Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Strategies to Promote Adherence
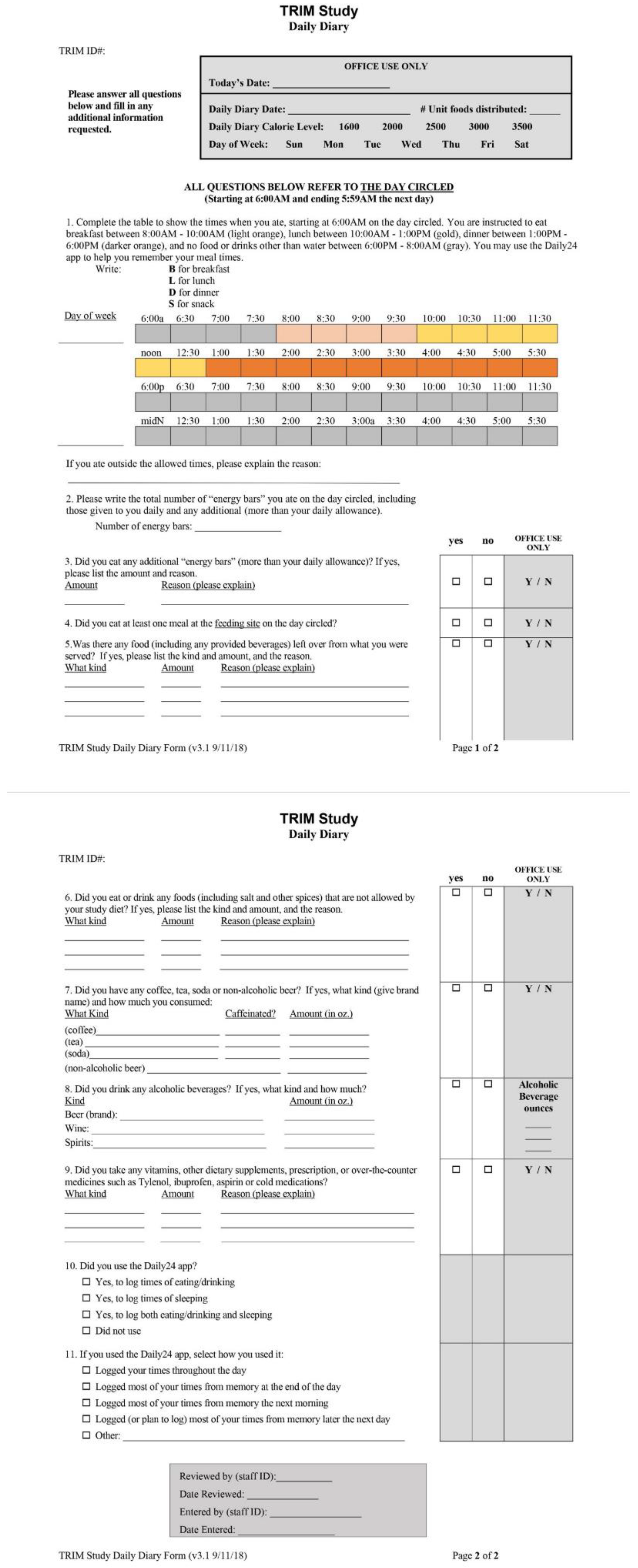
2.3. Measuring Adherence
2.3.1. Adherence to Meal Timing
2.3.2. Adherence to Food Consumption
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
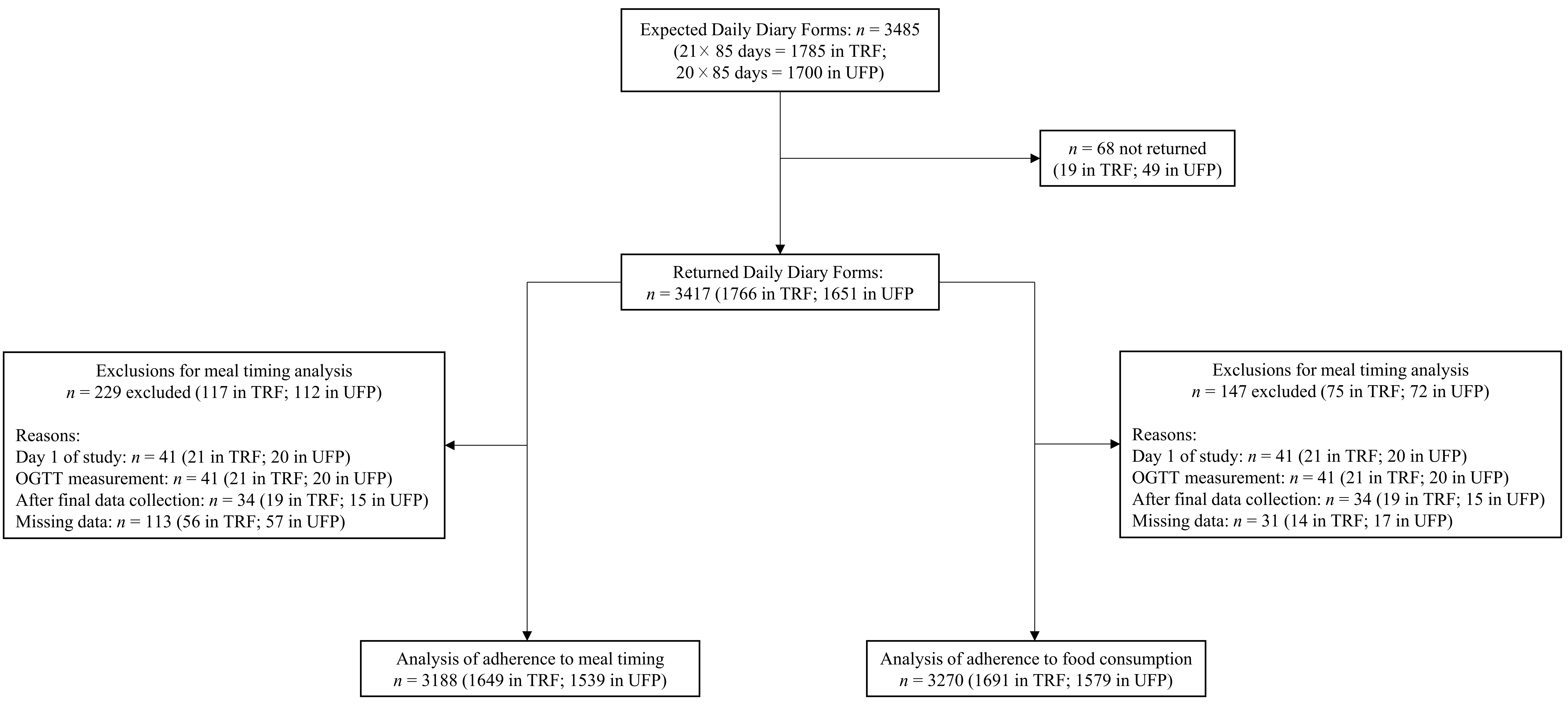
3.2. Completeness of Adherence Measurement
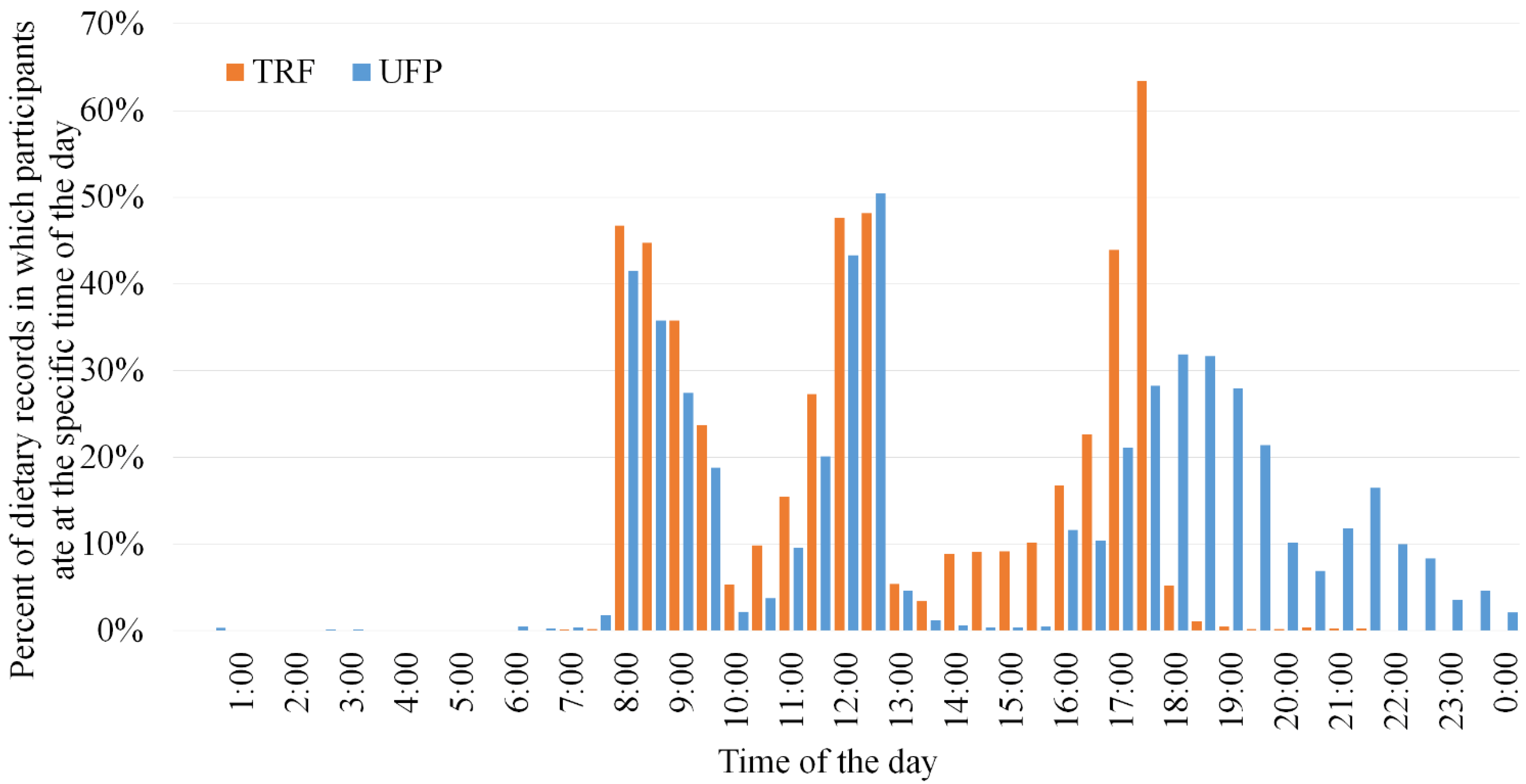
3.3. Adherence to Meal Timing
3.4. Adherence to Food Consumption
3.5. Differences in Adherence by Day of Week
3.6. Differences in Adherence across the Study Period
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRF | Time-restricted feeding |
| UFP | Usual feeding pattern |
| TRIM | Time-restricted intake of meals |
| BMI | Body mass index |
| GEE | Generalized estimating equations |
Appendix A
| Time-Restricted Feeding (TRF) | Usual Feeding Pattern (UFP) | |||
|---|---|---|---|---|
| Meal | Time Window | % of Total Calories | Time Window | % of Total Calories |
| Breakfast | 08:00–10:00 | 40 | 08:00–10:00 | 20 |
| Lunch | 10:00–13:00 | 40 | 10:00–13:00 | 25 |
| Dinner | 13:00–18:00 | 15 | 17:00–20:00 | 50 |
| Snack | 08:00–18:00 | 5 | 16:00–midnight | 5 |
| Perfect Adherence to Meal Timing Number (%) of Participants | Adherence to Food Consumption Number (%) of Participants | |||
|---|---|---|---|---|
| Proportion of Study Days Adherent | TRF 1 | UFP 2 | TRF 1 | UFP 2 |
| 100% | 2 (10) | 0 (0) | 5 (24) | 2 (10) |
| 90–99.9% | 12 (57) | 10 (50) | 12 (57) | 14 (70) |
| 80–89.9% | 4 (19) | 4 (20) | 1 (5) | 3 (15) |
| <80% | 3 (14) | 6 (30) | 3 (14) | 1 (5) |

References
