Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
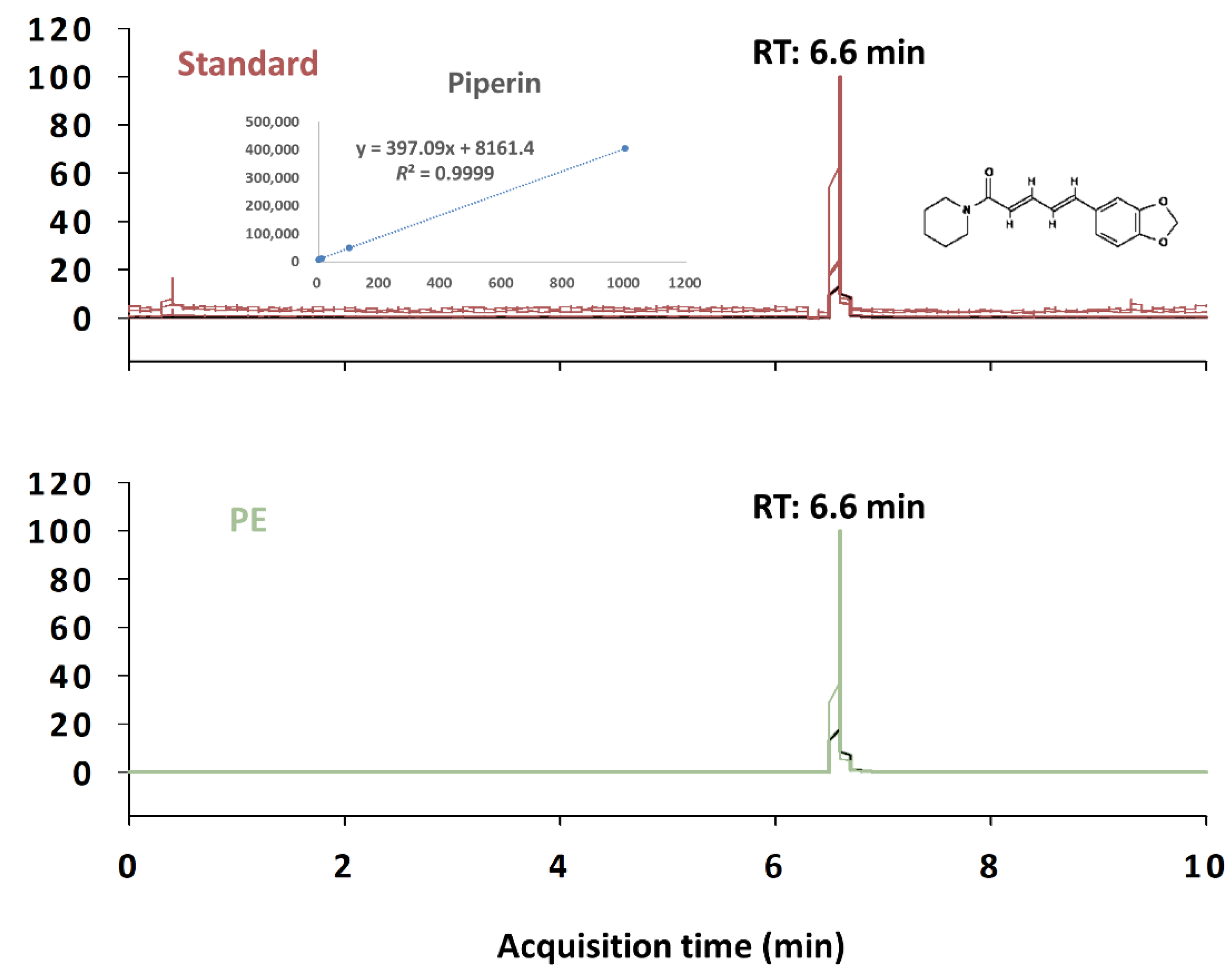
2.2. Preparation of PE and Liquid Chromatography with Tandem Mass Spectrometry Analysis
2.3. Animals and Treatments
2.4. Pentobarbital-Induced Sleep Accelerated Test
2.5. Sleep–Wake Profile Analysis
2.6. Open Field Test (OFT)
2.7. Data Analysis
3. Results
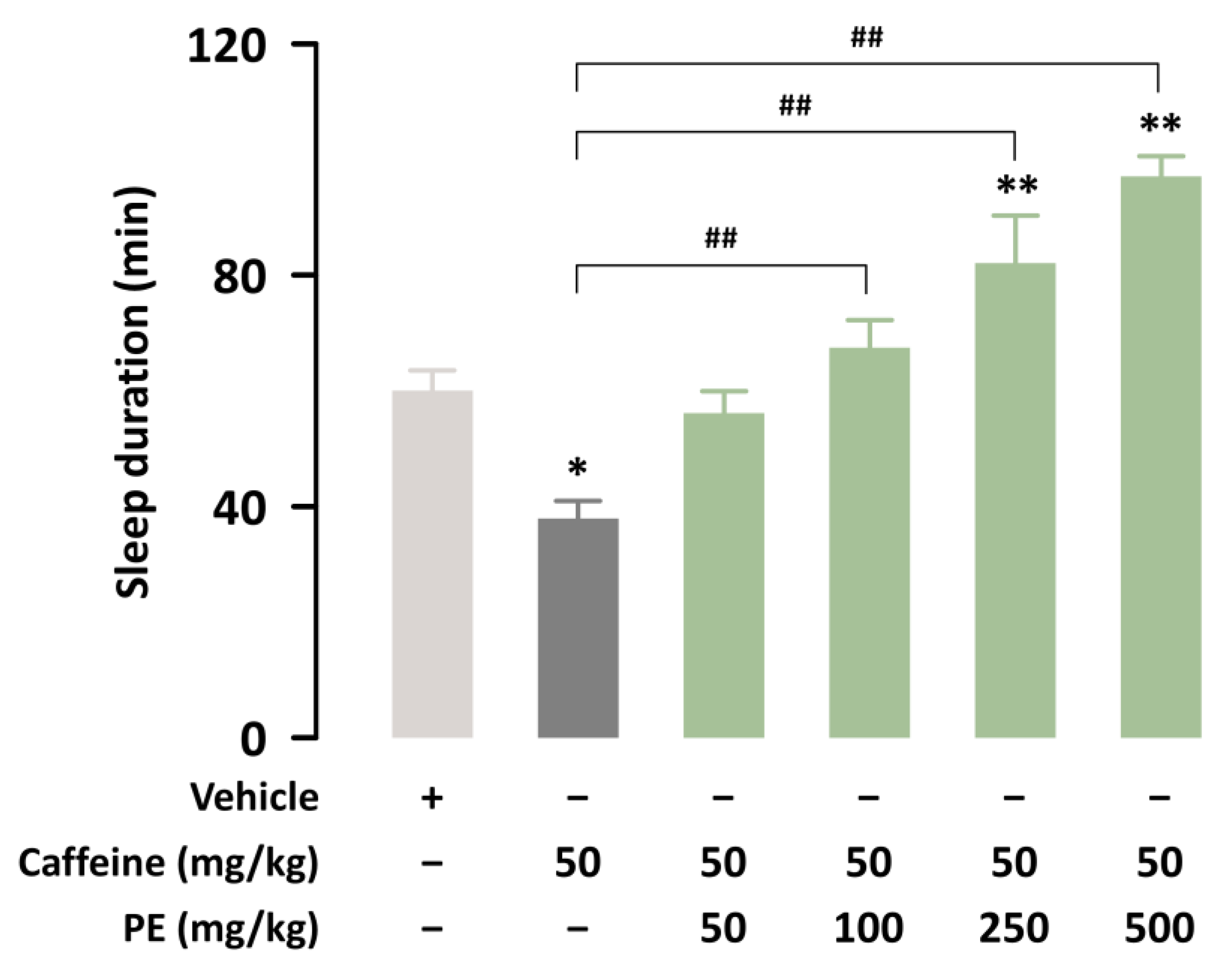
3.1. PE Attenuates Caffeine-Induced Sleep Disruption in ICR Mice
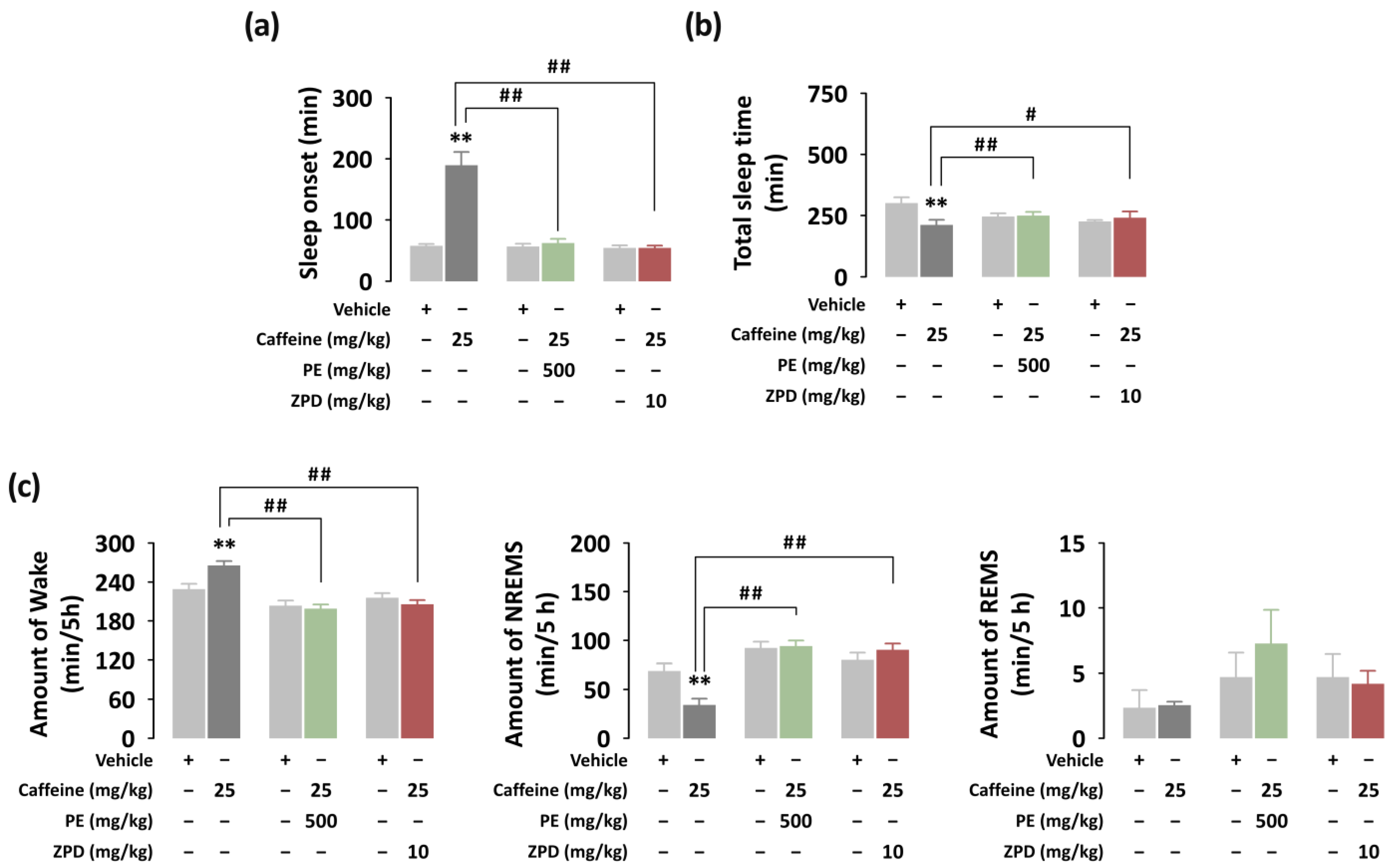
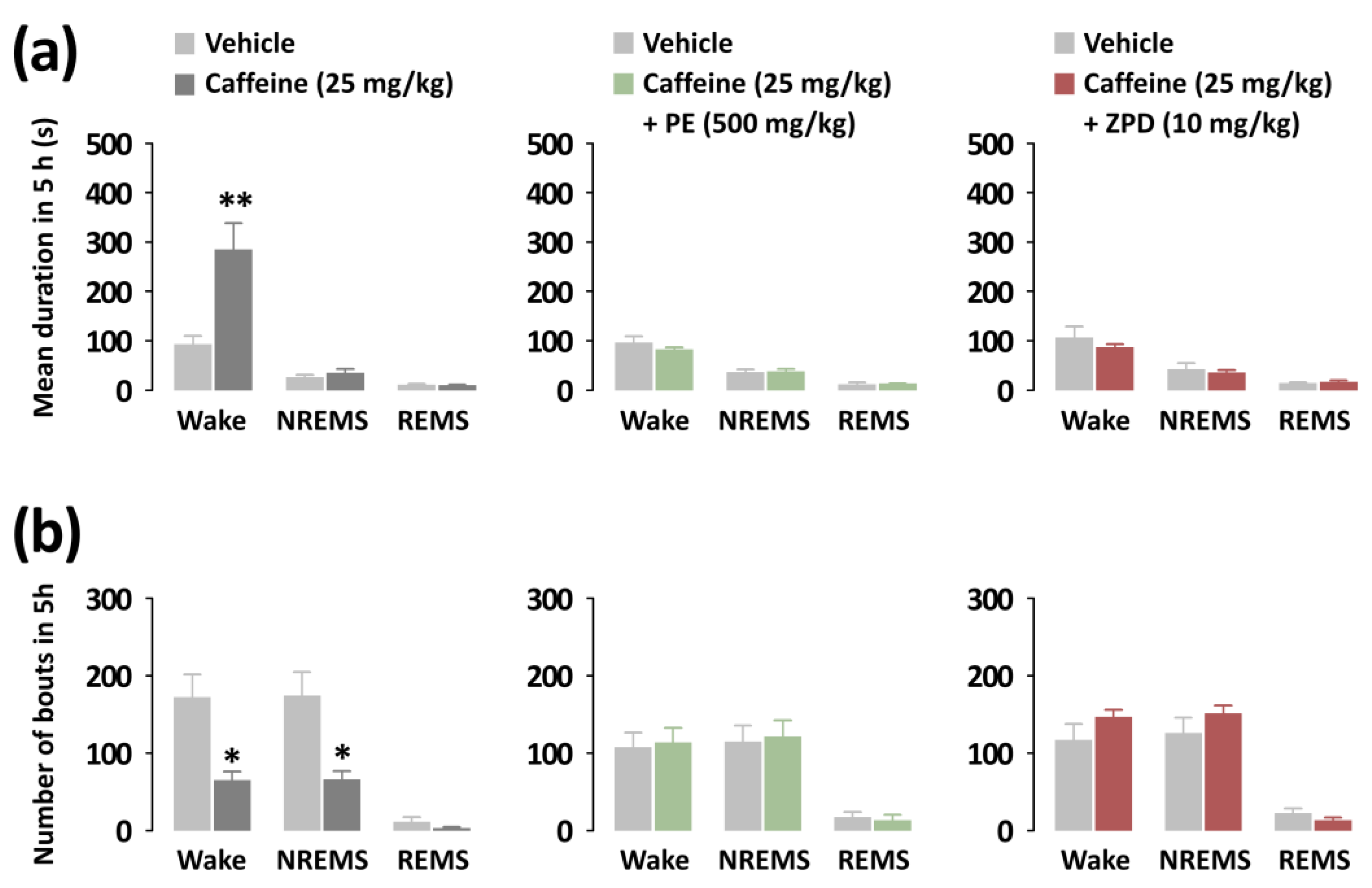
3.2. Effects of PE on Sleep Onset, Total Sleep Time, and Sleep Architecture of C57BL/6N Mice in Caffeine-Induced Sleep Disruption Model
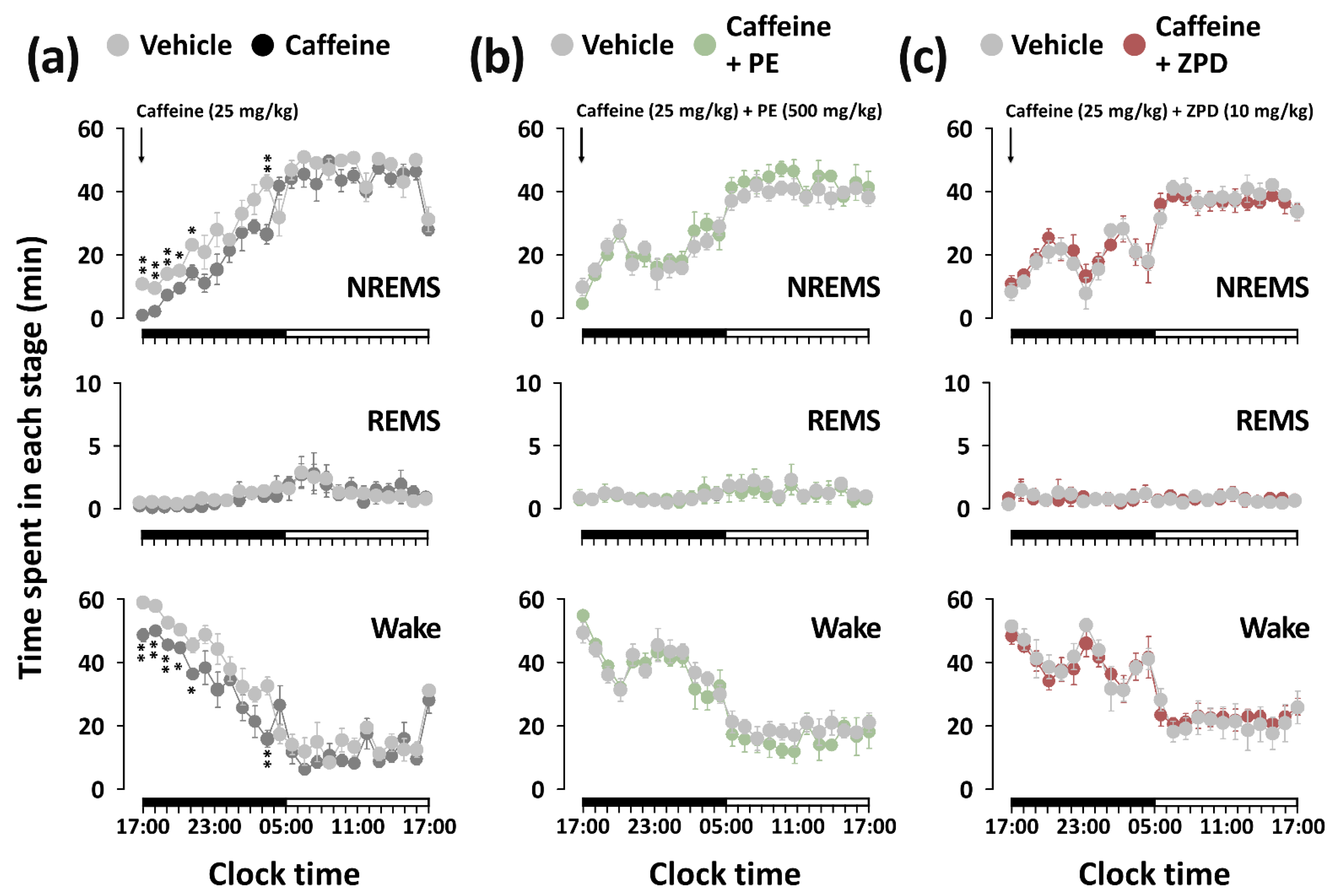
3.3. Effects of PE and ZPD on the Characteristics of Sleep–Wake Episodes of C57BL/6N Mice in Caffeine-Induced Sleep Disruption
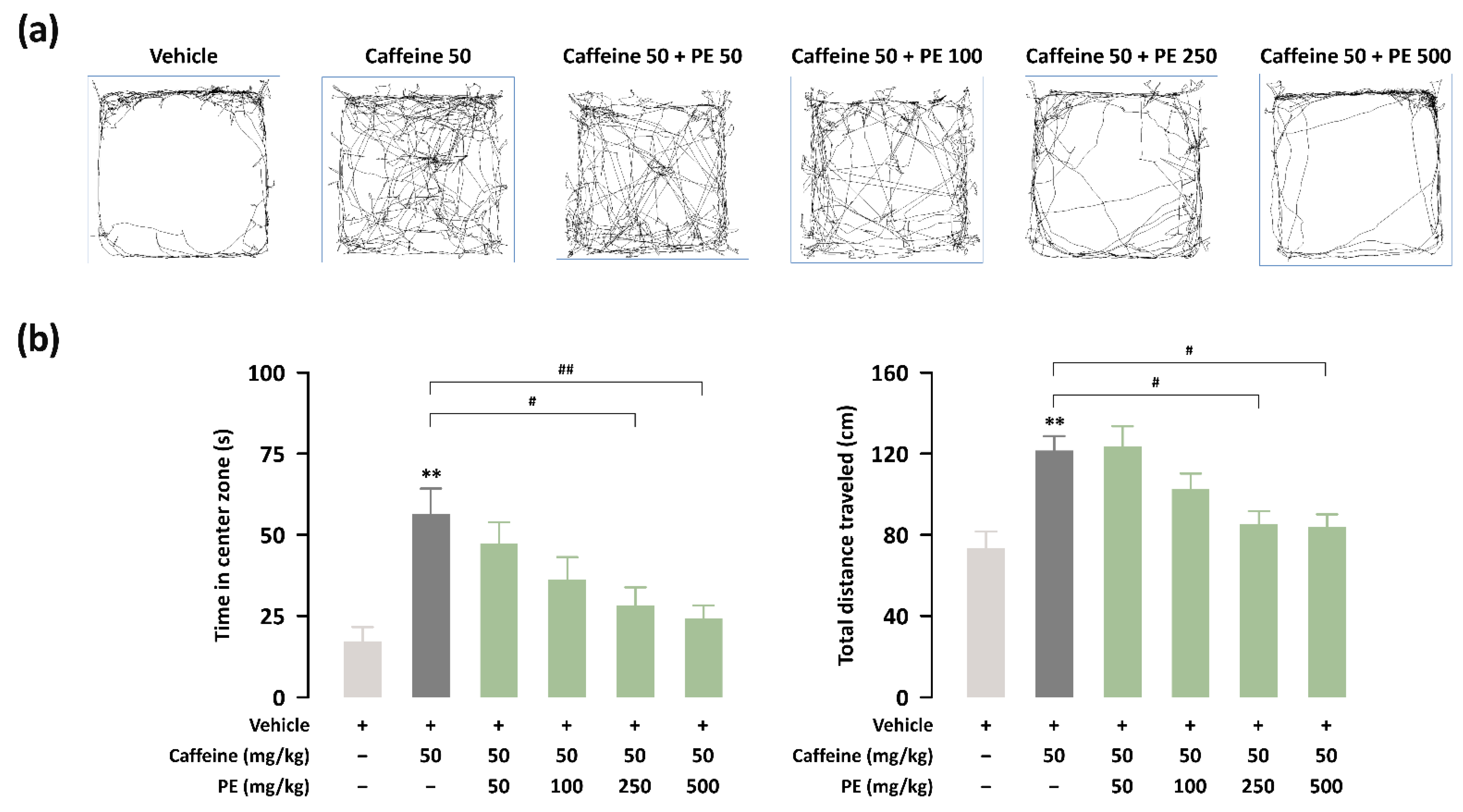
3.4. Effects of PE on Hyperlocomotion for Caffeine-Treated ICR Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matricciani, L.; Paquet, C.; Galland, B.; Short, M.; Olds, T. Children’s sleep and health: A meta-review. Sleep Med. Rev. 2019, 46, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef] [PubMed]
- Meredith, W.; Nicholas, K.; Martica, H.; Anne, G.; Karen, M.; Wendy, T.; Peter, F.; Daniel, B.; Kathryn, R.; Heather, G.; et al. Age Trends in Sleep Across the Lifespan: Findings from the Pittsburgh Lifespan Sleep Databank. Psychosom. Med. 2022, 84, 410–420. [Google Scholar]
