Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial
Abstract
:1. Introduction
2. Materials and Methods
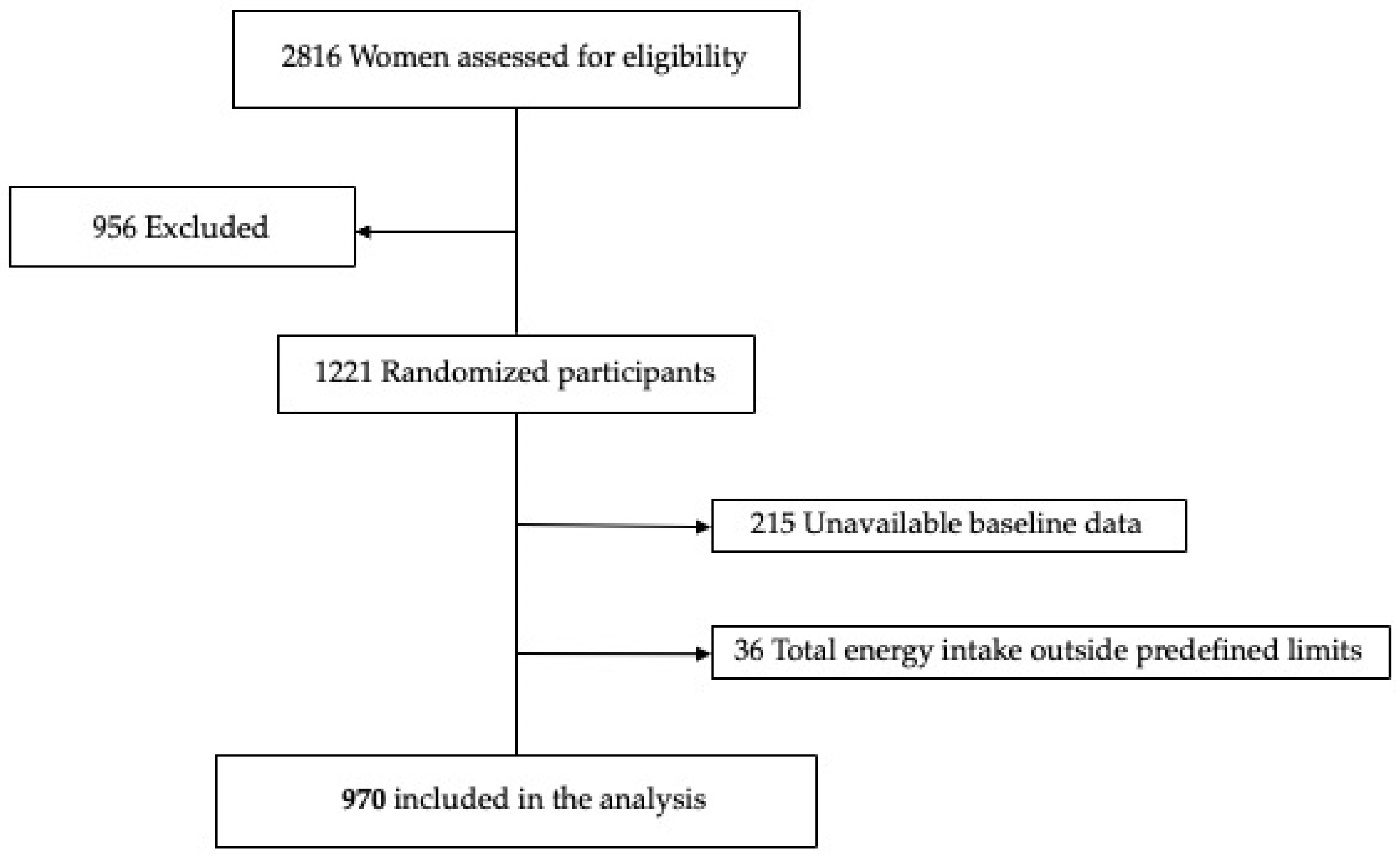
2.1. Study Design and Participants
2.2. Assessment of Dietary Intake
2.3. DII Assessment
2.4. Maternal Characteristics
2.5. Perinatal Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population According to DII Tertile
3.2. Maternal Dietary Characteristics and Adherence to MD According to DII Tertile
3.3. Association of DII with Maternal BMI and Newborn’s Birthweight
4. Discussion
4.1. Nutritional Intake According to DII Index
4.2. Mediterranean Diet Adherence
4.3. Perinatal Outcomes and Birthweight
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Bränn, E.; Edvinsson, Å.; Rostedt Punga, A.; Sundström-Poromaa, I.; Skalkidou, A. Inflammatory and Anti-Inflammatory Markers in Plasma: From Late Pregnancy to Early Postpartum. Sci. Rep. 2019, 9, 1863. [Google Scholar] [CrossRef]
- Brien, M.E.; Boufaied, I.; Bernard, N.; Forest, J.C.; Giguere, Y.; Girard, S. Specific inflammatory profile in each pregnancy complication: A comparative study. Am. J. Reprod. Immunol. 2020, 84, e13316. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial Dysfunction and Preeclampsia: Role of Oxidative Stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [Green Version]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of Oxidative Stress and Antioxidant Supplementation in Pregnancy Disorders. Am. J. Clin. Nutr. 2011, 94, 1980S–1985S. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30 (Suppl. A), 43–48. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.S.; Wang, C.C.R.; Tam, W.H.; Li, C.Y.; Chu, K.O.; Chu, C.Y. Oxidative Stress in Midpregnancy as a Predictor of Gestational Hypertension and Pre-Eclampsia. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1053–1059. [Google Scholar] [CrossRef]
- Shafiq, M.; Mathad, J.S.; Naik, S.; Alexander, M.; Yadana, S.; Araújo-Pereira, M.; Kulkarni, V.; Deshpande, P.; Kumar, N.P.; Babu, S.; et al. Association of Maternal Inflammation During Pregnancy with Birth Outcomes and Infant Growth among Women with or without HIV in India. JAMA Netw. Open 2021, 4, e2140584. [Google Scholar] [CrossRef]
- Moody, L.; Chen, H.; Pan, Y.X. Early-Life Nutritional Programming of Cognition—The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process. Adv. Nutr. 2017, 8, 337–350. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Acosta, P.; Marín-Jiménez, N.; Baena-García, L.; Aranda, P.; Aparicio, V.A. Influence of the Degree of Adherence to the Mediterranean Diet and Its Components on Cardiometabolic Risk during Pregnancy. The GESTAFIT Project. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2311–2318. [Google Scholar] [CrossRef]
- Timmermans, S.; Steegers-Theunissen, R.P.; Vujkovic, M.; den Breeijen, H.; Russcher, H.; Lindemans, J.; MacKenbach, J.; Hofman, A.; Lesaffre, E.E.; Jaddoe, V.V.; et al. The Mediterranean Diet and Fetal Size Parameters: The Generation R Study. Br. J. Nutr. 2012, 108, 1399–1409. [Google Scholar] [CrossRef] [Green Version]
