Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes
Abstract
:1. Introduction
2. Materials and Methods
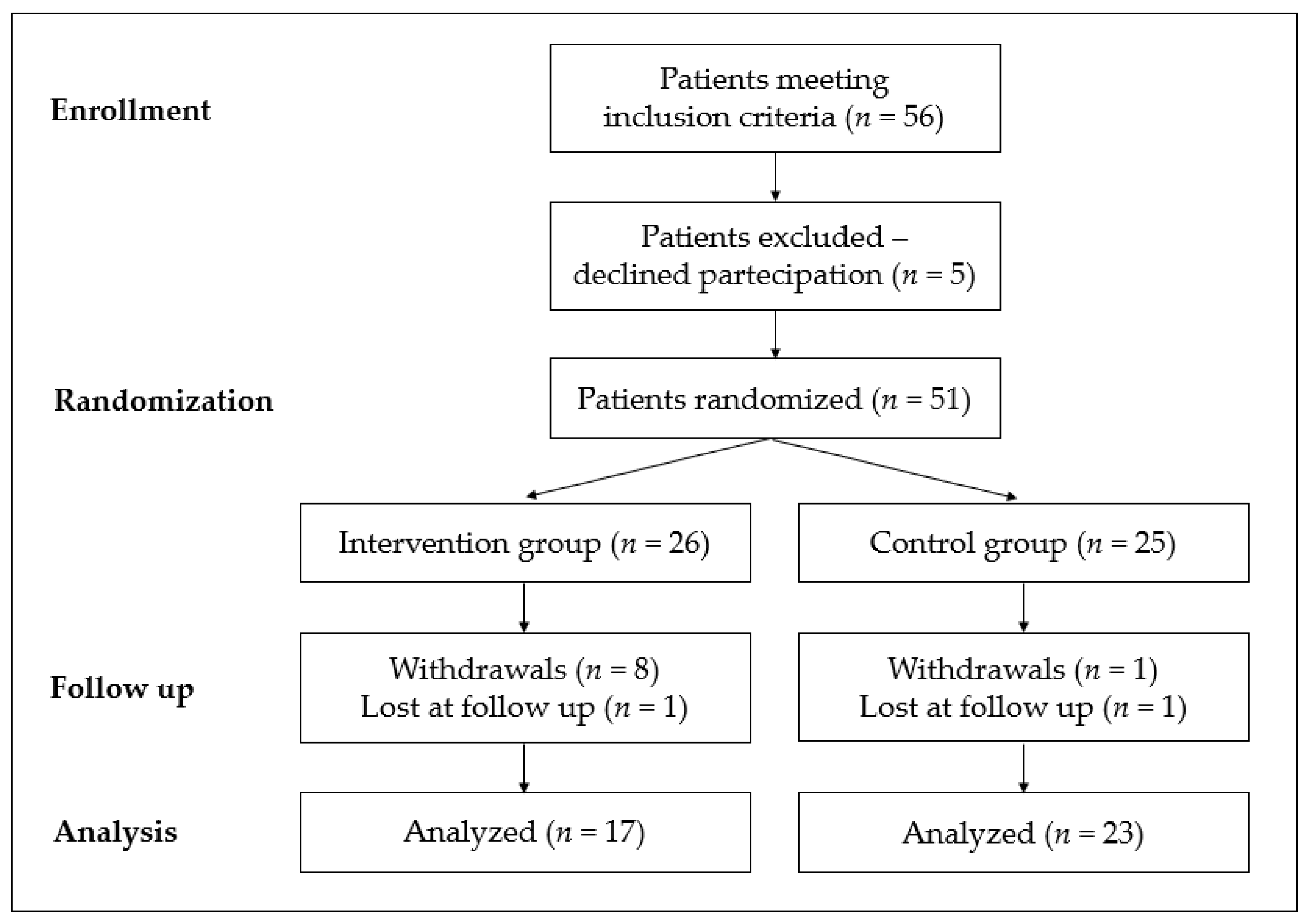
2.1. Study Design and Population
2.2. Randomization and Treatment Allocation
- Omega-3 fatty acids (pills, EnerZona Omega3Rx®, Enervit, Italia), at a daily dosage of 2.4 gr at breakfast;
- Anthocyanins (pills, EnerZona Maqui Response Capsule®, Enervit, Italia) at a total daily dosage of 108 mg divided into three equal intakes at breakfast, lunch, and dinner;
- Alpha-cyclodextrins (sachets, EnerZona Maqui Response Buste®, Enervit, Italia) at a total daily dosage of 15 gr divided into three equal intakes at breakfast, lunch, and dinner.