- Hall, D.M.; Most, M.M. Dietary Adherence in Well-Controlled Feeding Studies. J. Am. Diet. Assoc. 2005, 105, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Windhauser, M.M.; Evans, M.A.; McCullough, M.L.; Swain, J.F.; Lin, P.; Hoben, K.P.; Plaisted, C.S.; Karanja, N.M.; Vollmer, W.M. Dietary Adherence in the Dietary Approaches to Stop Hypertension Trial. J. Am. Diet. Assoc. 1999, 99, S76–S83. [Google Scholar] [CrossRef]
- Swain, J.F.; Windhauser, M.M.; Hoben, K.P.; Evans, M.A.; McGEE, B.B.; Steele, P.D. Menu Design and Selection for Multicenter Controlled Feeding Studies: Process Used in the Dietary Approaches to Stop Hypertension Trial. J. Am. Diet. Assoc. 1999, 99, S54–S59. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [Green Version]
- Most, M.M.; Ershow, A.G.; Clevidence, B.A. An overview of methodologies, proficiencies, and training resources for controlled feeding studies. J. Am. Diet. Assoc. 2003, 103, 729–735. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Carey, V.J.; Bishop, L.; Charleston, J.; Conlin, P.; Erlinger, T.; Laranjo, N.; McCarron, P.; Miller, E.; Rosner, B.; Swain, J.; et al. Rationale and design of the Optimal Macro-Nutrient Intake Heart Trial to Prevent Heart Disease (OMNI-Heart). Clin. Trials 2005, 2, 529–537. [Google Scholar] [CrossRef]
- Krishnan, S.; Lee, F.; Burnett, D.J.; Kan, A.; Bonnel, E.L.; Allen, L.H.; Adams, S.H.; Keim, N.L. Challenges in Designing and Delivering Diets and Assessing Adherence: A Randomized Controlled Trial Evaluating the 2010 Dietary Guidelines for Americans. Curr. Dev. Nutr. 2020, 4, nzaa022. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Sacks, F.M.; Obarzanek, E.; Vollmer, W.M.; Appel, L.J.; Lin, P.; Karanja, N.M.; Harsha, D.W.; Bray, G.A.; Aickin, M.; et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-Sodium): Rationale and Design. J. Am. Diet. Assoc. 1999, 99, S96–S104. [Google Scholar] [CrossRef]