- Assaf-Balut, C.; García De La Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean Diet with Additional Extra Virgin Olive Oil and Pistachios Reduces the Incidence of Gestational Diabetes Mellitus (GDM): A Randomized Controlled Trial: The St. Carlos GDM Prevention Study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Yeh, K.L.; Kautz, A.; Lohse, B.; Groth, S.W. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef]
- Spadafranca, A.; Piuri, G.; Bulfoni, C.; Liguori, I.; Battezzati, A.; Bertoli, S.; Speciani, A.F.; Ferrazzi, E. Adherence to the Mediterranean Diet and Serum Adiponectin Levels in Pregnancy: Results from a Cohort Study in Normal Weight Caucasian Women. Nutrients 2018, 10, 928. [Google Scholar] [CrossRef] [Green Version]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Mediterranean Diet, Dietary Polyphenols and Low Grade Inflammation: Results from the MOLI-SANI Study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Inflammatory Biomarkers and Immune Cell Populations: A Systematic Literature Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus Nutrition Intervention Randomized Trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Marfella, R.; Ciotola, M.; di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Dietary Inflammatory Potential during Pregnancy Is Associated with Lower Fetal Growth and Breastfeeding Failure: Results from Project Viva. J. Nutr. 2016, 146, 728–736. [Google Scholar] [CrossRef]
- De Andrade Miranda, D.E.G.; Santos, I.D.S.; Silva, C.A.; Carvalho, M.R.; Shivappa, N.; Hébert, J.R.; Crivellenti, L.C.; Sartorelli, D.S. Pro-inflammatory diet during pregnancy is associated with large-for-gestational-age infants. Nutr. Res. 2022, 100, 47–57, Epub 3 February 2022. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150–2160. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Borras, R.; Paules, C.; Casas, R.; Martín-Asuero, A.; Arranz, A.; Vieta, E.; Estruch, R.; Gratacós, E. Mediterranean Diet, Mindfulness-Based Stress Reduction and Usual Care during Pregnancy for Reducing Fetal Growth Restriction and Adverse Perinatal Outcomes: IMPACT BCN (Improving Mothers for a Better Prenatal Care Trial Barcelona): A Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 362. [Google Scholar] [CrossRef]
- Willet, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiological studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1231S. [Google Scholar] [CrossRef]
- Juton, C.; Castro-Barquero, S.; Casas, R.; Freitas, T.; Ruiz-León, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacós, E.; et al. Reliability and Concurrent and Construct Validity of a Food Frequency Questionnaire for Pregnant Women at High Risk to Develop Fetal Growth Restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef]
- Farrán, A.; Zamora, R.; Cervera, P. Tablas de Composición de Alimentos del CESNID; Edicions Universitat de Barcelona; McGraw-Hill Interamericana: Barcelona, Spain, 2003. [Google Scholar]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos. Guía de Prácticas, 19th ed.; Editorial Pirámide: Madrid, Spain, 2018. [Google Scholar]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The Classification, Diagnosis and Management of the Hypertensive Disorders of Pregnancy: A Revised Statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J.; Francis, A.; Gratacos, E.; Gardosi, J. Customized Birthweight Standards for a Spanish Population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef]