2.3. Dietary Intervention
2.4. Antenatal Monitoring and Treatment
2.5. Erythrocyte Fatty Acid Composition
2.6. Plasmatic PAF
2.7. Statistical Analysis
3. Results
3.1. Results by Randomization
3.2. Results by Severity of GD
4. Discussion
4.1. Maternal Anthropometric Parameters
4.2. Maternal Triglycerides
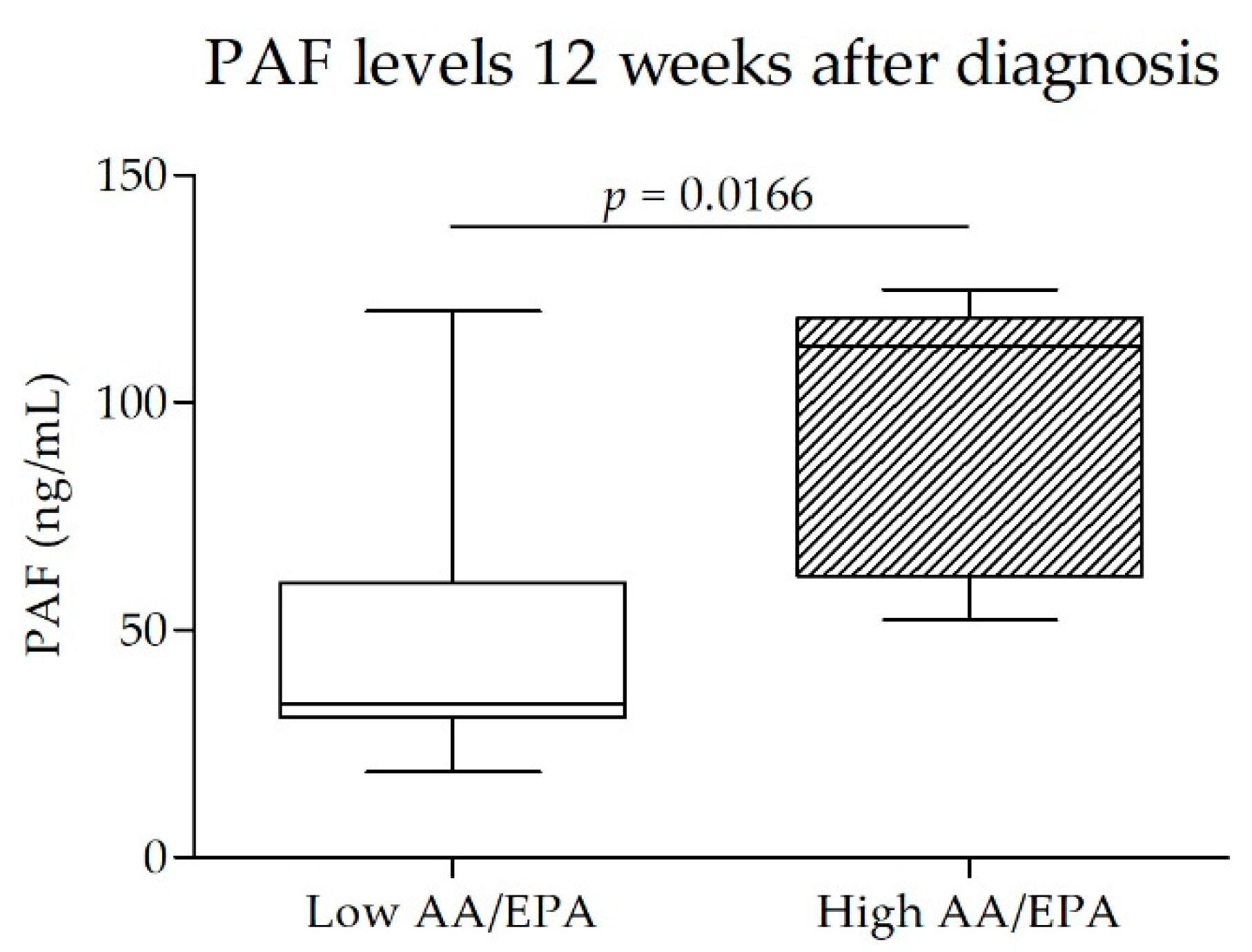
4.3. Maternal AA/EPA Ratio and PAF
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sears, B. Anti-inflammatory Diets. J. Am. Coll. Nutr. 2015, 34, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.; Leighton, F. Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzi, E.M.; Sears, B. Metabolic Syndrome and Complications of Pregnancy. The Potential Preventive Role of Nutrition, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 203–216. [Google Scholar]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.; Desoye, G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert. Rev. Endocrinol. Metab. 2019, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am. J. Obstet. Gynecol. 2010, 202, 643–649. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taschereau-Charron, A.; Da Silva, M.S.; Bilodeau, J.F.; Morisset, A.S.; Julien, P.; Rudkowska, I. Alterations of fatty acid profiles in gestational diabetes and influence of the diet. Maturitas 2017, 99, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [Green Version]
- McBurney, M.I.; Tintle, N.L.; Vasan, R.S.; Sala-Vila, A.; Harris, W.S. Using an erythrocyte fatty acid fingerprint to predict risk of all-cause mortality: The Framingham Offspring Cohort. Am. J. Clin. Nutr. 2021, 114, 1447–1454. [Google Scholar] [CrossRef]
- Nelson, J.R.; Budoff, M.J.; Wani, O.R.; Le, V.; Patel, D.K.; Nelson, A.; Nemiroff, R.L. EPA’s pleiotropic mechanisms of action: A narrative review. Postgrad. Med. 2021, 133, 651–664. [Google Scholar] [CrossRef]
- Katan, M.B.; Deslypere, J.P.; van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid. Res. 1997, 38, 2012–2022. [Google Scholar] [CrossRef]
- Patterson, A.C.; Chalil, A.; Aristizabal Henao, J.J.; Streit, I.T.; Stark, K.D. Omega-3 polyunsaturated fatty acid blood biomarkers increase linearly in men and women after tightly controlled intakes of 0.25, 0.5, and 1 g/d of EPA + DHA. Nutr. Res. 2015, 35, 1040–1051. [Google Scholar] [CrossRef]
