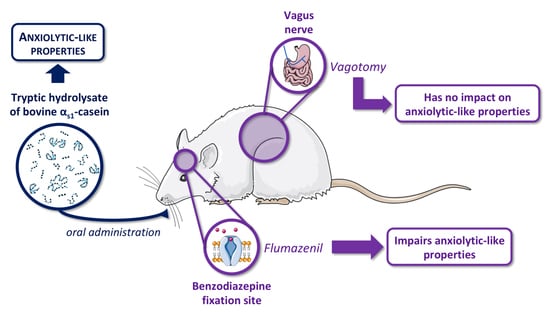
The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedure
2.2.1. Experiment 1: Vagus Nerve Involvement in Anxiolytic Activity of CH
2.2.2. Experiment 2: Benzodiazepine Site of GABAA Receptor Involvement in Anxiolytic Activity of CH
2.3. Conditioned Defensive Burying Test
2.3.1. Apparatus
2.3.2. Procedure
2.3.3. Studied Parameters
2.4. Statistical Analysis
3. Results
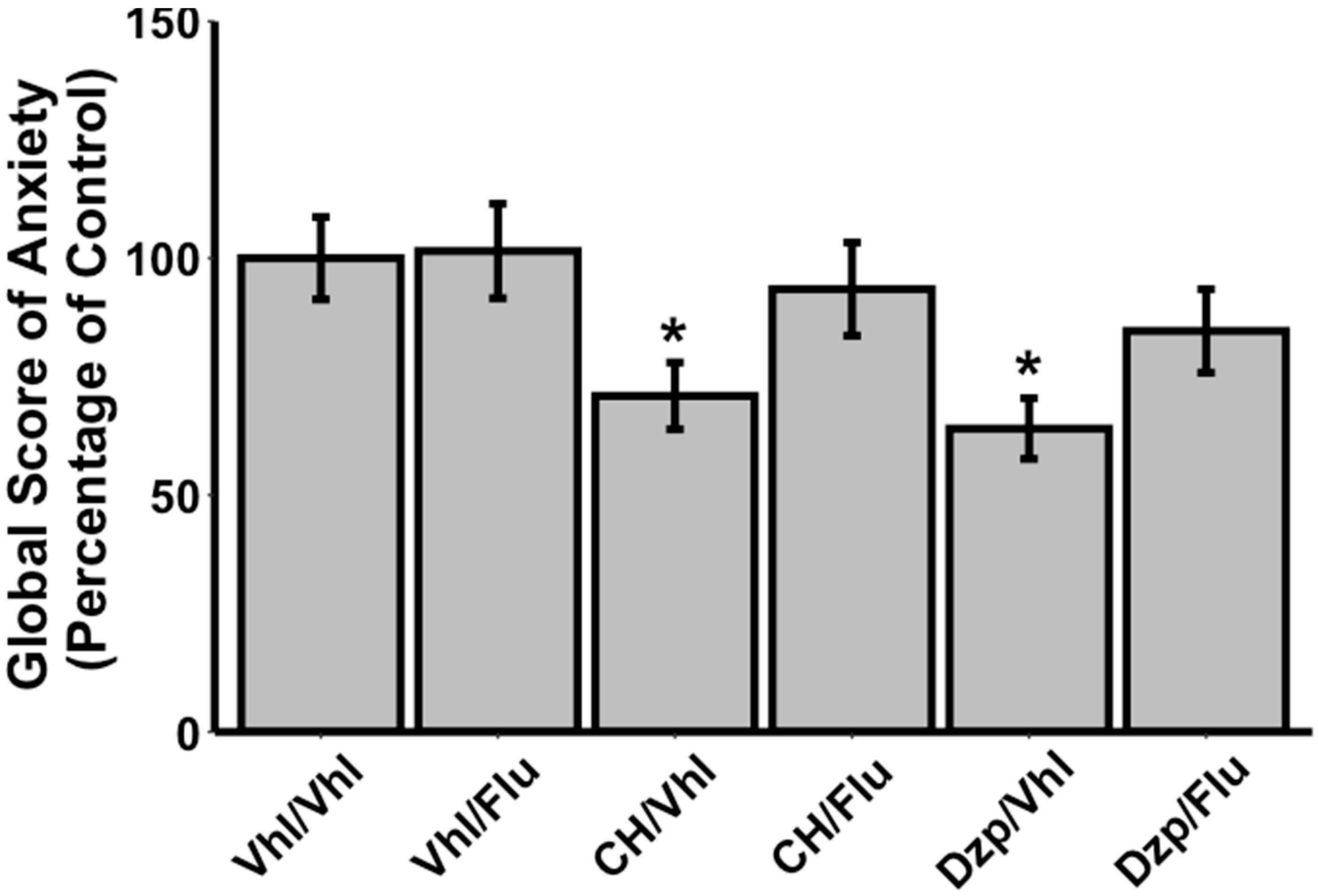
3.1. Evaluation of Anxiolytic Properties of CH after Oral Administration in Vagotomised Rats
3.2. Effects of Flumazenil on Anxiolytic Properties of Orally Administrated CH
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef] [Green Version]
- Miclo, L.; Perrin, E.; Driou, A.; Papadopoulos, V.; Boujrad, N.; Vanderesse, R.; Boudier, J.F.; Desor, D.; Linden, G.; Gaillard, J.-L. Characterization of alpha-casozepine, a tryptic peptide from bovine alpha(s1)-casein with benzodiazepine-like activity. FASEB J. 2001, 15, 1780–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violle, N.; Messaoudi, M.; Lefranc-Millot, C.; Desor, D.; Nejdi, A.; Demagny, B.; Schroeder, H. Ethological comparison of the effects of a bovine alpha s1-casein tryptic hydrolysate and diazepam on the behaviour of rats in two models of anxiety. Pharmacol. Biochem. Behav. 2006, 84, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Desor, D.; Kim, Y.T.; Yoon, W.J.; Kim, K.S.; Jun, J.S.; Pyun, K.H.; Shim, I. Efficacy of alphas1-casein hydrolysate on stress-related symptoms in women. Eur. J. Clin. Nutr. 2007, 61, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lefranc-Millot, C.; Desor, D.; Demagny, B.; Bourdon, L. Effects of a tryptic hydrolysate from bovine milk alphaS1-casein on hemodynamic responses in healthy human volunteers facing successive mental and physical stress situations. Eur. J. Nutr. 2005, 44, 128–132. [Google Scholar] [CrossRef]
- Guesdon, B.; Messaoudi, M.; Lefranc-Millot, C.; Fromentin, G.; Tomé, D.; Even, P.C. A tryptic hydrolysate from bovine milk alphaS1-casein improves sleep in rats subjected to chronic mild stress. Peptides 2006, 27, 1476–1482. [Google Scholar] [CrossRef]
- Beata, C.; Beaumont-Graff, E.; Coll, V.; Cordel, J.; Marion, M.; Massal, N.; Marlois, N.; Tauzin, J. Effect of alpha-casozepine (Zylkene) on anxiety in cats. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 40–46. [Google Scholar] [CrossRef]
- Beata, C.; Beaumont-Graff, E.; Diaz, C.; Marion, M.; Massal, N.; Marlois, N.; Muller, G.; Lefranc-Millot, C. Effects of alpha-casozepine (Zylkene) versus selegiline hydrochloride (Selgian, Anipryl) on anxiety disorders in dogs. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 175–183. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Miller, J.; Vaala, W. Calming benefit of short-term alpha-casozepine supplementation during acclimation to domestic environment and basic ground training of adult semi-feral ponies. J. Equine Vet. Sci. 2013, 33, 101–106. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Miller, J.; Vaala, W. Modestly improved compliance and apparent comfort of horses with aversions to mildly-aversive routine healthcare procedures following short-term alpha-casozepine supplementation. J. Equine Vet. Sci. 2014, 34, 1016–1020. [Google Scholar] [CrossRef]
- Benoit, S.; Chaumontet, C.; Schwarz, J.; Cakir-Kiefer, C.; Tomé, D.; Miclo, L. Mapping in mice the brain regions involved in the anxiolytic-like properties of α-casozepine, a tryptic peptide derived from bovine αs1-casein. J. Funct. Foods 2017, 38, 464–473. [Google Scholar] [CrossRef]
- Cakir-Kiefer, C.; Le Roux, Y.; Balandras, F.; Trabalon, M.; Dary, A.; Laurent, F.; Gaillard, J.-L.; Miclo, L. In vitro digestibility of α-casozepine, a benzodiazepine-like peptide from bovine casein, and biological activity of its main proteolytic fragment. J. Agric. Food Chem. 2011, 59, 4464–4472. [Google Scholar] [CrossRef]
- Benoit, S.; Chaumontet, C.; Schwarz, J.; Cakir-Kiefer, C.; Boulier, A.; Tomé, D.; Miclo, L. Anxiolytic activity and brain modulation pattern of the α-casozepine-derived pentapeptide YLGYL in mice. Nutrients 2020, 12, 1497. [Google Scholar] [CrossRef]
- Hafeez, Z.; Benoit, S.; Cakir-Kiefer, C.; Dary, A.; Miclo, L. Food protein-derived anxiolytic peptides: Their potential role in anxiety management. Food Funct. 2021, 12, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Steimer, T. The biology of fear- and anxiety-related behaviors. Dialogues Clin. Neurosci. 2002, 4, 231–249. [Google Scholar] [CrossRef]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dela Peña, I.J.I.; Kim, H.J.; de la Peña, J.B.; Kim, M.; Botanas, C.J.; You, K.Y.; Woo, T.; Lee, Y.S.; Jung, J.-C.; Kim, K.-M.; et al. A tryptic hydrolysate from bovine milk αs1-casein enhances pentobarbital-induced sleep in mice via the GABAA receptor. Behav. Brain Res. 2016, 313, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Schroeder, H.; Desor, D. Anxiolytic-like effects and safety profile of a tryptic hydrolysate from bovine alpha s1-casein in rats. Fundam. Clin. Pharmacol. 2009, 23, 323–330. [Google Scholar] [CrossRef]
- Klarer, M.; Arnold, M.; Günther, L.; Winter, C.; Langhans, W.; Meyer, U. Gut vagal afferents differentially modulate innate anxiety and learned fear. J. Neurosci. 2014, 34, 7067–7076. [Google Scholar] [CrossRef] [Green Version]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Ota, A.; Yamamoto, A.; Kimura, S.; Mori, Y.; Mizushige, T.; Nagashima, Y.; Sato, M.; Suzuki, H.; Odagiri, S.; Yamada, D.; et al. Rational identification of a novel soy-derived anxiolytic-like undecapeptide acting via gut-brain axis after oral administration. Neurochem. Int. 2017, 105, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bentué-Ferrer, D.; Bureau, M.; Patat, A.; Allain, H. Flumazenil. CNS Drug Rev. 1996, 2, 390–414. [Google Scholar] [CrossRef]