- Galan, H.; Grobman, W. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, E97–E109. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee Opinion No 579: Definition of Term Pregnancy. Obstet Gynecol. 2013, 122, 1139–1140. [Google Scholar] [CrossRef]
- Shin, D.; Hur, J.; Cho, E.H.; Chung, H.K.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Lee, K.W. Pre-Pregnancy Body Mass Index Is Associated with Dietary Inflammatory Index and C-Reactive Protein Concentrations during Pregnancy. Nutrients 2017, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Hebert, J.R.; Kivimaki, M.; Akbaraly, T. Alternative Healthy Eating Index 2010, Dietary Inflammatory Index and Risk of Mortality: Results from the Whitehall II Cohort Study and Meta-Analysis of Previous Dietary Inflammatory Index and Mortality Studies. Br. J. Nutr. 2017, 118, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Assmann, K.E.; Adjibade, M.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Touvier, M.; Akbaraly, T.; Hercberg, S.; Galan, P.; Julia, C.; et al. The Inflammatory Potential of the Diet at Midlife Is Associated with Later Healthy Aging in French Adults. J. Nutr. 2018, 148, 437–444. [Google Scholar] [CrossRef]
- Ernst, G.D.S.; de Jonge, L.L.; Hofman, A.; Lindemans, J.; Russcher, H.; Steegers, E.A.P.; Jaddoe, V.W.V. C-Reactive Protein Levels in Early Pregnancy, Fetal Growth Patterns, and the Risk for Neonatal Complications: The Generation R Study. Am. J. Obstet. Gynecol. 2011, 205, 132-e1. [Google Scholar] [CrossRef] [Green Version]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a Cholesterol-Lowering Diet on Maternal, Cord, and Neonatal Lipids, and Pregnancy Outcome: A Randomized Clinical Trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Zhong, C.; Zhou, X.; Liu, C.; Li, Q.; Chen, R.; Gao, Q.; Li, X.; Zhang, H.; et al. Association between Dietary Inflammatory Index and Gestational Diabetes Mellitus Risk in a Prospective Birth Cohort Study. Nutrition 2021, 87, 111193. [Google Scholar] [CrossRef]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between Dietary Inflammatory Index and Inflammatory Markers in the HELENA Study. Mol. Nutr. Food Res. 2017, 61, 1600707. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [Green Version]
- Casas, R.; Sacanella, E.; Urpí-Sardá, M.; Corella, D.; Castañr, O.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Martínez-Gonźalez, M.A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención Con DIeta MEDiterránea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal Dietary Patterns and Risk of Adverse Pregnancy (Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus) and Birth (Preterm Birth and Low Birth Weight) Outcomes: A Systematic Review and Meta-Analysis. Public Health Nutr. 2018, 22, 506–520. [Google Scholar] [CrossRef]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.M.; Bassett, J.K.; Dugué, P.A.; Shivappa, N.; Hébert, J.R.; Milne, R.L.; English, D.R.; Giles, G.G. Dietary Inflammatory Index or Mediterranean Diet Score as Risk Factors for Total and Cardiovascular Mortality. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 461–469. [Google Scholar] [CrossRef]
