Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Cell Treatments
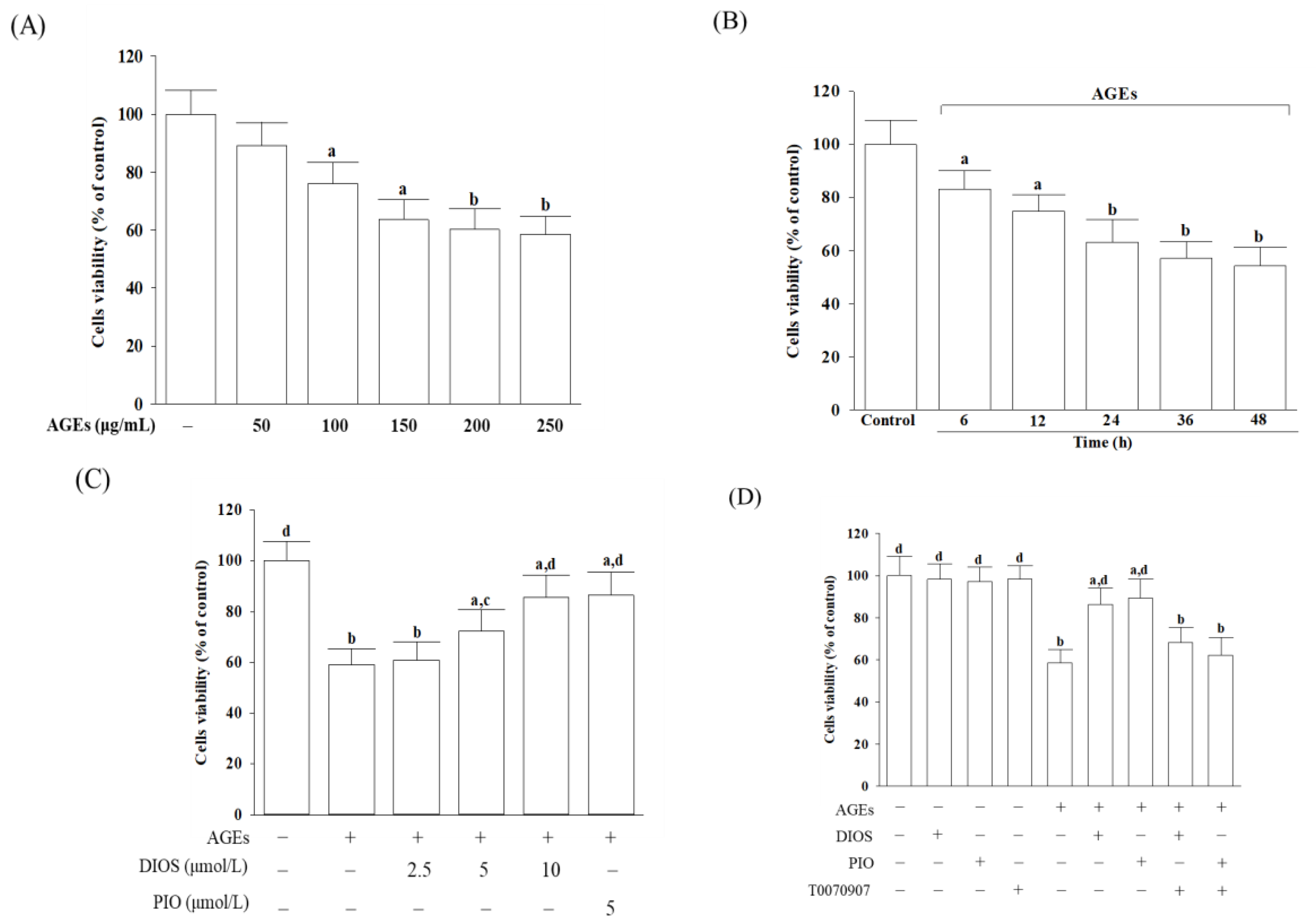
2.3. Cell Viability Assay
2.4. Reactive Oxygen Species Measurement
2.5. Antioxidant Enzyme Activities Determination
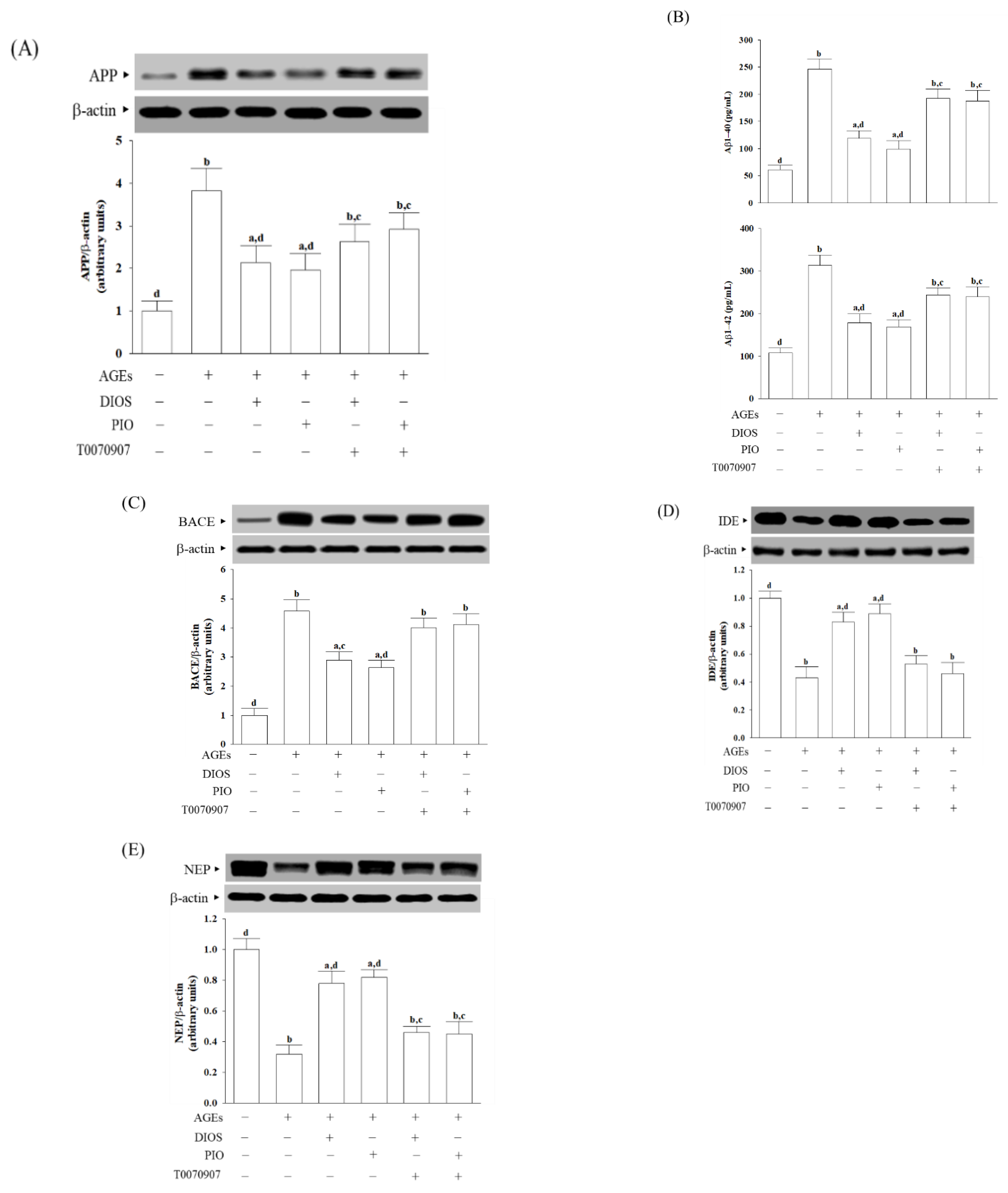
2.6. Aβ1–40 and Aβ1–42 Determination
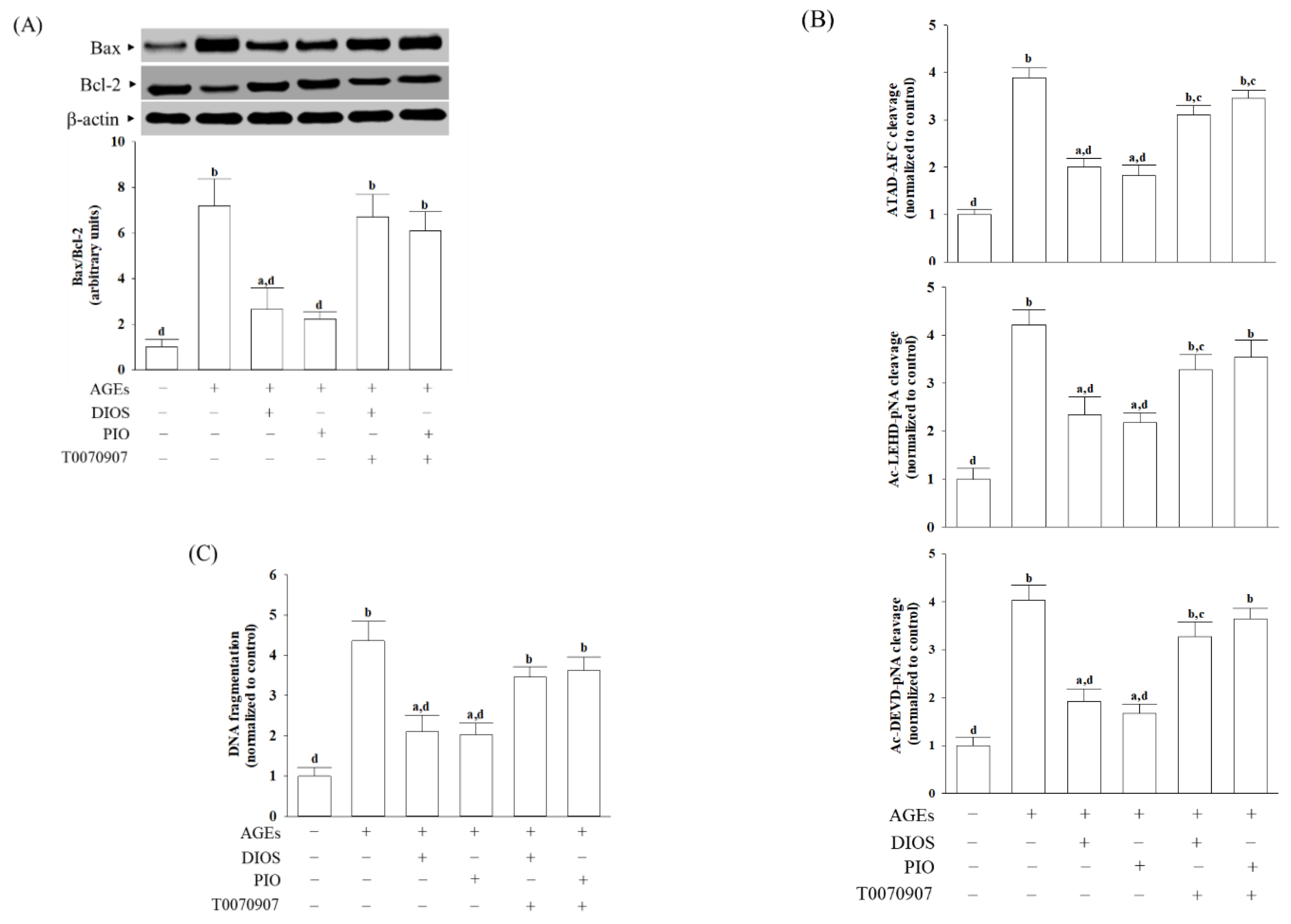
2.7. Caspases Activities Measurement
2.8. Apoptotic Cell Death Detection
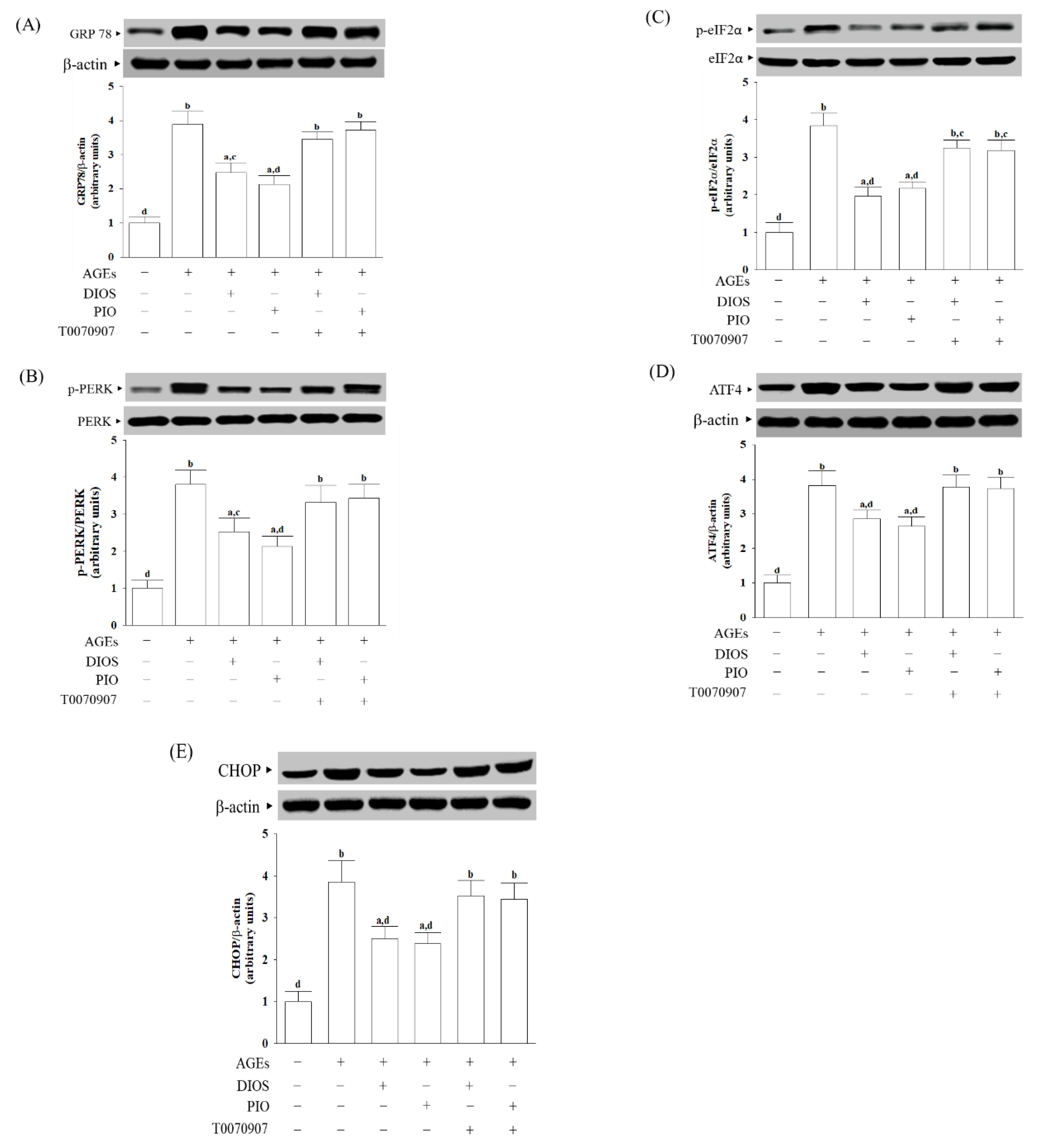
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Diosmetin Improved Cell Viability
3.2. Diosmetin Lowered the ROS Level and Enhanced the Antioxidant Enzymes Activities
3.3. Diosmetin Inhibited APP Processing and Aβ Production
3.4. Diosmetin Inhibited ER Stress-Activated Unfolded Protein Response
3.5. Diosmetin Suppressed ER Stress-Mediated Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Athar, T.; Al Balushi, K.; Khan, S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Lynn, J.; Park, M.; Ogunwale, C.; Acquaah-Mensah, G.K. A tale of two diseases: Exploring mechanisms linking diabetes mellitus with Alzheimer’s disease. J. Alzheimer Dis. 2022, 85, 485–501. [Google Scholar] [CrossRef]
- Prasad, K. AGE-RAGE stress: A changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell. Biochem. 2019, 459, 95–112. [Google Scholar] [CrossRef]
