; Jovanovski, E.; Ho, H.; Duvnjak, L.; Vuksan, V. The effect of ginseng (genus Panax) on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. J. Hum. Hypertens. 2016, 30, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Hong, M.; Sung, H.; Kim, S.; Suk, K.T. Effect of Korean red ginseng in chronic liver disease. J. Ginseng Res. 2017, 41, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gwak, S.R.; Joo, J.C.; Song, B.K.; Cha, S.W.; Song, Y.U.; Pyo, M.K.; Park, S.J. Effectiveness and safety of Panax ginseng extract on hepatic dysfunction: A randomized, double-blind, placebo-controlled clinical trial. Evid.-Based Complement. Altern. Med. 2020, 2020, 2689565. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).