- Paknahad, Z.; Fallah, A.; Moravejolahkami, A.R. Maternal Dietary Patterns and Their Association with Pregnancy Outcomes. Clin. Nutr. Res. 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, I. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Ness, R.B.; Harger, G.F.; Roberts, J.M. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am. J. Epidemiol. 2005, 162, 1198–1206. [Google Scholar] [CrossRef] [Green Version]
- Bullen, B.L.; Jones, N.M.; Holzman, C.B.; Tian, Y.; Senagore, P.K.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; Sikorskii, A. C-reactive protein and preterm delivery: Clues from placental findings and maternal weight. Reprod. Sci. 2013, 20, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003, 26, 819–824. [Google Scholar] [CrossRef] [Green Version]
- La Coursiere, D.Y.; Bloebaum, L.; Duncan, J.D.; Varner, M.W. Population-based trends and correlates of maternal overweight and obesity, Utah 1991–2001. Am. J. Obstet. Gynecol. 2005, 192, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, H. High prepregnancy body-mass index—A maternal-fetal risk factor. N. Engl. J. Med. 1998, 338, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Potdar, N.; Singh, R.; Mistry, V.; Evans, M.D.; Farmer, P.B.; Konje, J.C.; Cooke, M.S. First-Trimester Increase in Oxidative Stress and Risk of Small-for-Gestational-Age Fetus. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 637–642. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All n = 970 | DII | p y | ||
|---|---|---|---|---|---|
| Tertile 1 n = 323 | Tertile 2 n = 324 | Tertile 3 n = 323 | |||
| DII * | |||||
| Range | −5.71 to −0.33 | −5.71 to −3.48 | −3.47 to −2.34 | −2.33 to −0.33 | <0.001 |
| Mean ± SD | −2.94 ± 1.12 | −4.23 ± 0.51 | −2.89 ± 0.34 | −1.7 ± 0.45 | <0.001 |
| Maternal characteristics | |||||
| Age at enrollment (years) | 37.1 ± 4.70 | 37.4 ± 4.54 | 37.2 ± 4.70 | 36 ± 4.82 | 0.083 |
| ≤24 years | 16 (1.6) | 4 (1.2) | 4 (1.2) | 8 (2.5) | |
| 25–29 years | 61 (6.3) | 18 (5.6) | 19 (5.9) | 24 (7.4) | |
| ≥30 years | 893 (92.1) | 301 (93.2) | 301 (92.9) | 291 (90.1) | |
| Race and ethnicity a | 0.055 | ||||
| Asian | 15 (1.5) | 6 (1.9) | 6 (1.9) | 3 (0.9) | |
| Black | 15 (1.5) | 5 (1.5) | 5 (1.5) | 5 (1.5) | |
| Latin American | 128 (13.2) | 59 (18.3) | 35 (10.8) | 34 (10.5) | |
| White | 794 (81.9) | 245 (75.9) | 271 (83.6) | 278 (86.1) | |
| Maghreb | 18 (1.9) | 8 (2.5) | 7 (2.2) | 3 (0.9) | |
| Socioeconomic status b | 0.921 | ||||
| High | 618 (63.7) | 206 (63.8) | 209 (64.5) | 203 (62.8) | |
| Medium | 309 (31.9) | 105 (32.5) | 99 (30.6) | 105 (32.5) | |
| Low | 43 (4.4) | 12 (3.7) | 16 (4.9) | 15 (4.6) | |
| Pre-pregnancy BMI (kg/m2) | 23.88 ± 4.80 | 23.4 ± 4.98 | 23.7 ± 4.28 | 24.5 ± 5.05 | 0.011 |