- Sigga, S.J.; Kelton, M.; Sune, L. Gender differences in nighttime sleep patterns and variability across the adult lifespan: A global-scale wearables study. Sleep 2021, 44, zsaa169. [Google Scholar]
- Marissa, A.E.; Daniel, J.B.; Anna, L.M.; Aidan, G.C.W.; Jill, F.; Lucas, W.C.; Naina, K.; Rishabh, M.; Adam, J.; Swathi, S.; et al. Meta-analysis of age and actigraphy-assessed sleep characteristics across the lifespan. Sleep 2021, 44, zsab088. [Google Scholar]
- Baylan, S.; Griffiths, S.; Grant, N.; Broomfield, N.M.; Evans, J.J.; Gardani, M. Incidence and prevalence of post-stroke insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101222. [Google Scholar] [CrossRef]
- Fila-Witecka, K.; Malecka, M.; Senczyszyn, A.; Wieczorek, T.; Wieckiewicz, M.; Szczesniak, D.; Piotrowski, P.; Rymaszewska, J. Sleepless in Solitude-Insomnia Symptoms Severity and Psychopathological Symptoms among University Students during the COVID-19 Pandemic in Poland. Int. J. Environ. Res. Public Health 2022, 19, 2551. [Google Scholar] [CrossRef]
- Martynowicz, H.; Skomro, R.; Gać, P.; Mazur, G.; Porębska, I.; Bryłka, A.; Nowak, W.; Zieliński, M.; Wojakowska, A.; Poręba, R. The influence of hypertension on daytime sleepiness in obstructive sleep apnea. J. Am. Soc. Hypertens. 2017, 11, 295–302. [Google Scholar] [CrossRef]
- Doghramji, K. The epidemiology and diagnosis of insomnia. Am. J. Manag. Care 2006, 12, S214–S220. [Google Scholar]
