Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sampling and Biochemical Analyses
2.3. Statistics
2.4. Ethics
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Overweight (Body Mass Index ≥ 25), Age-Standardized (%) Estimates by Country. 2021. Available online: http://apps.who.int/gho/data/node.main.A896?lang=en (accessed on 1 February 2022).
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Mehta, T.S.; Davidson, L.E.; Hunt, S.C. All-cause and cause-specific mortality associated with bariatric surgery: A review. Curr. Atheroscler. Rep. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.; Sjöholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.A.; Taube, M.; Carlsson, B.; Peltonen, M. Life expectancy after bariatric surgery in the Swedish obese subjects study. N. Engl. J. Med. 2020, 383, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Beamish, A.J.; Olbers, T.; Kelly, A.S.; Inge, T.H. Cardiovascular effects of bariatric surgery. Nat. Rev. Cardiol. 2016, 13, 730–743. [Google Scholar] [CrossRef]
- Kjellmo, C.A.; Karlsson, H.; Nestvold, T.K.; Ljunggren, S.; Cederbrant, K.; Marcusson-Ståhl, M.; Mathisen, M.; Lappegård, K.T.; Hovland, A. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J. Clin. Lipidol. 2018, 12, 193–202. [Google Scholar]
- Lu, M.; Lu, Q.; Zhang, Y.; Tian, G. ApoB/apoA1 is an effective predictor of coronary heart disease risk in overweight and obesity. J. Biomed. Res. 2011, 25, 266–273. [Google Scholar] [CrossRef]
- Enas, E.A.; Varkey, B.; Dharmarajan, T.S.; Pare, G.; Bahl, V.K. Lipoprotein (a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019, 71, 99–112. [Google Scholar] [CrossRef]
- Jansen, A.; Berg, J.P.; Klungsoyr, O.; Muller, M.H.B.; Lyche, J.L.; Aaseth, J.O. The Influence of Persistent Organic Pollutants on Thyroidal, Reproductive and Adrenal Hormones After Bariatric Surgery. Obes. Surg. 2020, 30, 1368–1378. [Google Scholar] [CrossRef]
- Aasbrenn, M.; Lydersen, S.; Farup, P.G. A Conservative Weight Loss Intervention Relieves Bowel Symptoms in Morbidly Obese Subjects with Irritable Bowel Syndrome: A Prospective Cohort Study. J. Obes. 2018, 2018, 3732753. [Google Scholar] [CrossRef]
- Kligman, M.D.; Dexter, D.J.; Omer, S.; Park, A.E. Shrinking cardiovascular risk through bariatric surgery: Application of Framingham risk score in gastric bypass. Surgery 2008, 143, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Komorniak, N.; Hoffmann, M.; Walczak, J.; Jaroszek, A.; Kowalewski, B.; Kaseja, K.; Jamioł-Milc, D.; Stachowska, E. Body weight reduction and biochemical parameters of the patients