- Baranello, R.J.; Bharani, K.L.; Padmaraju, V.; Chopra, N.; Lahiri, D.K.; Greig, N.H.; Pappolla, M.A.; Sambamurti, K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xu, Y. Therapeutic effects of natural compounds and small molecule inhibitors targeting endoplasmic reticulum stress in Alzheimer’s disease. Front. Cell Dev. Biol. 2021, 9, 745011. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Sun, H.; Wang, H.; Peng, W.; Zhou, Z.; Wang, H.; Pi, C.; Shi, Y.; He, X. Metabolism: A novel shared link between diabetes mellitus and Alzheimer’s disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Masciopinto, F.; Di Pietro, N.; Corona, C.; Bomba, M.; Pipino, C.; Curcio, M.; Di Castelnuovo, A.; Ciavardelli, D.; Silvestri, E.; Canzoniero, L.M.; et al. Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death Dis. 2012, 3, e448. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Burns, D.K.; Gottschalk, W.K. Reassessment of pioglitazone for Alzheimer’s disease. Front. Neurosci. 2021, 15, 666958. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.N.; Jia, J.P. Peroxisome proliferator-activated receptor-gamma agonists for Alzheimer’s disease and amnestic mild cognitive impairment: A systematic review and meta-analysis. Drugs Aging 2015, 32, 57–65. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-mediated toxicology and applied pharmacology. Cells 2020, 9, 352. [Google Scholar] [PubMed] [Green Version]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef]