| <18.5 (n (%)) | 61(6.3) | 28 (8.7) | 14 (4.3) | 19 (5.9) | |
| 18.5–24.9 | 593 (61.1) | 201 (62.2) | 207 (63.9) | 185 (57.3) | |
| ≥25 | 316 (32.6) | 94 (29.1) | 103 (31.8) | 119 (36.8) | |
| Systolic blood pressure (mmHg) | 105.36 ± 12.02 | 105.79 ± 13.37 | 105.48 ± 11.67 | 104.79 ± 10.85 | 0.564 |
| Diastolic blood pressure (mmHg) | 67.73 ± 8.58 | 67.3 ± 8.97 | 68.18 ± 8.37 | 67.71 ± 8.37 | 0.424 |
| Education level (schooling years) | 0.500 | ||||
| University (yes (%)) | 653 (67.3) | 220 (68.1) | 220 (67.9) | 213 (65.9) | |
| Vocational (yes (%)) | 76 (7.8) | 23 (7.1) | 29 (9) | 24 (7.4) | |
| Secondary (yes (%)) | 198 (20.4) | 68 (21.1) | 59 (18.2) | 71 (22) | |
| Primary (yes (%)) | 34 (3.5) | 7 (2.2) | 13 (4) | 14 (4.3) | |
| No education (yes (%)) | 9 (0.9) | 5 (1.5) | 3 (0.9) | 1 (0.3) | |
| Use of assisted reproductive technologies (yes (%)) | 262 (27.0) | 82 (25.4) | 89 (27.5) | 91 (28.2) | 0.709 |
| Medical history | |||||
| Autoimmune disease (yes (%)) | 159 (16.4) | 43 (13.3) | 59 (18.2) | 57 (17.6) | 0.184 |
| Obesity c (yes (%)) | 109 (11.2) | 29 (9) | 31 (9.6) | 49 (15.2) | 0.106 |
| Thyroid disorder (yes (%)) | 110 (11.3) | 34 (10.5) | 38 (11.7) | 38 (11.8) | 0.852 |
| Diabetes (yes (%)) | 48 (4.9) | 18 (5.6) | 14 (4.3) | 16 (5) | 0.764 |
| Minor psychiatric disorder d (yes (%)) | 38 (3.9) | 10 (3.1) | 16 (4.9) | 12 (3.7) | 0.470 |
| Chronic hypertension (yes (%)) | 41 (4.2) | 15 (4.6) | 14 (4.3) | 12 (3.7) | 0.837 |
| Chronic kidney disease (yes (%)) | 23 (2.4) | 6 (1.9) | 10 (3.1) | 7 (2.2) | 0.565 |
| Adverse obstetric history | |||||
| Previous SGA (yes (%)) | 157 (16.2) | 44 (13.6) | 55 (17) | 58 (18) | 0.292 |
| Previous preterm birth (yes (%)) | 54 (5.6) | 21 (6.5) | 15 (4.6) | 18 (5.6) | 0.583 |
| Previous preeclampsia (yes (%)) | 49 (5.1) | 17 (5.3) | 21 (6.5) | 11 (3.4) | 0.198 |
| Previous stillbirth (yes (%)) | 26 (2.7) | 6 (1.9) | 11 (3.4) | 9 (2.8) | 0.476 |
| Nulliparous (yes (%)) | 400 (41.2) | 128 (39.6) | 146 (45.1) | 126 (39) | 0.227 |
| During pregnancy | |||||
| Smoking habit (yes (%)) | 64 (6.6) | 16 (5) | 23 (7.1) | 25 (7.7) | 0.648 |
| Alcohol consumption (yes (%)) | 14 (1.4) | 6 (1.9) | 3 (0.9) | 5 (1.5) | 0.066 |
| Drug consumption (yes (%)) | 3 (0.3) | 0 (0) | 2 (0.6) | 1 (0.3) | 0.296 |
| Physical exercise (yes (%)) | 219 (22.6) | 98 (30.3) | 72 (22.2) | 49 (15.2) | <0.001 |
| Yoga or pilates (yes (%)) | 189 (19.5) | 91 (28.2) | 64 (19.8) | 34 (10.5) | <0.001 |
| Gestational age at randomization, mean (SD), weeks | 20.85 ± 0.68 | 20.85 ± 0.69 | 20.85 ± 0.68 | 20.85 ± 0.67 | 0.999 |
| Perinatal outcome | |||||
| GDM (yes (%)) | 93 (9.6) | 23 (7) | 42 (12.6) | 28 (9) | 0.282 |
| Preeclampsia (yes (%)) | 72 (7.4) | 33 (10.1) | 19 (5.7) | 20 (6.5) | 0.587 |
| Birthweight, mean (SD), g | 3195.74 ± 523.4 | 3246.79 ± 501.73 | 3185.14 ± 500.5 | 3155.28 ± 562.91 | 0.077 |
| Cesarean section (yes (%)) | 327 (33.7) | 104 (31.8) | 121 (36.3) | 102 (32.9) | 0.126 |
| Gestational age at delivery, mean (SD), weeks | 39.42 ± 1.76 | 39.54 ± 1.51 | 39.4 ± 1.8 | 39.31 ± 1.94 | 0.246 |