- Botteman, M. Health economics of insomnia therapy: Implications for policy. Sleep Med. 2009, 10, S22–S25. [Google Scholar] [CrossRef]
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of Sleep Disturbance During COVID-19 Pandemic: A Systematic Review. Nat. Sci. Sleep 2021, 13, 933–966. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C.D.; Zabriskie, N. Natural approaches for optimal sleep. Altern. Complement. Ther. 2008, 14, 181–188. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Gottowik, J.; Gatti, S.; Wettstein, J.G.; Moreau, J.L. Rodent models of insomnia: A review of experimental procedures that induce sleep disturbances. Neurosci. Biobehav. Rev. 2009, 33, 874–899. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Caffeine use as a model of acute and chronic insomnia. Sleep 1992, 15, 526–536. [Google Scholar]
- Kwon, S.; Yoon, M.; Lee, J.; Moon, K.D.; Kim, D.; Kim, S.B.; Cho, S. A standardized phlorotannin supplement attenuates caffeine-induced sleep disruption in mice. Nutrients 2019, 11, 556. [Google Scholar] [CrossRef]
- Jo, K.; Kim, S.; Hong, K.B.; Suh, H.J. Nelumbo nucifera promotes non-rapid eye movement sleep by regulating GABAergic receptors in rat model. J. Ethnopharmacol. 2021, 267, 113511. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef]
- Son, J.; Park, S.J.; Ha, T.; Lee, S.N.; Cho, H.Y.; Choi, J.W. Electrophysiological Monitoring of Neurochemical-Based Neural Signal Transmission in a Human Brain–Spinal Cord Assembloid. ACS Sens. 2022, 7, 409–414. [Google Scholar] [CrossRef]