- Sawmiller, D.; Habib, A.; Li, S.; Darlington, D.; Hou, H.; Tian, J.; Shytle, R.D.; Smith, A.; Giunta, B.; Mori, T.; et al. Diosmin reduces cerebral Aβ levels, tau hyperphosphorylation, neuroinflammation, and cognitive impairment in the 3xTg-AD mice. J. Neuroimmunol. 2016, 299, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Cova, D.; De Angelis, L.; Giavarini, F.; Palladini, G.; Perego, R. Pharmacokinetics and metabolism of oral diosmin in healthy volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992, 30, 29–33. [Google Scholar]
- Russo, R.; Chandradhara, D.; De Tommasi, N. Comparative bioavailability of two diosmin formulations after oral administration to healthy volunteers. Molecules 2018, 23, 2174. [Google Scholar] [CrossRef] [Green Version]
- Campanero, M.A.; Escolar, M.; Perez, G.; Garcia-Quetglas, E.; Sadaba, B.; Azanza, J.R. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 875–881. [Google Scholar] [CrossRef]
- Villa, P.; Cova, D.; De Francesco, L.; Guaitani, A.; Palladin, I.G.; Perego, R. Protective effect of diosmetin on in vitro cell membrane damage and oxidative stress in cultured rat hepatocytes. Toxicology 1992, 73, 179–189. [Google Scholar] [CrossRef]
- Kuhla, B.; Loske, C.; De Garcia Arriba, S.; Schinzel, R.; Huber, J.; Münch, G. Differential effects of “advanced glycation endproducts” and beta-amyloid peptide on glucose utilization and ATP levels in the neuronal cell line SH-SY5Y. J. Neural Transm. 