- Bandín, C.; Scheer, F.A.J.L.; Luque, A.J.; Ávila-Gandía, V.; Zamora, S.; Madrid, J.A.; Gómez-Abellán, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 382. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.W.; Wong, M.C.; Wang, H.H.; Liu, K.Q.; Lee, C.L.; Yan, B.P.; Yu, C.; Griffiths, S.M. Compliance with the Dietary Approaches to Stop Hypertension (DASH) Diet: A Systematic Review. PLoS ONE 2013, 8, e78412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crumb-Johnson, R.; Smith-Banes, M.; Hatcher, L.; Hagan, D.W. Assessment of differences between compliers and noncompliers in outpatient research diet studies. J. Am. Diet. Assoc. 1993, 93, 1041–1042. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- LeCheminant, J.D.; Christenson, E.; Bailey, B.W.; Tucker, L.A. Restricting night-time eating reduces daily energy intake in healthy young men: A short-term cross-over study. Br. J. Nutr. 2013, 110, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.M.; Shi, J.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of Protein, Monounsaturated Fat, and Carbohydrate Intake on Blood Pressure and Serum LipidsResults of the OmniHeart Randomized Trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Anderson, C.A.; Cobb, L.K.; Miller, E.R., III; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.E.; Dunbar-Jacob, J. Adherence to medication, diet, and activity recommendations: From assessment to maintenance. J. Cardiovasc. Nurs. 1995, 9, 62–79. [Google Scholar] [CrossRef]