after RYGB and SG bariatric procedures in 12-month observation. Obes. Surg. 2017, 27, 940–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liaskos, C.; Koliaki, C.; Alexiadou, K.; Argyrakopoulou, G.; Tentolouris, N.; Diamantis, T.; Alexandrou, A.; Katsilambros, N.; Kokkinos, A. Roux-en-Y gastric bypass is more effective than sleeve gastrectomy in improving postprandial glycaemia and lipaemia in non-diabetic morbidly obese patients: A short-term follow-up analysis. Obes. Surg. 2018, 28, 3997–4005. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: A population-based retrospective cohort study. Circulation 2021, 143, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Insull, W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South. Med. J. 2006, 99, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Gordts, P.L.; Nora, C.; Yeang, C.; Witztum, J.L. Statin therapy increases lipoprotein (a) levels. Eur. Heart J. 2020, 41, 2275–2284. [Google Scholar] [CrossRef]
- Lin, B.X.; Weiss, M.C.; Parikh, M.; Berger, J.S.; Fisher, E.A.; Heffron, S.P. Changes in lipoprotein (a) following bariatric surgery. Am. Heart J. 2018, 197, 175. [Google Scholar] [CrossRef]
- Ferrebee, C.B.; Dawson, P.A. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm. Sin. B 2015, 5, 129–134. [Google Scholar] [CrossRef]
- Kumari, A.; Pathak, D.P.; Asthana, S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism. Int. J. Biol. Sci. 2020, 16, 2308–2322. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Haas, M.J.; Wehmeier, K.R.; Wong, N.C. Obesity-related changes in high-density lipoprotein metabolism. Obesity 2008, 16, 1152–1160. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, P.M.; Heinecke, J.W. Cholesterol efflux capacity, macrophage reverse cholesterol transport and cardioprotective HDL. Curr. Opin. Lipidol. 2015, 26, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Lemieux, I.; Després, J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
| Characteristics (Number (n) If Less Than 111) | Result Mean (SD) or n (%) | Results Gastric Bypass (n 90) | Results Sleeve Gastrectomy (n 21) | Statistics * p-Values |
|---|---|---|---|---|
| Sex (male/female) | 23 (21%)/88 (79%) | 22 (24%)/68 (76%) | 1 (5%)/20 (95%) | 0.069 |
| Age (years) | 43.0 (8.2) | 42.9 (8.6) | 43.3 (6.8) | 0.825 |
| BMI (kg/m2) | 38.8 (3.8) | 39.1 (3.6) | 37.2 (4.1) | 0.825 |
| Diabetes | 19 (17%) | 17 (20%) | 2 (9.5%) | 0.351 |
| HbA1c (%) (n 101) | 5.48 (0.87) | 5.46 (0.67) | 5.55 (1.43) | 0.689 |
| CRP (mg/L) | 4.41 (3.92) | 4.36 (4.09) | 4.62 (3.14) | 0.783 |