- Piuri, G.; Basello, K.; Rossi, G.; Soldavini, C.M.; Duiella, S.; Privitera, G.; Spadafranca, A.; Costanzi, A.; Tognon, E.; Cappelletti, M.; et al. Methylglyoxal, Glycated Albumin, PAF, and TNF-α: Possible Inflammatory and Metabolic Biomarkers for Management of Gestational Diabetes. Nutrients 2020, 12, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Nutrition, Harvard T.H Chan School of Public Health. The Healthy Eating Plate; Harvard University: Cambridge, MA, USA, 2011; Available online: https://www.hsph.harvard.edu/nutritionsource/healthy-eating-plate/ (accessed on 1 March 2022).
- Italian Society of Human Nutrition. Reference Intake Levels of Nutrients and Energy for the Italian Population (LARN), 4th ed.; SICS: Rome, Italy, 2014. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rondanelli, M.; Klersy, C.; Perna, S.; Faliva, M.A.; Montorfano, G.; Roderi, P.; Colombo, I.; Corsetto, P.A.; Fioravanti, M.; Solerte, S.B.; et al. Effects of two-months balanced diet in metabolically healthy obesity: Lipid correlations with gender and BMI-related differences. Lipids Health Dis. 2015, 14, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S.; Johnston, D.T. Virtual non-compliance with Omega-3 treatment results in null effects: The RANGER study. Prostaglandins Leukot Essent Fat. Acids 2021, 171, 102319. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meshel, S.; Schejter, E.; Harel, T.; Maslovitz, S.; Germez, N.; Elimelech, B.; Cohen, B.; Azuri, J. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J. Matern. Fetal Neonatal. Med. 2016, 29, 2062–2066. [Google Scholar] [CrossRef]
- Langer, O.; Yogev, Y.; Xenakis, E.M.; Brustman, L. Overweight and obese in gestational diabetes: The impact on pregnancy outcome. Am. J. Obstet. Gynecol. 2005, 192, 1768–1776. [Google Scholar] [CrossRef]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Jalaludin, B.B.; Wong, V.W.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia 2016, 59, 2331–2338. [Google Scholar] [CrossRef]
- Velardo, C.; Clifton, D.; Hamblin, S.; Khan, R.; Tarassenko, L.; Mackillop, L. Toward a Multivariate Prediction Model of Pharmacological Treatment for Women With Gestational Diabetes Mellitus: Algorithm Development and Validation. J. Med. Internet Res. 2021, 23, e21435. [Google Scholar] [CrossRef]