- Treit, D.; Pinel, J.P.; Fibiger, H.C. Conditioned defensive burying: A new paradigm for the study of anxiolytic agents. Pharmacol. Biochem. Behav. 1981, 15, 619–626. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Moran, T.H.; Baldessarini, A.R.; Salorio, C.F.; Lowery, T.; Schwartz, G.J. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997, 272, R1245–R1251. [Google Scholar] [CrossRef]
- Mizushige, T. Neuromodulatory peptides: Orally active anxiolytic-like and antidepressant-like peptides derived from dietary plant proteins. Peptides 2021, 142, 170569. [Google Scholar] [CrossRef]
- Cakir-Kiefer, C.; Miclo, L.; Balandras, F.; Dary, A.; Soligot, C.; Le Roux, Y. Transport across Caco-2 cell monolayer and sensitivity to hydrolysis of two anxiolytic peptides from αs1-casein, α-casozepine, and αs1-casein-f91-97: Effect of bile salts. J. Agric. Food Chem. 2011, 59, 11956–11965. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, L.; Su, G.; Huang, M.; Luo, D.; Zhao, M. Identification and Screening of Potential Bioactive Peptides with Sleep-Enhancing Effects in Bovine Milk Casein Hydrolysate. J. Agric. Food Chem. 2021, 69, 11246–11258. [Google Scholar] [CrossRef]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- Amundarain, M.J.; Ribeiro, R.P.; Costabel, M.D.; Giorgetti, A. GABAA receptor family: Overview on structural characterization. Future Med. Chem. 2019, 11, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Löw, K.; Crestani, F.; Keist, R.; Benke, D.; Brünig, I.; Benson, J.A.; Fritschy, J.M.; Rülicke, T.; Bluethmann, H.; Möhler, H.; et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 2000, 290, 131–134. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Kozuska, J.L.; Paulsen, I.M.; Dunn, S.M.J. Benzodiazepine modulation of the rat GABAA receptor α4β3γ2L subtype expressed in Xenopus oocytes. Neuropharmacology 2010, 59, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, T.; Sawashi, Y.; Yamada, A.; Kanamoto, R.; Ohinata, K. Characterization of Tyr-Leu-Gly, a novel anxiolytic-like peptide released from bovine αS-casein. FASEB J. 2013, 27, 2911–2917. [Google Scholar] [CrossRef]
- Vonderviszt, F.; Mátrai, G.; Simon, I. Characteristic sequential residue environment of amino acids in proteins. Int. J. Pept. Protein Res. 1986, 27, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Kanegawa, N.; Suzuki, C.; Ohinata, K. Dipeptide Tyr-Leu (YL) exhibits anxiolytic-like activity after oral administration via activating serotonin 5-HT1A, dopamine D1 and GABAA receptors in mice. FEBS Lett. 2010, 584, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.V.; Dawson, G.R.; Reynolds, D.S.; Atack, J.R.; Stephens, D.N. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur. J. Neurosci. 2006, 23, 2495–2504. [Google Scholar] [CrossRef]