| Birthweight percentile, mean (SD) | 42.66 ± 29.96 | 44.22 ± 30.56 | 42.33 ± 28.79 | 41.43 ± 30.5 | 0.481 |
| SGA (yes (%)) | 161 (16.6) | 65 (19.9) | 46 (13.8) | 50 (16.1) | 0.400 |
| Severe SGA (yes (%)) | 60 (6.2) | 31 (9.5) | 15 (4.5) | 14 (4.5) | 0.228 |
| Prematurity (yes (%)) | 60 (6.2) | 20 (6.1) | 19 (5.7) | 21 (6.8) | 0.695 |
| Combined adverse perinatal outcome (yes (%)) | 209 (21.5) | 85 (26) | 61 (18.3) | 63 (20.3) | 0.485 |
| Characteristics | All n = 97 | DII | p y | ||
|---|---|---|---|---|---|
| Tertile 1 n = 323 | Tertile 2 n = 324 | Tertile 3 n = 323 | |||
| EVOO (g/day) | 50 (25 to 50) | 50 (25 to 50) | 50 (25 to 50) | 25 (10 to 50) | 0.001 |
| Refined olive oil (g/day) | 0 (0 to 6.96) | 0 (0 to 0) | 0 (0 to 9.46) | 0 (0 to 10) | 0.073 |
| Total nuts (g/day) | 12.86 (4.29 to 27.71) | 25.71 (12.86 to 42.57) | 12.86 (4.29 to 25.71) | 6 (0 to 12.86) | <0.001 |
| Vegetables (g/day) | 269.15 (198.67 to 355.9) | 376.81 (307.07 to 446.77) | 269.04 (214.76 to 327.8) | 192.78 (141.93 to 234.81) | <0.001 |
| Legumes (g/day) | 42.86 (30 to 64.29) | 56.19 (41.43 to 79.52) | 42.86 (30 to 57.98) | 33.33 (20 to 43.33) | <0.001 |
| Fruits (g/day) | 306.43 (207.14 to 412.5) | 388.57 (303.57 to 513) | 307.71 (218.57 to 406.67) | 218.86 (148.21 to 307.82) | <0.001 |
| Refined cereals (g/day) | 60 (32.14 to 89.71) | 51.43 (17.14 to 77.14) | 60 (34.14 to 94.29) | 72.57 (38.29 to 94.29) | <0.001 |
| Whole grain cereals (g/day) | 25.71 (0 to 60) | 47.14 (12.79 to 70.61) | 31.07 (4.29 to 60) | 8.57 (0 to 47.14) | <0.001 |
| Fish or seafood (g/day) | 68 (42.6 to 95.9) | 83.14 (55.98 to 113.95) | 65.9 (42.33 to 92.57) | 55.67 (34.39 to 77.87) | <0.001 |
| Blue fish (g/day) | 8.33 (0 to 17.86) | 17.86 (8.33 to 17.86) | 8.33 (0 to 17.86) | 8.33 (0 to 17.86) | <0.001 |
| Lean meat (g/day) | 74.29 (42.86 to 85.71) | 74.29 (52.86 to 85.71) | 74.29 (52.86 to 85.71) | 69.29 (41.43 to 85.71) | 0.106 |
| Processed meat (g/day) | 28.57 (14.29 to 50) | 24.76 (10.48 to 49.29) | 28.57 (21.43 to 50) | 28.57 (14.29 to 50) | 0.085 |
| Pastries, cakes, or sweets (g/day) | 30.1 (13.74 to 55.76) | 28.57 (12.42 to 55.71) | 30.24 (14.29 to 50.59) | 30.48 (12.41 to 60.71) | 0.782 |
| Dairy products (g/day) | 300 (184.42 to 410.18) | 312.5 (174.64 to 440.48) | 310.36 (196.61 to 427.32) | 275 (187.86 to 366.13) | 0.019 |
| Onion (g/day) | 15 (5 to 15) | 15 (15 to 27.5) | 15 (15 to 15) | 15 (5 to 15) | <0.001 |
| Garlic (g/day) | 0.3 (0 to 0.9) | 0.9 (0.3 to 1.65) | 0.9 (0.14 to 0.9) | 0.14 (0 to 0.3) | <0.001 |
| Oregano (g/day) | 2.14 (0 to 2.14) | 0.21 (0.07 to 0.21) | 0.21 (0 to 0.21) | 0.07 (0 to 0.21) | <0.001 |
| Pepper (g/day) | 0 (0 to 2.14) | 0.33 (0 to 2.14) | 0 (0 to 2.14) | 0 (0 to 0.71) | <0.001 |
| Alcohol (g/day) | 0 (0 to 0.09) | 0 (0 to 0.19) | 0 (0 to 0.09) | 0 (0 to 0.09) | 0.002 |
| MD Score | 8 (6 to 10) | 9 (7 to 11) | 8 (6 to 9) | 6 (4.25 to 8) | <0.001 |
| Characteristics | All n = 970 | DII | p y | ||
|---|---|---|---|---|---|
| Tertile 1 n = 323 | Tertile 2 n = 324 | Tertile 3 n = 323 | |||
| Energy (kcal/day) | 2412.32 ± 449.4 | 2717.83 ± 361.31 | 2421.29 ± 365.41 | 2097.82 ± 387.77 | <0.001 |