- Pertot, T.; Molyneaux, L.; Tan, K.; Ross, G.P.; Yue, D.K.; Wong, J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus. Diabetes Care 2011, 34, 2214–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapienza, A.D.; Fransisco, R.P.V.; Trindade, T.C.; Zugaib, M. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 88, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Martin-Nguyen, A.; Fong, T.M.; Gallagher, D.; Pietrobelli, A. Body circumferences: Clinical implications emerging from a new geometric model. Nutr. Metab. 2008, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piuri, G.; Ferrazzi, E.; Bulfoni, C.; Mastricci, L.; Di Martino, D.; Speciani, A.F. Longitudinal changes and correlations of bioimpedance and anthropometric measurements in pregnancy: Simple possible bed-side tools to assess pregnancy evolution. J. Matern. Fetal Neonatal. Med. 2017, 30, 2824–2830. [Google Scholar] [CrossRef]
- Kannieappan, L.M.; Deussen, A.R.; Grivell, R.M.; Yelland, L.; Dodd, J.M. Developing a tool for obtaining maternal skinfold thickness measurements and assessing inter-observer variability among pregnant women who are overweight and obese. BMC Pregnancy Childbirth 2013, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Fakier, A.; Petro, G.; Fawcus, S. Mid-upper arm circumference: A surrogate for body mass index in pregnant women. S. Afr. Med. J. 2017, 107, 606–610. [Google Scholar] [CrossRef]
- Ducarme, G.; Desroys du Roure, F.; Grange, J.; Vital, M.; Le Thuaut, A.; Crespin-Delcourt, I. Predictive factors of subsequent insulin requirement for glycemic control during pregnancy at diagnosis of gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2019, 14, 265–270. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Estimating the risk of gestational diabetes mellitus based on the 2013 WHO criteria: A prediction model based on clinical and biochemical variables in early pregnancy. Acta Diabetol. 2020, 57, 661–671. [Google Scholar] [CrossRef]
- Eppel, D.; Feichtinger, M.; Lindner, T.; Kotzaeridi, G.; Rosicky, I.; Yerlikaya-Schatten, G.; Eppel, W.; Husslein, P.; Tura, A.; Göbl, C.S. Association between maternal triglycerides and disturbed glucose metabolism in pregnancy. Acta Diabetol. 2020, 58, 459–465. [Google Scholar] [CrossRef]
