Cytokine Pattern of Peripheral Blood Mononuclear Cells Isolated from Children Affected by Generalized Epilepsy Treated with Different Protein Fractions of Meat Sources
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Recruitment Procedure
2.2. Separation of Meat and Fish Proteins
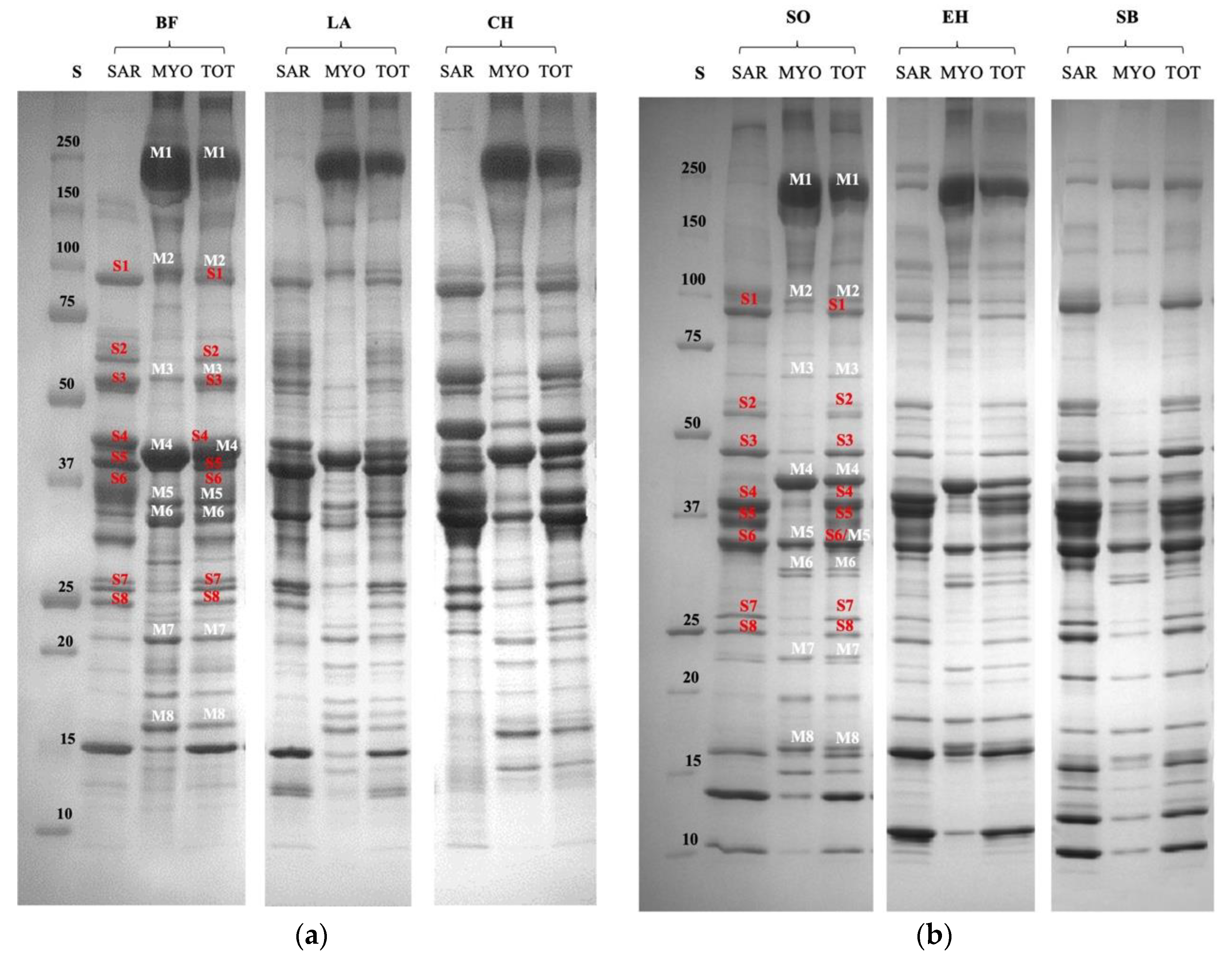
2.3. SDS-PAGE Analysis of Fish and Meat Samples
2.4. Determination of Cytokine Profile from PBMCs Supernatants
2.5. Statistical Analysis
3. Results
3.1. Characterization of Proteomic Profile of Meat and Fish Extracts
3.2. Cytokine Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plevin, D.; Smith, N. Assessment and management of depression and anxiety in children and adolescents with epilepsy. Behav. Neurol. 2019, 2019, 2571368. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.R.; Almeida, N.C.; Prather, K.Y.; O’Neal, C.M.; Wells, A.A.; Chen, S.; Conner, A.K. Resting-state functional magnetic resonance imaging with independent component analysis for presurgical seizure onset zone localization: A systematic review and meta-analysis. Epilepsia 2020, 61, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Santillo, A.; Polito, R.; Messina, G.; Albenzio, M. The Role of Milk Nutrition and Ketogenic Diet in Epileptic Disorders; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-6453320-4-9. [Google Scholar] [CrossRef]
- Ünalp, A. Ketogenic diet practices in childhood epilepsies. İzmir Dr. Behçet Uz Çocuk Hastanesi Dergisi 2017, 7, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Westmark, C.J. A role for amino acid balance in dietary treatments for epilepsy. J. Nutr. 2018, 148, 307–308. [Google Scholar] [CrossRef]
- Gietzen, D.W.; Dixon, K.D.; Truong, B.G.; Jones, A.C.; Barrett, J.A.; Washburn, D.S. Indispensable amino acid deficiency and increased seizure susceptibility in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 271, R18–R24. [Google Scholar] [CrossRef]
- Rudell, J.B.; Rechs, A.J.; Kelman, T.J.; Ross-Inta, C.M.; Hao, S.; Gietzen, D.W. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J. Neurosci. 2011, 31, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Gaby, A.R. Natural approaches to epilepsy. Altern. Med. Rev. 2007, 12, 9. [Google Scholar]
- Beghi, E.; Shorvon, S. Antiepileptic drugs and the immune system. Epilepsia 2011, 52, 40–44. [Google Scholar] [CrossRef]
- Vezzani, A.; Granata, T. Brain inflammation in epilepsy: Experimental and clinical evidence. Epilepsia 2005, 46, 1724–1743. [Google Scholar] [CrossRef]
- Muñoz-Fernández, M.; Fresno, M. The role of tumour necrosis factor, interleukin 6, interferon-γ and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998, 56, 307–340. [Google Scholar] [CrossRef]
- Lehtimäki, K.A.; Keränen, T.; Palmio, J.; Mäkinen, R.; Hurme, M.; Honkaniemi, J.; Peltola, J. Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol. Scand. 2007, 116, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Conti, M.; De Luigi, A.; Ravizza, T.; Moneta, D.; Marchesi, F.; De Simoni, M.G. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999, 19, 5054–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penkowa, M.; Molinero, A.; Carrasco, J.; Hidalgo, J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience 2001, 102, 805–818. [Google Scholar] [CrossRef]
- Kamali, A.N.; Zian, Z.; Bautista, J.M.; Hamedifar, H.; Hossein-Khannazer, N.; Hosseinzadeh, R.; Yazdani, R.; Azizi, G. The potential role of pro-inflammatory and anti-inflammatory cytokines in epilepsy pathogenesis. Endocr. Metab. Immune Disord.—Drug Targets 2021, 21, 1760–1774. [Google Scholar] [CrossRef]