- Rudolph, U.; Crestani, F.; Benke, D.; Brünig, I.; Benson, J.A.; Fritschy, J.M.; Martin, J.R.; Bluethmann, H.; Möhler, H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 1999, 401, 796–800. [Google Scholar] [CrossRef]
- Scott, S.; Aricescu, A.R. A structural perspective on GABAA receptor pharmacology. Curr. Opin. Pharmacol. 2019, 54, 189–197. [Google Scholar] [CrossRef]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Rasoel, S.S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef]
- Sieghart, W.; Savić, M.M. International Union of Basic and Clinical Pharmacology. CVI: GABA A receptor subtype- and function-selective ligands: Key issues in translation to humans. Pharmacol. Rev. 2018, 70, 836–878. [Google Scholar] [CrossRef] [Green Version]

| Antagonist | Test Product | Initial n | Final n | |||
|---|---|---|---|---|---|---|
| Group Name | Dose, Administration | Time before Test | Dose, Administration | Time before Test | ||
| Vhl/Vhl | Vehicle 2 (2 mL/kg, i.p.) | 80 min | Vehicle 1 (5 mL/kg, p.o.) | 60 min | 16 | 12 |
| Vhl/Flu | Flumazenil (10 mg/kg, i.p.) | 80 min | Vehicle 1 (5 mL/kg, p.o.) | 60 min | 16 | 15 |
| CH/Vhl | Vehicle 2 (2 mL/kg, i.p.) | 80 min | CH (15 mg/kg, p.o.) | 60 min | 16 | 11 |
| CH/Flu | Flumazenil (10 mg/kg, i.p.) | 80 min | CH (15 mg/kg, p.o.) | 60 min | 16 | 13 |
| Dzp/Vhl | Vehicle 2 (2 mL/kg, i.p.) | 80 min | Diazepam (3 mg/kg, p.o.) | 60 min | 16 | 13 |
| Dzp/Flu | Flumazenil (10 mg/kg, i.p.) | 80 min | Diazepam (3 mg/kg, p.o.) | 60 min | 16 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benoit, S.; Chaumontet, C.; Violle, N.; Boulier, A.; Hafeez, Z.; Cakir-Kiefer, C.; Tomé, D.; Schwarz, J.; Miclo, L. The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve. Nutrients 2022, 14, 2212. https://doi.org/10.3390/nu14112212
Benoit S, Chaumontet C, Violle N, Boulier A, Hafeez Z, Cakir-Kiefer C, Tomé D, Schwarz J, Miclo L. The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve. Nutrients. 2022; 14(11):2212. https://doi.org/10.3390/nu14112212
Chicago/Turabian StyleBenoit, Simon, Catherine Chaumontet, Nicolas Violle, Audrey Boulier, Zeeshan Hafeez, Céline Cakir-Kiefer, Daniel Tomé, Jessica Schwarz, and Laurent Miclo. 2022. "The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve" Nutrients 14, no. 11: 2212. https://doi.org/10.3390/nu14112212
APA StyleBenoit, S., Chaumontet, C., Violle, N., Boulier, A., Hafeez, Z., Cakir-Kiefer, C., Tomé, D., Schwarz, J., & Miclo, L. (2022). The Anxiolytic-like Properties of a Tryptic Hydrolysate of Bovine αs1 Casein Containing α-Casozepine Rely on GABAA Receptor Benzodiazepine Binding Sites but Not the Vagus Nerve. Nutrients, 14(11), 2212. https://doi.org/10.3390/nu14112212