- Chaudhary, N.S.; Grandner, M.A.; Jackson, N.J.; Chakravorty, S. Caffeine consumption, insomnia, and sleep duration: Results from a nationally representative sample. Nutrition 2016, 32, 1193–1199. [Google Scholar] [CrossRef]
- Ko, Y.H.; Shim, K.Y.; Lee, S.Y.; Jang, C.G. Evodiamine reduces caffeine-induced sleep disturbances and excitation in mice. Biomol. Ther. 2018, 26, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Prashant, A.; Rangaswamy, C.; Yadav, A.K.; Reddy, V.; Sowmya, M.N.; Madhunapantula, S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int. J. Appl. Basic Med. Res. 2017, 7, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef]
- Belemkar, S.; Kumar, A.; Pata, M.K. Pharmacological screening of herbal extract of piper nigrum (Maricha) and Cinnamomum zeylanicum (Dalchini) for anticonvulsant activity. Ethnopharmacology 2013, 2, 1–5. [Google Scholar]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef]
- Woo, W.S.; Shin, K.H.; Kim, I.C. The effects of irritating spices on drug metabolizing enzyme activity-effects on hexobarbital hypnosis in mice. Korean J. Pharmacogn. 1977, 8, 115–119. [Google Scholar]
- Mujumdar, A.M.; Dhuley, J.N.; Deshmukh, V.K.; Raman, P.H.; Thorat, S.L.; Naik, S.R. Effect of piperine on pentobarbitone induced hypnosis in rats. Indian J. Exp. Biol. 1990, 28, 486–487. [Google Scholar]