2004, 111, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Ning, Z.; Chen, L.; Wei, Q.; Yuan, E.; Yang, J.; Ren, J. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J. Agric. Food Chem. 2014, 62, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, L.; Zhou, Z.; Ge, J.; Yao, T.; Shen, C. Pioglitazone alleviates inflammation in diabetic mice fed a high-fat diet via inhibiting advanced glycation end-product-induced classical macrophage activation. FEBS J. 2016, 283, 2295–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Z.; Muthusami, S.; Yu, J.R.; Park, W.Y. T0070907, a PPAR γ inhibitor, induced G2/M arrest enhances the effect of radiation in human cervical cancer cells through mitotic catastrophe. Reprod. Sci. 2014, 21, 1352–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, B.N. Medical use of dimethyl sulfoxide (DMSO). Rev. Clin. Basic Pharm. 1985, 5, 1–33. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 20,70-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Durak, I.; Yurtarslanl, Z.; Canbolat, O.; Akyol, O. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin. Chim. Acta 1993, 214, 103–104. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Matassov, D.; Kagan, T.; Leblanc, J.; Sikorska, M.; Zakeri, Z. Measurement of apoptosis by DNA fragmentation. Methods Mol. Biol. 2004, 282, 1–17. [Google Scholar] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Roy, S. The physical approximation of APP and BACE-1: A key event in alzheimer’s disease pathogenesis. Dev. Neurobiol. 