The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
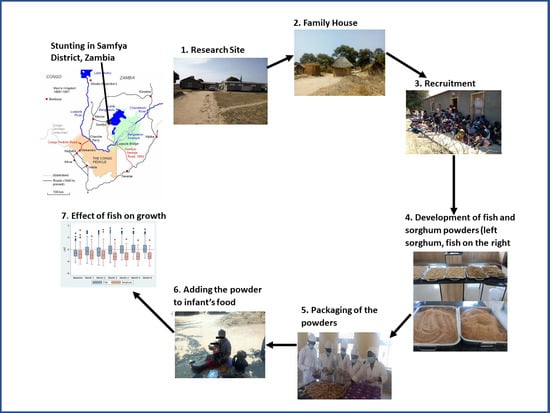
2.1. Study Area
2.2. Participants and Study Design
2.3. Sample Size Determination
2.4. Participant Recruitment
2.5. Preparation of the Fish and Sorghum Powders
2.6. Nutrient Content of the Fish and Sorghum Powder
2.7. Pilot Study
2.8. Measurements
2.8.1. Socio-Demographic Information
2.8.2. Anthropometry
2.8.3. Dietary Data
2.8.4. Skin Prick Test (SPT)
Procedure
2.8.5. Adherence to the Intervention
2.9. Statistical Analysis
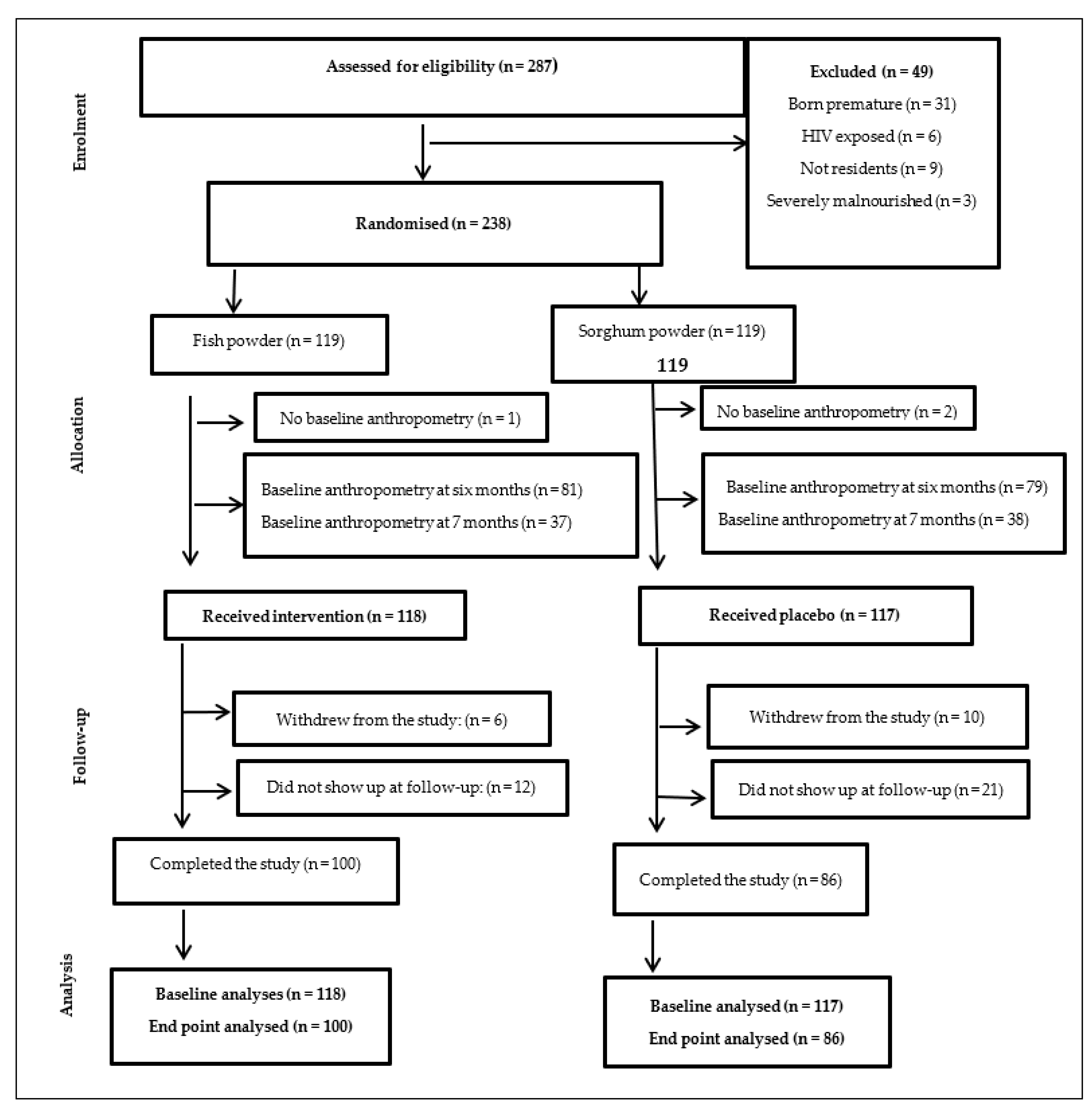
3. Results
3.1. Baseline Characteristics of the Study Population
3.1.1. Baseline Characteristics of Participating Infants and Their Mothers by Group
3.1.2. Socio-Economic Characteristics of Mothers of Infants in the Study
3.2. The Effect of the Fish Powder on the Growth of Infants
Infants Growth for the Study Duration and the Effect of the Intervention