- Khom, S.; Strommer, B.; Schöffmann, A.; Hintersteiner, J.; Baburin, I.; Erker, T.; Schwarz, T.; Schwarzer, C.; Zaugg, J.; Hamburger, M.; et al. GABAA receptor modulation by piperine and a non-TRPV1 activating derivative. Biochem. Pharmacol. 2013, 85, 1827–1836. [Google Scholar] [CrossRef]
- Zaugg, J.; Baburin, I.; Strommer, B.; Kim, H.J.; Hering, S.; Hamburger, M. HPLC-based activity profiling: Discovery of piperine as a positive GABAA receptor modulator targeting a benzodiazepine-independent binding site. J. Nat. Prod. 2010, 73, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yoon, M.; Um, M.Y.; Lee, J.; Jung, J.; Lee, C.; Kim, Y.T.; Kwon, S.; Kim, B.; Cho, S. Sleep-promoting effects and possible mechanisms of action associated with a standardized rice bran supplement. Nutrients 2017, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Yoon, M.; Lee, J.; Jung, J.; Cho, S. A novel potent sleep-promoting effect of turmeric: Turmeric increases non-rapid eye movement sleep in mice via histamine H1 receptor blockade. Mol. Nutr. Food Res. 2021, 65, 2100100. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Han, T.; Um, M.Y.; Yoon, M.; Kim, T.E.; Kim, Y.T.; Han, D.; Lee, J.; Lee, C.H. Administration of Asian herb bennet (Geum japonicum) extract reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Nutrients 2019, 11, 2841. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Kreiger, N.; Darlington, G.A.; Sloan, M. Misclassification of exposure: Coffee as a surrogate for caffeine intake. Am. J. Epidemiol. 