| Protein (g/day) | 102 ± 23.09 | 115.35 ± 21.25 | 102.05 ± 19.81 | 88.6 ± 19.99 | <0.001 |
| Carbohydrate (g/day) | 211.8 (179.53 to 248.39) | 243.56 (213.2 to 278.39) | 212.07 (186.52 to 241.83) | 181.07 (155.53 to 211.33) | <0.001 |
| Fiber (g/day) | 32.04 (25.36 to 39.48) | 41.83 (37.53 to 47.19) | 32.21 (28.28 to 35.54) | 23.64 (19.83 to 26.74) | <0.001 |
| Total fat (g/day) | 129.54 (110.8 to 151.81) | 144.41 (126.82 to 164.92) | 129.81 (113.25 to 150.84) | 115.27 (97.8 to 132.27) | <0.001 |
| SFAs (g/day) | 34.13 (28.47 to 40.52) | 36.73 (31.31 to 42.69) | 34.53 (29.03 to 40.47) | 30.71 (26.69 to 38.29) | <0.001 |
| MUFAs (g/day) | 66.17 ± 17.23 | 72.91 ± 15.51 | 66.74 ± 17.28 | 58.86 ± 15.92 | <0.001 |
| PUFAs (g/day) | 18.04 (14.75 to 23.1) | 22.82 (18.55 to 27.77) | 18.2 (15.5 to 22.16) | 14.67 (12.91 to 17.48) | <0.001 |
| Linoleic acid (g/day) | 13.07 (10.53 to 17.1) | 16.55 (12.69 to 20.3) | 13.07 (10.66 to 16.64) | 10.91 (9.39 to 13.34) | <0.001 |
| α-Linolenic acid (g/day) | 1.18 (0.94 to 1.72) | 1.7 (1.17 to 2.04) | 1.17 (0.98 to 1.67) | 0.96 (0.79 to 1.19) | <0.001 |
| EPA (g/day) | 0.13 (0.08 to 0.19) | 0.17 (0.12 to 0.23) | 0.13 (0.08 to 0.18) | 0.11 (0.06 to 0.15) | <0.001 |
| DHA (g/day) | 0.28 (0.15 to 0.38) | 0.34 (0.21 to 0.46) | 0.27 (0.15 to 0.37) | 0.2 (0.1 to 0.33) | <0.001 |
| Trans-FA (g/day) | 1.39 (0.9 to 1.93) | 1.33 (0.83 to 1.77) | 1.37 (0.85 to 1.9) | 1.51 (0.98 to 2.1) | 0.006 |
| Cholesterol (mg/day) | 329.37 (271.94 to 387.11) | 352.56 (290.57 to 419.63) | 332.07 (280.83 to 390.09) | 301.47 (242.94 to 357.17) | <0.001 |
| Vitamins | |||||
| Vitamin A (µg/day) | 1232.06 (925.37 to 1605.67) | 1647.04 (1353.22 to 1995.93) | 1239.6 (1018.95 to 1514.61) | 880.25 (696.21 to 1093.78) | <0.001 |
| Vitamin C (mg/day) | 241.62 (172.22 to 326.08) | 348.1 (278.38 to 423.19) | 246.67 (191.3 to 295.85) | 159.67 (122.7 to 203.88) | <0.001 |
| Vitamin D (µg/day) | 4.56 (3.46 to 5.91) | 5.65 (4.3 to 7.16) | 4.49 (3.44 to 5.66) | 3.83 (2.92 to 5) | <0.001 |
| Vitamin E (mg/day) | 17.94 (14.85 to 21.73) | 22.62 (19.46 to 26.79) | 17.7 (15.68 to 20.11) | 14.12 (12.07 to 16.39) | <0.001 |
| Vitamin B1 (mg/day) | 1.81 (1.54 to 2.11) | 2.12 (1.87 to 2.39) | 1.81 (1.59 to 2.04) | 1.52 (1.31 to 1.77) | <0.001 |
| Vitamin B2 (mg/day) | 2.09 (1.76 to 2.45) | 2.42 (2.16 to 2.76) | 2.09 (1.81 to 2.38) | 1.73 (1.48 to 1.99) | <0.001 |
| Vitamin B3 (mg/day) | 23.74 (20.21 to 27.28) | 27.74 (24.74 to 30.69) | 23.49 (20.81 to 26.05) | 20.28 (16.84 to 22.98) | <0.001 |
| Vitamin B6 (mg/day) | 2.79 ± 0.67 | 3.4 ± 0.51 | 2.76 ± 0.43 | 2.22 ± 0.45 | <0.001 |
| Vitamin B9 (µg/day) | 471.52 (376.29 to 581.24) | 613.43 (555.07 to 700.19) | 470.34 (420.74 to 519.52) | 347.85 (298.9 to 393.19) | <0.001 |
| Vitamin B12 (µg/day) | 6.33 (4.89 to 8.3) | 7.5 (5.8 to 9.56) | 7.5 (5.8 to 9.56) | 7.5 (5.8 to 9.56) | <0.001 |
| Β-carotene (µg/day) | 5481.21 (3853.48 to 7339.81) | 7627.1 (6353.83 to 9270.43) | 5447.07 (4429.53 to 6880.58) | 3561.99 (2744.51 to 4845.43) | <0.001 |
| Minerals | |||||