- Albenzio, M.; Polito, A.N.; Santillo, A. The role of milk nutrition in childhood epilepsy. Intern. J. Food Nutr. Sciences. 2016, 5, 141–150. [Google Scholar]
- Albenzio, M.; Campanozzi, A.; D’Apolito, M.; Santillo, A.; Mantovani, M.P.; Sevi, A. Differences in protein fraction from goat and cow milk and their role on cytokine production in children with cow's milk protein allergy. Small Rumin. Res. 2012, 105, 202–205. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Polito, A.N. Role of Milk from Small Ruminant Species on Human Health. In Nutrients in Dairy and their Implications on Health and Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 435–440. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Ciliberti, M.G.; Figliola, L.; Caroprese, M.; Marino, R.; Polito, A. Milk from different species: Relationship between protein fractions and inflammatory response in infants affected by generalized epilepsy. J. Dairy Sci. 2016, 99, 5032–5038. [Google Scholar] [CrossRef] [Green Version]
- Della Malva, A.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for extraction of muscle proteins from meat and fish using denaturing and nondenaturing solutions. J. Food Qual. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Hashimoto, K.; Watabe, S.; KoNo, M.; Shiro, K. Muscle protein composition of sardine and mackerel. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–601. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Enterprise Guide: Statistics, Version 6.1 ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- De Vries, E.E.; Van den Munckhof, B.; Braun, K.P.; Van Royen-Kerkhof, A.; De Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Takamatsu, T.; Morichi, S.; Yamazaki, T.; Mizoguchi, I.; Ohno, K.; Watanabe, Y.; Ishida, Y.; Oana, S.; Suzuki, S.; et al. Interleukin-1β in peripheral monocytes is associated with seizure frequency in pediatric drug-resistant epilepsy. J. Neuroimmunol. 2021, 352, 577475. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125, S53–S72. [Google Scholar] [CrossRef] [PubMed]
- Albenzio, M.; Santillo, A.; Ciliberti, M.G.; Figliola, L.; Caroprese, M.; Polito, A.; Messina, G. Milk nutrition and childhood epilepsy: An ex vivo study on cytokines and oxidative stress in response to milk protein fractions. J. Dairy Sci. 2018, 101, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Dundar, N.O.; Aktekin, B.; Ekinci, N.C.; Sahinturk, D.; Yavuzer, U.; Yegin, O.; Haspolat, S. Interleukin-1β secretion in hippocampal sclerosis patients with mesial temporal lobe epilepsy. Neurol. Int. 2013, 5, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Helminen, M.; Vesikari, T. Increased Interleukin-1 (IL-1) Production from LPS-stimulated peripheral blood monocytes in children with febrile convulsions. Acta Paediatr. 1990, 79, 810–816. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, K.; Ichimaru, T.; Nakazato, S.; Hamasaki, Y. Increased IL-1β production from dsRNA-stimulated leukocytes in febrile seizures. Pediatr. Neurol. 2006, 35, 102–106. [Google Scholar] [CrossRef]
- Nur, B.G.; Sahinturk, D.; Coskun, M.; Duman, O.; Yavuzer, U.; Haspolat, S. Single nucleotide polymorphism and production of IL-1β and IL-10 cytokines in febrile seizures. Neuropediatrics 2012, 43, 194–200. [Google Scholar] [CrossRef]
- Pacifici, R.; Paris, L.; Di Carlo, S.; Bacosi, A.; Pichini, S.; Zuccaro, P. Cytokine production in blood mononuclear cells from epileptic patients. Epilepsia 1995, 36, 384–387. [Google Scholar] [CrossRef]