- Chiu, S.; Bergeron, N.; Williams, P.T.; Bray, G.A.; Sutherland, B.; Krauss, R.M. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hand, R.K.; Perzynski, A.T. Ecologic Momentary Assessment: Perspectives on Applications and Opportunities in Research and Practice Regarding Nutrition Behaviors. J. Nutr. Educ. Behav. 2016, 48, 568–577.e1. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Participants Mean (SD) or Number (%) | TRF 1 Mean (SD) or Number (%) | UFP 2 Mean (SD) or Number (%) |
|---|---|---|---|
| Age, years | 59.4 (7.1) | 59.7 (7.0) | 59.1 (7.5) |
| Age categories | |||
| 40–49 years | 4 (9.8) | 2 (9.5) | 2 (10.0) |
| 50–59 years | 13 (31.7) | 6 (28.6) | 7 (35.0) |
| 60–69 years | 24 (58.5) | 13 (61.9) | 11 (55.0) |
| Sex | |||
| Female | 38 (92.7) | 19 (90.5) | 19 (95.0) |
| Male | 3 (7.3) | 2 (9.5) | 1 (5.0) |
| Race | |||
| Black | 38 (92.7) | 20 (95.2) | 18 (90.0) |
| White | 3 (7.3) | 1 (4.8) | 2 (10.0) |
| Marital status | |||
| Single | 12 (29.3) | 5 (23.8) | 7 (35.0) |
| Married | 18 (43.9) | 8 (38.1) | 10 (50.0) |
| Widowed | 3 (7.3) | 1 (4.76) | 2 (10.0) |
| Divorced\Separated | 8 (19.5) | 7 (33.3) | 1 (5.0) |
| Education | |||
| <Bachelor | 19 (46.3) | 6 (28.6) | 13 (65.0) |
| Bachelor | 12 (29.3) | 9 (42.9) | 3 (15.0) |
| >Bachelor | 10 (24.4) | 6 (28.6) | 4 (20.0) |
| Employment status | |||
| Full-time | 24 (58.5) | 14 (66.7) | 10 (50.0) |
| Part-time | 5 (12.2) | 2 (9.5) | 3 (15.0) |
| None | 12 (29.3) | 5 (23.8) | 7 (35.0) |
| Yearly household income 3 | |||
| <$45,000 | 9 (23.7) | 4 (20.0) | 5 (27.8) |
| $45,000–<$75,000 | 14 (36.8) | 7 (35.0) | 7 (38.9) |
| >$75,000 | 15 (39.5) | 9 (45.0) | 6 (33.3) |
| Number (%) of Participant-Days | ||||
|---|---|---|---|---|
| All Participants | TRF 1 | UFP 2 | p-Value 3 | |
| Adherence to all meal | 3188 | 1649 | 1539 | 0.36 |
| times | ||||
| Perfect adherence | 2763 (87) | 1448 (88) | 1315 (85) | |
| Good adherence | 291 (9) | 135 (8) | 156 (10) | |
| Poor adherence | 134 (4) | 66 (4) | 68 (4) | |