- Nasioudis, D.; Doulaveris, G.; Kanninen, T.T. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. 2019, 71, 155–162. [Google Scholar] [CrossRef]
- Gorban de Lapertosa, S.; Alvariñas, J.; Elgart, J.F.; Salzberg, S.; Gagliardino, J.J.; EduGest group. The triad macrosomia, obesity, and hypertriglyceridemia in gestational diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ambrosi, F.; Rossi, G.; Soldavini, C.M.; Carbone, I.F.; Cetera, G.E.; Cesano, N.; Di Maso, M.; Ferrazzi, E. Evaluation of fetal cardiac function in pregnancies with well-controlled gestational diabetes. Arch. Gynecol. Obstet. 2021, 304, 337–344. [Google Scholar] [CrossRef] [PubMed]


| IG T12 (N = 17) | PG T12 (N = 23) | p | |
|---|---|---|---|
| Arm circumference (cm) | 28.6 (26.8–29.3) | 28.5 (28.0–30.0) | 0.2202 |
| Wrist circumference (cm) | 15 (14.5–15.5) | 15 (14.5–16.0) | 0.3312 |
| Waist circumference (cm) | 94.5 (91–99) | 97 (93.0–102) | 0.1590 |
| Bicipital skin fold (mm) | 8.5 (7.3–10.3) | 10.4 (7.7.15.8) | 0.3162 |
| Triceps skin fold (mm) | 20.0 (19.0–23.4) | 23.8 (18.4–27.3) | 0.1195 |
| Subscapular skin fold (mm) | 15.8 (14.4–19.0) | 16.4 (14.4–24) | 0.2795 |
| Glycemia (mg/dL) | 72 (68–82) | 71 (66–78) | 0.6073 |
| HbA1c (mmol/mol) | 33 (32–36) | 33 (31–34) | 0.2034 |
| Insulin (µU/mL) | 9.7 (7.4–14.9) | 9.3 (5.8–15.1) | 0.5333 |
| Total cholesterol (mg/dL) | 269 (228–313) | 250 (221–278) | 0.4722 |
| LDL cholesterol (mg/dL) | 146 (114–188) | 126 (106–154) | 0.1166 |
| HDL cholesterol (mg/dL) | 69 (57–80) | 73 (65–87) | 0.5457 |
| Triglycerides (mg/dL) | 229 (181–271) | 227 (195–283) | 0.5490 |
| RBC AA/EPA ratio | 17.2 (11.1–30.1) | 24.2 (14.9–40.2) | 0.5716 |
| Cortisol (µg/L) | 29.8 (22.8–38.5) | 26.3 (23.4–32.9) | 0.5849 |
| C-reactive protein (mg/dL) | 0.27 (0.19–0.47) | 0.23 (0.17–0.52) | 0.8691 |
| PAF (ng/dL) | 33.9 (29.1–51.2) | 37.7 (31.3–56.8) | 0.4160 |
| GD (N = 45) | GD+PT (N = 6) | p | |
|---|---|---|---|
| Age (years) | 34 (32–37) | 36 (32–38) | 0.5985 |
| Height (cm) | 163 (160–170) | 165 (162–167) | 0.6358 |
| Pre-pregnancy weight (kg) | 60.0 (52.5–68.0) | 69.5 (65.3–103) | 0.0149 |
| Pre-pregnancy BMI (kg/m2) | 22.0 (20.1–24.0) | 25.8 (24.5–37.2) | 0.0043 |
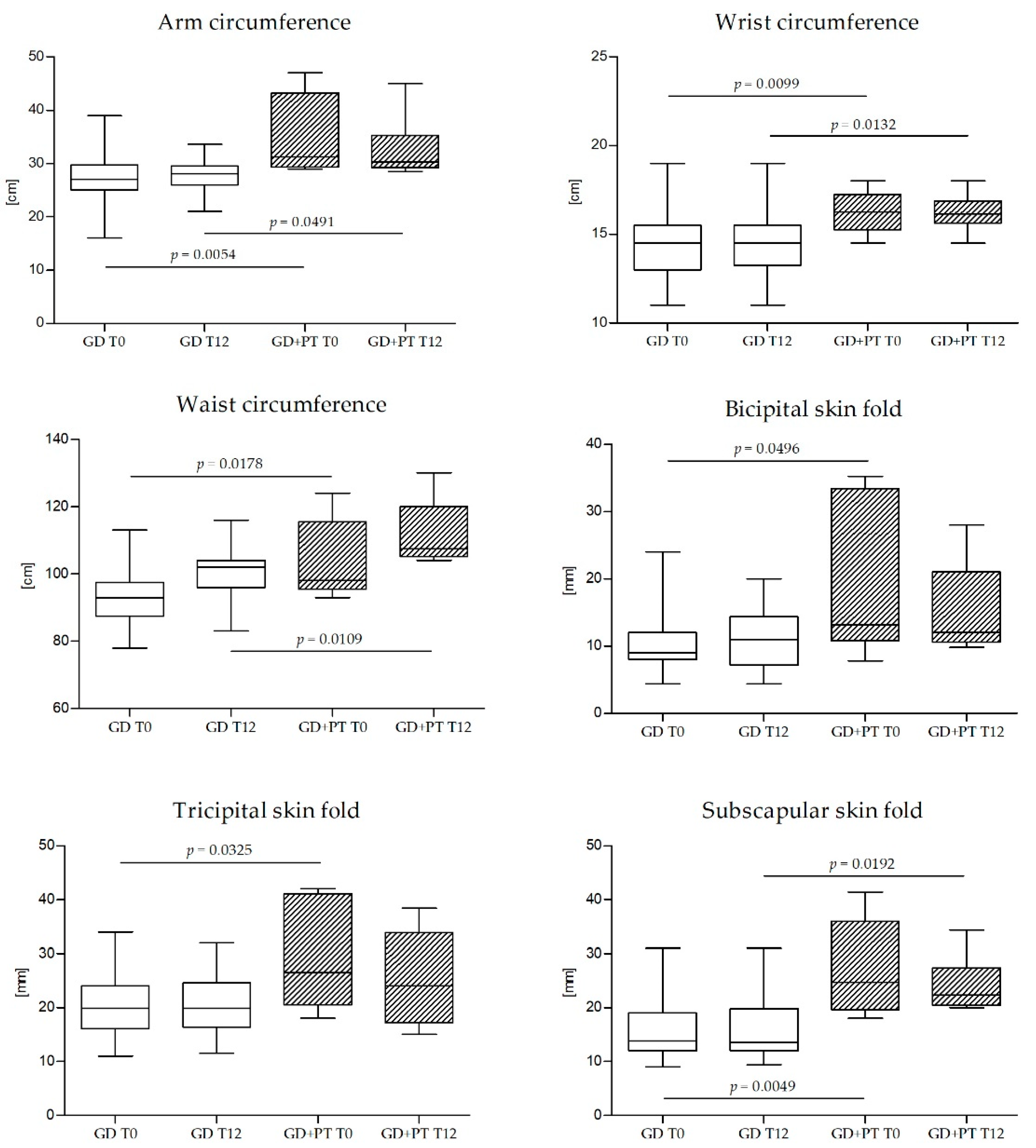