2001, 153, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, H.S.; Han, S.; Tana, G.; Chang, M.J. Study on relationship between caffeine intake level and metabolic syndrome and related diseases in Korean adults: 2013~2016 Korea National Health and Nutrition Examination Survey. J. Nutr. Health 2019, 52, 227–241. [Google Scholar] [CrossRef]
- Mabunga, D.F.; Gonzales, E.L.; Kim, H.J.; Choung, S.Y. Treatment of GABA from fermented rice germ ameliorates caffeine-induced sleep disturbance in mice. Biomol. Ther. 2015, 23, 268. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Wagatsuma, K.; Toyohara, J.; Ishiwata, K.; Ishii, K. Adenosine A2A receptor occupancy by caffeine after coffee intake in Parkinson’s disease. Mov. Disord. 2022, 37, 853–857. [Google Scholar] [CrossRef]
- Paterson, L.M.; Wilson, S.J.; Nutt, D.J.; Hutson, P.H.; Ivarsson, M. Characterisation of the effects of caffeine on sleep in the rat: A potential model of sleep disruption. J. Psychopharmacol. 2009, 23, 475–486. [Google Scholar] [CrossRef]
- Jang, H.S.; Jung, J.Y.; Jang, I.S.; Jang, K.H.; Kim, S.H.; Ha, J.H.; Suk, K.; Lee, M.G. L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharmacol. Biochem. Behav. 2012, 101, 217–221. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Yoon, M.; Kim, J.; Kim, D.; Kim, J.; Kim, S.B. Arousal inhibitory effect of phlorotannins on caffeine in pentobarbital-induced mice. Fish. Aquat. Sci. 2014, 17, 13–18. [Google Scholar] [CrossRef][Green Version]