2018, 78, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Rozpędek, W.; Pytel, D.; Popławski, T.; Walczak, A.; Gradzik, K.; Wawrzynkiewicz, A.; Wojtczak, R.; Mucha, B.; Diehl, J.A.; Majsterek, I. Inhibition of the PERK-dependent unfolded protein response signaling pathway involved in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 209–218. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Troy, C.M.; Jean, Y.Y. Caspases: Therapeutic targets in neurologic disease. Neurotherapeutics 2015, 12, 42–48. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.; Yin, H. Signaling pathways involved in endoplasmic reticulum stress-induced neuronal apoptosis. Int. J. Neurosci. 2013, 123, 155–162. [Google Scholar] [CrossRef]
- Ding, Y.; Kang, J.; Liu, S.; Xu, Y.; Shao, B. The protective effects of peroxisome proliferator-activated receptor gamma in cerebral ischemia-reperfusion injury. Front. Neurol. 2020, 11, 588516. [Google Scholar] [CrossRef]
- Ikeda, J.; Ichiki, T.; Takahara, Y.; Kojima, H.; Sankoda, C.; Kitamoto, S.; Tokunou, T.; Sunagawa, K. PPARγ Agonists Attenuate Palmitate-Induced ER Stress through Up-Regulation of SCD-1 in Macrophages. PLoS ONE 2015, 10, e0128546. [Google Scholar] [CrossRef]