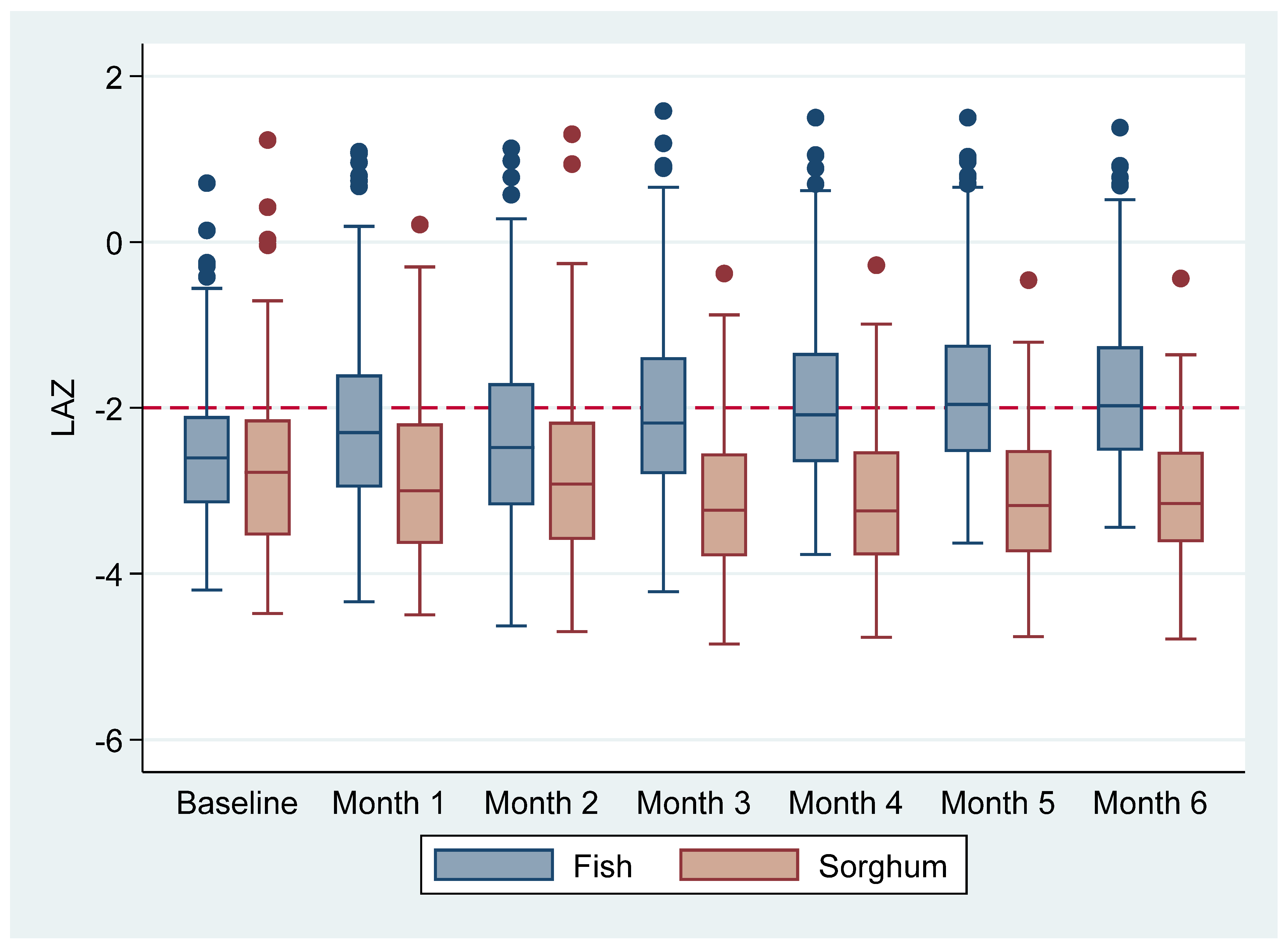
Length for-Age z-Scores
3.3. Morbidity Reported in the Infants during the Six-Month Intervention Period
3.4. Dietary Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, A.L.; Sacco, L.; Hyder, A.; Black, R.E. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 2000, 78, 1207–1221. [Google Scholar] [PubMed]
- Yohannes, T.; Laelago, T.; Ayele, M.; Tamrat, T. Mortality and morbidity trends and predictors of mortality in under-five children with severe acute malnutrition in Hadiya zone, South Ethiopia: A four-year retrospective review of hospital-based records (2012–2015). BMC Nutr. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- UNICEF/WHO/World Bank Group. Levels and Trends in Child Malnutrition. Key Findings of the 2018 Edition. 2018, pp. 1–16. Available online: http://www.who.int/nutgrowthdb/jme_brochoure2017.pdf?ua=1 (accessed on 2 February 2018).