| Breakfast | 3268 | 1693 | 1575 | 0.14 |
| Perfect adherence | 3170 (97) | 1654 (98) | 1516 (96) | |
| Good adherence | 61 (2) | 24 (1) | 37 (2) | |
| Poor adherence | 37 (1) | 15 (1) | 22 (1) | |
| Lunch | 3272 | 1692 | 1580 | 0.75 |
| Perfect adherence | 3059 (93) | 1582 (94) | 1477 (93) | |
| Good adherence | 123 (4) | 54 (3) | 69 (4) | |
| Poor adherence | 90 (3) | 56 (3) | 34 (2) | |
| Dinner | 3254 | 1678 | 1576 | 0.10 |
| Perfect adherence | 3123 (96) | 1630 (97) | 1493 (95) | |
| Good adherence | 83 (3) | 22 (1) | 61 (4) | |
| Poor adherence | 48 (1) | 26 (2) | 22 (1) | |
| Snack | 3219 | 1665 | 1554 | 0.10 |
| Perfect adherence | 3053 (95) | 1572 (94) | 1481 (95) | |
| Good adherence | 109 (3) | 69 (4) | 40 (3) | |
| Poor adherence | 57 (2) | 24 (1) | 33 (2) | |
| Number (%) of Participant-Days | ||||
|---|---|---|---|---|
| All Participants | TRF 1 | UFP 2 | p-Value 3 | |
| Adherence to study diet | 3270 | 1691 | 1579 | |
| Adherence | 3063 (94) | 1570 (93) | 1493 (95) | 0.65 |
| Non-adherence | ||||
| Had food left-over only | 126 (4) | 79 (5) | 47 (3) | 0.39 |
| Ate outside food only | 56 (2) | 27 (2) | 29 (2) | 0.50 |
| Had left-over and ate outside food | 25 (1) | 15 (1) | 10 (1) | 0.80 |
| Perfect Adherence to Meal Timing, % of Participant-Days | Adherence to Food Consumption, % of Participant-Days | |||||||
|---|---|---|---|---|---|---|---|---|
| TRF 1 | p-Value 3 | UFP 2 | p-Value 3 | TRF 1 | p-Value 3 | UFP 2 | p-Value 3 | |
| Monday | 87 | 0.72 | 89 | 0.002 | 91 | 0.05 | 93 | 0.89 |
| Tuesday | 88 | 87 | 95 | 94 | ||||
| Wednesday | 87 | 89 | 95 | 96 | ||||
| Thursday | 90 | 87 | 93 | 94 | ||||
| Friday | 88 | 87 | 95 | 94 | ||||
| Saturday | 88 | 81 | 94 | 94 | ||||
| Sunday | 86 | 78 | 89 | 96 | ||||