| Zinc (mg/day) | 11.92 (10.21 to 14.07) | 14.34 (12.25 to 15.85) | 12.1 (10.84 to 13.6) | 10.06 (8.7 to 11.32) | <0.001 |
| Iron (mg/day) | 15.95 (13.51 to 18.59) | 19.46 (17.65 to 21.74) | 15.94 (14.43 to 17.45) | 12.71 (11.27 to 14.04) | <0.001 |
| Magnesium (mg/day) | 437.41 (359.96 to 510.06) | 545.09 (488.22 to 622.47) | 440.92 (394.65 to 477.09) | 331.41 (294.23 to 379.52) | <0.001 |
| Selenium (µg/day) | 101.32 (84.15 to 121.61) | 117.71 (98.4 to 136.98) | 101.39 (87.62 to 116.1) | 86.98 (72.85 to 103.87) | <0.001 |
| Tertiles of DII Score (1 = Lower DII Score and 3 = Higher DII Score) | ||||||
|---|---|---|---|---|---|---|
| Outcome | n = 970 | Model 1 | Model 2 | Model 3 | Model 4 | |
| Pre-pregnancy BMI, kg/m2 | ||||||
| DII continuous | 970 | 22.43 ± 4.1 | 0.41 (−0.07 to 0.9) | 0.30 (0.10 to 0.49) | 0.29 (0.09 to 0.49) | 0.32 (0.12 to 0.52) |
| DII tertile | ||||||
| Tertile 1 | 323 | 23.41 ± 4.98 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Tertile 2 | 324 | 23.72 ± 4.28 | 0.45 (−0.77 to 1.67) | 0.44 (−0.06 to 0.93) | 0.41 (−0.09 to 0.92) | 0.42 (−0.08 to 0.92) |
| Tertile 3 | 323 | 24.50 ± 5.05 | 1.35 (−0.03 to 2.72) | 0.82 (0.26 to 1.39) | 0.80 (0.24 to 1.37) | 0.88 (0.31 to 1.45) |
| Birthweight percentile | ||||||
| DII continuous | 970 | 39.87 ± 29.05 | −3.17 (−6.57 to 0.24) | −3.37 (−6.70 to −0.04) | −3.62 (−6.94 to −0.30) | −4.05 (−7.42 to −0.68) |
| DII tertile | ||||||
| Tertile 1 | 323 | 44.22 ± 30.56 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Tertile 2 | 324 | 42.33 ± 28.79 | −2.51 (−11.20 to 6.18) | −2.52 (−10.99 to 5.96) | −3.12 (−11.59 to 5.36) | −3.86 (−12.36 to 4.65) |
| Tertile 3 | 323 | 41.43 ± 30.5 | −7.16 (−16.96 to 2.64) | −8.08 (−17.67 to 1.51) | −8.56 (−18.12 to 0.99) | −9.84 (−19.57 to −0.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas, R.; Castro-Barquero, S.; Crovetto, F.; Larroya, M.; Ruiz-León, A.M.; Segalés, L.; Nakaki, A.; Youssef, L.; Benitez, L.; Casanovas-Garriga, F.; et al. Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial. Nutrients 2022, 14, 2284. https://doi.org/10.3390/nu14112284
Casas R, Castro-Barquero S, Crovetto F, Larroya M, Ruiz-León AM, Segalés L, Nakaki A, Youssef L, Benitez L, Casanovas-Garriga F, et al. Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial. Nutrients. 2022; 14(11):2284. https://doi.org/10.3390/nu14112284
Chicago/Turabian StyleCasas, Rosa, Sara Castro-Barquero, Francesca Crovetto, Marta Larroya, Ana Maria Ruiz-León, Laura Segalés, Ayako Nakaki, Lina Youssef, Leticia Benitez, Francesc Casanovas-Garriga, and et al. 2022. "Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial" Nutrients 14, no. 11: 2284. https://doi.org/10.3390/nu14112284
APA StyleCasas, R., Castro-Barquero, S., Crovetto, F., Larroya, M., Ruiz-León, A. M., Segalés, L., Nakaki, A., Youssef, L., Benitez, L., Casanovas-Garriga, F., Vieta, E., Crispi, F., Gratacós, E., & Estruch, R. (2022). Maternal Dietary Inflammatory Index during Pregnancy Is Associated with Perinatal Outcomes: Results from the IMPACT BCN Trial. Nutrients, 14(11), 2284. https://doi.org/10.3390/nu14112284