| Arm circumference (cm) | 27.0 (25.0–29.8) | 31.3 (29.4–43.3) | 0.0054 |
| Wrist circumference (cm) | 14.5 (13–15.5) | 16.25 (15.25–17.25) | 0.0099 |
| Waist circumference (cm) | 92.0 (87.0–95.6) | 98.0 (95.5–115.5) | 0.0178 |
| Bicipital skin fold (mm) | 9.0 (8.0–12.0) | 13.2 (10.8–33.4) | 0.0496 |
| Triceps skin fold (mm) | 19.9 (16.1–24.0) | 26.5 (20.6–40.9) | 0.0325 |
| Subscapular skin fold (mm) | 13.8 (12.0–19.0) | 24.7 (19.7–36.0) | 0.0049 |
| GD (N = 45) | GD+PT (N = 6) | p | |
|---|---|---|---|
| Glycemia (mg/dL) | 73 (66–76) | 74 (67–89) | 0.2683 |
| HbA1c (mmol/mol) | 30 (28–32) | 36 (30–38) | 0.0272 |
| Insulin (µU/mL) | 7.5 (5.7–12.6) | 17.3 (7.2–25.2) | 0.0409 |
| Total cholesterol (mg/dL) | 252 (222–275) | 267 (239–277) | 0.2819 |
| LDL cholesterol (mg/dL) | 140 (109–162) | 135 (115–171) | 0.5158 |
| HDL cholesterol (mg/dL) | 80 (66–88) | 60 (50–88) | 0.0865 |
| Triglycerides (mg/dL) | 165 (144–203) | 260 (201–295) | 0.0029 |
| C-reactive protein (mg/dL) | 0.24 (0.15–0.49) | 0.5 (0.41–0.63) | 0.0478 |
| Cortisol (µg/L) | 28.6 (22.8–31.9) | 24.8 (20.5–29.0) | 0.1230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldavini, C.M.; Piuri, G.; Rossi, G.; Corsetto, P.A.; Benzoni, L.; Maggi, V.; Privitera, G.; Spadafranca, A.; Rizzo, A.M.; Ferrazzi, E. Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes. Nutrients 2022, 14, 2502. https://doi.org/10.3390/nu14122502
Soldavini CM, Piuri G, Rossi G, Corsetto PA, Benzoni L, Maggi V, Privitera G, Spadafranca A, Rizzo AM, Ferrazzi E. Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes. Nutrients. 2022; 14(12):2502. https://doi.org/10.3390/nu14122502
Chicago/Turabian StyleSoldavini, Chiara Maria, Gabriele Piuri, Gabriele Rossi, Paola Antonia Corsetto, Linda Benzoni, Valeria Maggi, Giulia Privitera, Angela Spadafranca, Angela Maria Rizzo, and Enrico Ferrazzi. 2022. "Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes" Nutrients 14, no. 12: 2502. https://doi.org/10.3390/nu14122502
APA StyleSoldavini, C. M., Piuri, G., Rossi, G., Corsetto, P. A., Benzoni, L., Maggi, V., Privitera, G., Spadafranca, A., Rizzo, A. M., & Ferrazzi, E. (2022). Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes. Nutrients, 14(12), 2502. https://doi.org/10.3390/nu14122502