- Straussberg, R.; Amir, J.; Harel, L.; Punsky, I.; Bessler, H. Pro- and anti-inflammatory cytokines in children with febrile convulsions. Pediatr. Neurol. 2001, 24, 49–53. [Google Scholar] [CrossRef]
- Librizzi, L.; Noè, F.; Vezzani, A.; De Curtis, M.; Ravizza, T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann. Neurol. 2012, 72, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Moneta, D.; Richichi, C.; Aliprandi, M.; Burrows, S.J.; Ravizza, T.; Perego, C.; de Simoni, M.G. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 2002, 43, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Moneta, D.; Conti, M.; Richichi, C.; Ravizza, T.; De Luigi, A.; De Simoni, M.G.; Sperk, G.; Andell-Jonsson, S.; Lundkvist, J.; et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11534–11539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchi, N.; Fan, Q.; Ghosh, C.; Fazio, V.; Bertolini, F.; Betto, G.; Batra, A.; Carlton, E.; Najm, I.; Granata, T.; et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis. 2009, 33, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, S.; Gokhale, J. Immunity boosting nutraceuticals: Current trends and challenges. J. Food Biochem. 2021, 46, e13902. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R. Potential for diet to prevent and remediate cognitive deficits in neurological disorders. Nutr. Rev. 2018, 76, 204–217. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Maroso, M.; Zardoni, D.; Noé, F.; Ravizza, T. ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Curr. Opin. Investig. Drugs 2010, 11, 43–50. [Google Scholar]
- Oby, E.; Janigro, D. The blood–brain barrier and epilepsy. Epilepsia 2006, 47, 1761–1774. [Google Scholar] [CrossRef]
- Ravizza, T.; Gagliardi, B.; Noe’, F.M.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Ravizza, T.; Boer, K.; Redeker, S.; Spliet, W.G.M.; Van Rijen, P.C.; Troost, D.; Vezzani, A.; Aronica, E. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 2006, 24, 128–143. [Google Scholar] [CrossRef]
- Boer, K.; Jansen, F.; Nellist, M.; Van den Redeker, S.; Ouweland, A.M.W.; Spliet, W.G.M.; Van Nieuwenhuizen, O.; Troost, D.; Crino, P.B.; Aronica, E. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008, 78, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Cho, K.-O. Functional nutrients for epilepsy. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albenzio, M.; Santillo, A.; Avondo, M.; Nudda, A.; Chessa, S.; Pirisi, A.; Banni, S. Nutritional properties of small ruminant food products and their role on human health. Small Rumin. Res. 2015, 135, 3–12. [Google Scholar] [CrossRef]
- Pilon, G.; Ruzzin, J.; Rioux, L.E.; Lavigne, C.; White, P.J.; Frøyland, H.J.; Bryle, P.; Beaulieu, L.; Marette, A. Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat–fed rats. Metabolism 2011, 60, 1122–1130. [Google Scholar] [CrossRef]
- Demonty, I.; Deshaies, Y.; Jacques, H. Dietary proteins modulate the effects of fish oil on triglyceridemia in the rat. Lipids 1998, 33, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Pilon, G.; Dallaire, P.; Marette, A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: A new mechanism of action of insulin-sensitizing drugs. J. Biol. Chem. 2004, 279, 20767–20774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, S.G.; Godukhin, O.V. Protective effects of interleukin-10 on the development of epileptiform activity evoked by transient episodes of hypoxia in rat hippocampal slices. Neurosci. Behav. Physiol. 2007, 37, 467–470. [Google Scholar] [CrossRef]