- Piemontese, L. An innovative approach for the treatment of Alzheimer’s disease: The role of peroxisome proliferator-activated receptors and their ligands in development of alternative therapeutic interventions. Neural Regen. Res. 2019, 14, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, E.; Nasiri Boroujeni, S.; Safavi, P.; Borjian Boroujeni, Z.; Bijad, E. Diosmetin mitigates cognitive and memory impairment provoked by chronic unpredictable mild stress in mice. Evid. Based Complementary Altern. Med. 2020, 2020, 5725361. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, C.T.; Yu, X.N.; Guo, J.B.; Ma, H.; Liu, K.; Luo, P.; Ren, J. Preparation and pharmacokinetic study of diosmetin long-circulating liposomes modified with lactoferrin. J. Funct. Foods 2022, 91, 105027. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.C.; Liu, W.Y.; Liou, S.-S.; Liu, I.-M. Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury. Nutrients 2022, 14, 2248. https://doi.org/10.3390/nu14112248
Lai MC, Liu WY, Liou S-S, Liu I-M. Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury. Nutrients. 2022; 14(11):2248. https://doi.org/10.3390/nu14112248
Chicago/Turabian StyleLai, Mei Chou, Wayne Young Liu, Shorong-Shii Liou, and I-Min Liu. 2022. "Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury" Nutrients 14, no. 11: 2248. https://doi.org/10.3390/nu14112248
APA StyleLai, M. C., Liu, W. Y., Liou, S.-S., & Liu, I.-M. (2022). Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury. Nutrients, 14(11), 2248. https://doi.org/10.3390/nu14112248