- Eigenmann, D.E.; Dürig, C.; Jähne, E.A.; Smieško, M.; Culot, M.; Gosselet, F.; Cecchelli, R.; Helms, H.C.; Brodin, B.; Wimmer, L.; et al. In vitro blood–brain barrier permeability predictions for GABAA receptor modulating piperine analogs. Eur. J. Pharm. Biopharm. 2016, 103, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Paterson, L.M.; Nutt, D.J.; Ivarsson, M.; Hutson, P.H.; Wilson, S.J. Effects on sleep stages and microarchitecture of caffeine and its combination with zolpidem or trazodone in healthy volunteers. J. Psychopharmacol. 2009, 23, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers phytochemicals: A valuable forest with therapeutic potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Paterson, L.M.; Wilson, S.J.; Nutt, D.J.; Hutson, P.H.; Ivarsson, M. A translational, caffeine-induced model of onset insomnia in rats and healthy volunteers. Psychopharmacology 2007, 191, 943–950. [Google Scholar] [CrossRef]
- Oatess, T.L.; Harrison, F.E.; Himmel, L.E.; Jones, C.P. Effects of acrylic tunnel enrichment on anxiety-like behavior, neurogenesis, and physiology of C57BL/6J mice. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 44–53. [Google Scholar] [CrossRef]
- Batista, L.A.; Viana, T.G.; Silveira, V.T.; Aguiar, D.C.; Moreira, F.A. Effects of aripiprazole on caffeine-induced hyperlocomotion and neural activation in the striatum. Naunyn Schmiedeberg’s Arch. Pharmacol. 2016, 389, 11–16. [Google Scholar] [CrossRef]