- Opp, M.R.; Smith, E.M.; Hughes, T.K., Jr. Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J. Neuroimmunol. 1995, 60, 165–168. [Google Scholar] [CrossRef]
- Mkhize, N.V.P.; Qulu, L.; Mabandla, M.V. The effect of quercetin on pro- and anti-inflammatory cytokines in a prenatally stressed rat model of febrile seizures. J. Exp. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Youn, Y.A.; Kim, S.J.; Sung, I.K.; Chung, S.Y.; Kim, Y.H.; Lee, I.G. Serial examination of serum IL-8, IL-10 and IL-1Ra levels is significant in neonatal seizures induced by hypoxic-ischaemic encephalopathy1. Scand. J. Immunol. 2012, 76, 286–293. [Google Scholar] [CrossRef]
- Virta, M.; Hurme, M.; Helminen, M. Increased Plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 2002, 43, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-Y.; Ding, J.; Peng, W.-F.; Ma, Y.; Zhang, Y.-H.; Fan, W.; Wang, X. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia 2013, 54, e142–e145. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Gao, Y.; Zhang, S.-J.; Zhe, X.; Meng, F.-L.; Qian, H.; Zhang, B.; Li, Y.-J. Alteration of plasma cytokines in patients with active epilepsy. Acta Neurol. Scand. 2017, 135, 663–669. [Google Scholar] [CrossRef] [PubMed]





| EC | CC | p-Value | |
|---|---|---|---|
| M/F | 8/8 | 8/8 | ns |
| AGE (MO) | 32.4 ± 4.4 | 33.6 ± 5.8 | ns |
| PERCENTILE | 25° | 25° | ns |
| GLYCEMIA (MG/DL) | 75.5 ± 2.8 | 79.8 ± 3.6 | ns |
| HEMOGLOBIN (G/DL) | 12.9 ± 2.3 | 11.9 ±1.2 | ns |
| TOTAL CHOLESTEROL (MG/DL) | 110 ± 5.6 | 115 ± 3.4 | ns |
| TRIGLYCERIDES (MG/DL) | 90.6 ± 6.1 | 109.7 ± 4.3 | ns |
| ALBUMIN (G/DL) | 3.5 ± 0.8 | 4 ± 2.1 | ns |
| CRP (MG/DL) | <0.6 | <0.6 | ns |
| Class 1 | |||
|---|---|---|---|
| Cytokines | LL-EC | ML-EC | HL-EC |
| TNF-α | 60 | 20 | 20 |
| IL-6 | 60 | 20 | 20 |
| IL-1β | 60 | 30 | 10 |
| IL-10 | 70 | 20 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilberti, M.G.; Santillo, A.; Polito, A.N.; Messina, G.; Della Malva, A.; Caroprese, M.; Sevi, A.; Albenzio, M. Cytokine Pattern of Peripheral Blood Mononuclear Cells Isolated from Children Affected by Generalized Epilepsy Treated with Different Protein Fractions of Meat Sources. Nutrients 2022, 14, 2243. https://doi.org/10.3390/nu14112243
Cilberti MG, Santillo A, Polito AN, Messina G, Della Malva A, Caroprese M, Sevi A, Albenzio M. Cytokine Pattern of Peripheral Blood Mononuclear Cells Isolated from Children Affected by Generalized Epilepsy Treated with Different Protein Fractions of Meat Sources. Nutrients. 2022; 14(11):2243. https://doi.org/10.3390/nu14112243
Chicago/Turabian StyleCilberti, Maria Giovanna, Antonella Santillo, Anna N. Polito, Giovanni Messina, Antonella Della Malva, Mariangela Caroprese, Agostino Sevi, and Marzia Albenzio. 2022. "Cytokine Pattern of Peripheral Blood Mononuclear Cells Isolated from Children Affected by Generalized Epilepsy Treated with Different Protein Fractions of Meat Sources" Nutrients 14, no. 11: 2243. https://doi.org/10.3390/nu14112243
APA StyleCilberti, M. G., Santillo, A., Polito, A. N., Messina, G., Della Malva, A., Caroprese, M., Sevi, A., & Albenzio, M. (2022). Cytokine Pattern of Peripheral Blood Mononuclear Cells Isolated from Children Affected by Generalized Epilepsy Treated with Different Protein Fractions of Meat Sources. Nutrients, 14(11), 2243. https://doi.org/10.3390/nu14112243