| Triglycerides (mmol/L) (n 101) | 1.33 (0.52) | 1.38 (0.54) | 1.09 (0.35) | 0.005 |
| Cholesterol (mmol/L) (n 101) | 4.36 (0.88) | 4.36 (0.91) | 4.38 (0.77) | 0.930 |
| HDL (mmol/L) (n 101) | 1.11 (0.30) | 1.06 (0.29) | 1.30 (0.26) | 0.001 |
| Non-HDL (mmol/L) (n 101) | 3.26 (0.89) | 3.08 (0.77) | 3.30 (0.92) | 0.327 |
| LDL (mmol/L) (n 101) | 2.78 (0.82) | 2.81 (0.84) | 2.69 (0.71) | 0.548 |
| LDL/HDL ratio (n 101) | 2.73 (1.16) | 2.86 (1.19) | 2.17 (0.84) | 0.016 |
| ApoA1 (g/L) (n 99) | 1.14 (0.19) | 1.12 (0.19) | 1.22 (0.17) | 0.032 |
| ApoB (g/L) (n 99) | 0.86 (0.21) | 0.86 (0.21) | 0.85 (0.21) | 0.778 |
| ApoB/ApoA1 ratio (n 99) | 0.77 (0.23) | 0.79 (0.24) | 0.70 (0.19) | 0.126 |
| Lp(a) (nmol/L) (n 99) | 53.6 (63.0) | 52.4 (61.4) | 58.7 (70.8) | 0.700 |
| Dependent Variables | Changes * † All Subjects | Changes * Gastric Bypass | Changes * Sleeve Gastrectomy | Change * Differences (Bypass Minus Sleeve) |
|---|---|---|---|---|
| BMI (kg/m2) | −9.57 (−10.08; −9.07) p < 0.001 | −9.70 (−10.26; −9.13) p < 0.001 | −9.04 (−10.21; −7.87) p < 0.001 | −0.65 (−1.95; 0.64) p = 0.320 |
| CRP (mg/L) | −2.99 (−3.52; −2.45) p < 0.001 | −3.02 (−3.60; −2.43) p < 0.001 | −2.85 (−4.11; 1.26) p < 0.001 | −0.16 (−1.55; 1–23) p = 0.819 |
| Triglycerides (mmol/L | −0.35 (−0.42; −0.28) p < 0.001 | −0.38 (−0.46; −0.31) p < 0.001 | −0.18 (−0.34; −0.02) p = 0.026 | −0.20 (−0.38; −0.03) p = 0.025 |
| Cholesterol (mmol/L) | −0.02 (−0.16; 0.12) p = 0.752 | −0.12 (−0.28; 0.03) p = 0.103 | 0.43 (0.11; 0.74) p = 0.008 | −0.55 (−0.90; −0.20) p = 0.002 |
| HDL (mmol/L) | 0.36 (0.32; 0.40) p < 0.001 | 0.36 (0.31; 0.40) p < 0.001 | 0.39 (0.29; 0.49) p < 0.001 | −0.03 (−0.14; 0.08) p = 0.548 |
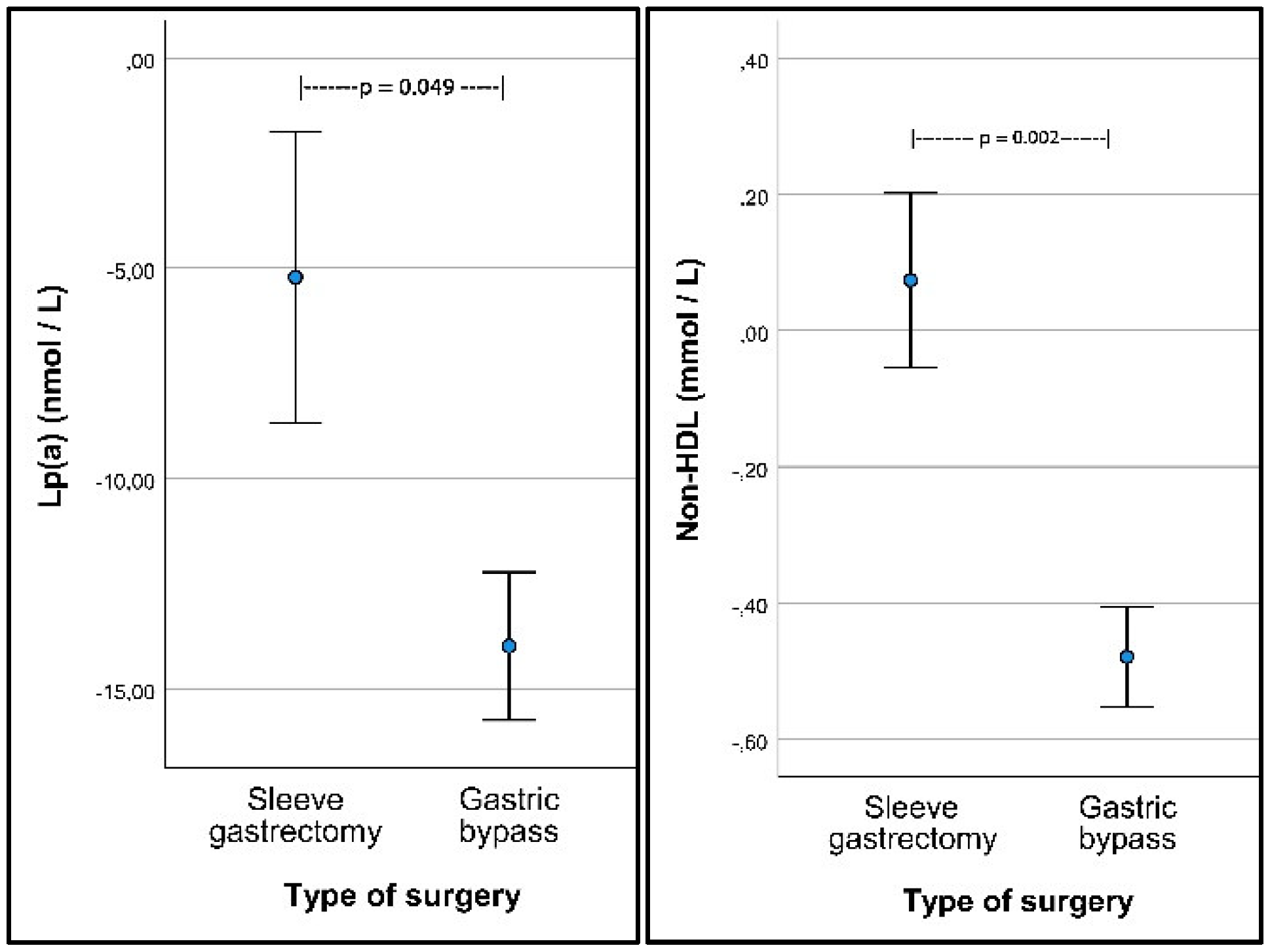
| Non-HDL (mmol/L) | −0.38 (−0.52; −0.25) p < 0.001 | −0.48 (−0.62; −0.33) p < 0.001 | 0.04 (−0.26; 0.34) p = 0.789 | −0.52 (−0.86; −0.19) p = 0.002 |
| LDL (mmol/L) | −0.12 (−0.25; 0.004) p = 0.057 | −0.21 (−0.35; −0.07) p = 0.003 | 0.26 (−0.03; 0.54) p = 0.076 | −0.47 (−0.78; −0.15) p = 0.004 |
| LDL/HDL ratio | −0.80 (−0.94; −0.66) p < 0.001 | −0.90 (−1.05; −0.75) p < 0.001 | −0.35 (−0.67; −0.04) p = 0.027 | −0.54 (−0.89; −0.20) p = 0.002 |
| ApoA1 (g/L) | 0.23 (0.20–0.26) p < 0.001 | 0.23 (0.19; 0.26) p < 0.001 | 0.26 (0.18; 0.33) p < 0.001 | −0.03 (−0.11; 0.05) p = 0.463 |
| ApoB (g/L) | −0.04 (−0.07; −0.01) p = 0.007 | −0.06 (−0.09; −0.02) p = 0.001 | (−0.06; 0.09) p = 0.706 | −0.07 (−0.15; 0.01) p = 0.086 |
| ApoB/ApoA1 ratio | −0.16 (−0.19; −0.13) p < 0.001 | −0.17 (−0.21; −0.14) p < 0.001 | −0.11 (−0.18; −0.05) p = 0.001 | −0.06 (−0.14; 0.02) p = 0.134 |