- Powell, K.R.; Holtzman, S.G. Lack of NMDA receptor involvement in caffeine-induced locomotor stimulation and tolerance in rats. Pharmacol. Biochem. Behav. 1998, 59, 433–438. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Poddar, M.K. Caffeine-induced locomotor activity: Possible involvement of GABAergic-dopaminergic-adenosinergic interaction. Neurochem. Res. 1995, 20, 39–44. [Google Scholar] [CrossRef]
| Methodology | Parameter | PE Treatment (1) |
|---|---|---|
| Pentobarbital-induced sleep test | Sleep duration | Increased |
| Sleep–wake profile analysis | Sleep onset (min) | Decreased |
| Total sleep time (min) | Increased | |
| Wake amount (min/5 h) | Decreased | |
| NREMS amount (min/5 h) | Increased | |
| REMS amount (min/5 h) | No change | |
| Open field test | Locomotor activity | Decreased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, M.; Jung, J.; Kim, M.; Lee, C.; Cho, S.; Um, M. Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients 2022, 14, 2249. https://doi.org/10.3390/nu14112249
Yoon M, Jung J, Kim M, Lee C, Cho S, Um M. Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients. 2022; 14(11):2249. https://doi.org/10.3390/nu14112249
Chicago/Turabian StyleYoon, Minseok, Jonghoon Jung, Minjung Kim, Changho Lee, Suengmok Cho, and Minyoung Um. 2022. "Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice" Nutrients 14, no. 11: 2249. https://doi.org/10.3390/nu14112249
APA StyleYoon, M., Jung, J., Kim, M., Lee, C., Cho, S., & Um, M. (2022). Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients, 14(11), 2249. https://doi.org/10.3390/nu14112249