| Perfect Adherence to Meal Timing, % of Participant-Days | Adherence to Food Consumption, % of Participant-Days | |||||||
|---|---|---|---|---|---|---|---|---|
| Week | TRF 1 | p-Value 3 | UFP 2 | p-Value 3 | TRF 1 | p-Value 3 | UFP 2 | p-Value 3 |
| 1 | 85 | 0.39 | 75 | 0.03 | 90 | 0.15 | 92 | 0.42 |
| 2 | 84 | 79 | 93 | 94 | ||||
| 3 | 89 | 90 | 93 | 92 | ||||
| 4 | 83 | 84 | 93 | 98 | ||||
| 5 | 86 | 91 | 95 | 97 | ||||
| 6 | 89 | 85 | 95 | 96 | ||||
| 7 | 91 | 84 | 86 | 94 | ||||
| 8 | 90 | 90 | 94 | 98 | ||||
| 9 | 87 | 81 | 92 | 95 | ||||
| 10 | 90 | 87 | 93 | 93 | ||||
| 11 | 92 | 88 | 95 | 94 | ||||
| 12 | 88 | 92 | 96 | 93 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; White, K.; Maw, M.T.T.; Charleston, J.; Zhao, D.; Guallar, E.; Appel, L.J.; Clark, J.M.; Maruthur, N.M.; Pilla, S.J. Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding. Nutrients 2022, 14, 2283. https://doi.org/10.3390/nu14112283
Wu B, White K, Maw MTT, Charleston J, Zhao D, Guallar E, Appel LJ, Clark JM, Maruthur NM, Pilla SJ. Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding. Nutrients. 2022; 14(11):2283. https://doi.org/10.3390/nu14112283
Chicago/Turabian StyleWu, Beiwen, Karen White, May Thu Thu Maw, Jeanne Charleston, Di Zhao, Eliseo Guallar, Lawrence J. Appel, Jeanne M. Clark, Nisa M. Maruthur, and Scott J. Pilla. 2022. "Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding" Nutrients 14, no. 11: 2283. https://doi.org/10.3390/nu14112283
APA StyleWu, B., White, K., Maw, M. T. T., Charleston, J., Zhao, D., Guallar, E., Appel, L. J., Clark, J. M., Maruthur, N. M., & Pilla, S. J. (2022). Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding. Nutrients, 14(11), 2283. https://doi.org/10.3390/nu14112283