| Lp(a) (nmol/L) | −12.3 (−15.6; −9.0) p < 0.001 | −13.8. (−17.4; −10.2) p < 0.001 | −5.3 (−13.0; 2.4) p = 0.174 | −8.5 (−17.0; −0.04) p = 0.049 |
| Dependent Variables | Independent Variables * | |||
|---|---|---|---|---|
| BMI | Diabetes | Sex (Male) | Age | |
| Triglycerides (mmol/L) | 0.02 (0.01; 0.04) p = 0.005 | 0.31 (0.13; 0.50) p = 0.001 | −0.02 (−0.21; 0.17) p = 0.853 | −0.003 (−0.01; 0.01) p = 0.441 |
| Cholesterol (mmol/L) | 0.03 (−0.003; 0.06) p = 0.08 | 0.05 (−0.28; 0.37) p = 0.781 | −0.33 (−0.66; −0.01) p = 0.046 | 0.02 (0.001; 0.03) p = 0.037 |
| HDL (mmol/L) | −0.014 (−0.025; −0.004) p = 0.006 | −0.02 (−0.16; 0.13) p = 0.827 | −0.13 (−0.27; 0.02) p = 0.087 | 0.014 (0.007; 0.021) p < 0.001 |
| Non-HDL (mmol/L) | 0.04 (0.01; 0.07) p = 0.006 | 0.06 (−0.28; 0.40) p = 0.714 | −0.21 (−0.55; 0.13) p = 0.226 | 0.002 (−0.01;0.02) p = 0.778 |
| LDL (mmol/L) | 0.03 (0.005; 0.06) p = 0.022 | −0.03 (−0.34; 0.28) p = 0.243 | −0.22 (−0.53; 0.09) p = 0.164 | 0.005 (−0.001; 0.02) p = 0.518 |
| LDL/HDL ratio | 0.06 (0.03; 0.10) p < 0.001 | 0.10 (−0.31; 0.51) p = 0.637 | 0.06 (−0.35; 0.47) p = 0.764) | −0.02 (−0.04; −0.01) p = 0.034 |
| ApoA1 (g/L) | −0.004 (−0.012; 0.003) p = 0.269 | 0.04 (−0.06; 0.14) p = 0.418 | −0.10 (−0.20; 0.002) p = 0.055 | 0.008 (0.003; 0.012) p = 0.001 |
| ApoB (g/L) | 0.009 (0.002; 0.017) p = 0.014 | 0.02 (−0.07; 0.11) p = 0.646 | −0.06 (−0.15; 0.02) p = 0.156 | 0.001 (−0.003; 0.005) p = 0.551 |
| ApoB/ApoA1 ratio | 0.012 (0.005; 0.019) p = 0.001 | −0.001 (−0.11; 0.09) p = 0.673 | 0.003 (−0.089; 0.094)p = 0.953 | −0.003 (−0.008; 0.001) p = 0.148 |
| Lp(a) (nmol/L) | 0.37 (−0.73; 1.47) p = 0.505 | −0.79 (−34.5; 32.9) p = 0.963 | 13.0 (−20.2; 46.3) p = 0.438 | 0.71 (−0.86; 2.27) p = 0.373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaseth, J.O.; Rootwelt, H.; Retterstøl, K.; Hestad, K.; Farup, P.G. Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients 2022, 14, 2381. https://doi.org/10.3390/nu14122381
Aaseth JO, Rootwelt H, Retterstøl K, Hestad K, Farup PG. Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients. 2022; 14(12):2381. https://doi.org/10.3390/nu14122381
Chicago/Turabian StyleAaseth, Jan O., Helge Rootwelt, Kjetil Retterstøl, Knut Hestad, and Per G. Farup. 2022. "Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy" Nutrients 14, no. 12: 2381. https://doi.org/10.3390/nu14122381
APA StyleAaseth, J. O., Rootwelt, H., Retterstøl, K., Hestad, K., & Farup, P. G. (2022). Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients, 14(12), 2381. https://doi.org/10.3390/nu14122381