- de Onis, M.; Martines, J.; Onyango, A.; Pinol, A.; World Health Organization; Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO: Geneva, Switzerland, 2006; p. 339.
- de Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern Child Nutr. 2016, 12, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.; Onyango, A. Contextualising complementary feeding in a broader framework for stunting prevention. Matern Child Nutr. 2013, 9, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Begum, K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011, 7, 5–18. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund. Causes and Consequences of Stunting: An Opportunity to Improve Health and Economic Development; United Nations Children’s Fund: New York, NY, USA, 2012; pp. 8–11. [Google Scholar]
- Oot, L.; Sommerfelt, A.E.; Sethuraman, K.; Ross, J. The Effect of Chronic Malnutrition (Stunting) on Learning Ability, a Measure of Human Capital: A Model in PROFILES for Country-Level Advocacy; Food and Nutrition Technical Assistance (FANTA) III Project: Washington, DC, USA, 2016. [Google Scholar]
- Akombi, B.J.; Agho, K.E.; Hall, J.J.; Wali, N.; Renzaho, A.M.N.; Merom, D. Stunting, wasting and underweight in Sub-Saharan Africa: A systematic review. Int. J. Environ. Res. Public Health 2017, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Keino, S.; Plasqui, G.; Ettyang, G.; Van Den Borne, B. Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr. Bull. 2014, 35, 167–178. [Google Scholar] [CrossRef]
- De Onis, M.; Dewey, K.G.; Borghi, E.; Onyango, A.W.; Blössner, M.; Daelmans, B.; Piwoz, E.; Branca, F. The world health organization’s global target for reducing childhood stunting by 2025: Rationale and proposed actions. Matern Child Nutr. 2013, 9, 6–26. [Google Scholar] [CrossRef]
- Weise, A. Global Nutrition Targets 2025: Low Birth Weight Policy Brief; World Health Organisation: Geneva, Switzerland, 2012; pp. 1–7. Available online: https://www.who.int/nutrition/publications/globaltargets2025_policybrief_lbw/en/ (accessed on 2 February 2018).
- Zambia Statistics Agency Ministry of Health (MOH) Zambia; ICF. Zambia Demographic and Health Survey 2018; Zambia Statistics Agency Ministry of Health (MOH) Zambia: Lusaka, Zambia; ICF: Rockville, MD, USA, 2019.
- Phiri, B.E. “When Children Have Children”—A Documentary by the Civil Society Scaling-Up Nutrition on Mulnutrition in Samfya District; Zambia National Broadcasting Corporation: Lusaka, Zambia, 2015; p. 2. [Google Scholar]
- World Health Organization, Complementary Feeding: Report of the Global Consultation, Summary of Guiding Principles for Complementary Feeding of the Breastfed Child; World Health Organization: Geneva, Switzerland, 2001. [CrossRef]
- Dewey, K.; Lutter, C.; Martines, J.; Daelmans, B. Guiding Principles for Complementary Feeding of the Breastfed (PAHO and WHO); Pan American Health Organization; World Health Organization: Washington, DC, USA, 2001.
- Allen, L.H. Global dietary patterns and diets in childhood: Implications for health outcomes. Ann. Nutr. Metab. 2012, 61, 29–37. [Google Scholar] [CrossRef]
- Skau, J.K.H.; Touch, B.; Chhoun, C.; Chea, M.; Unni, U.S.; Makurat, J.; Filteau, S.; Wieringa, F.T.; Dijkhuizen, M.A.; Ritz, C.; et al. Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: A randomized trial in Cambodia. Am. J. Clin. Nutr. 2015, 101, 742–751. [Google Scholar] [CrossRef]
- Haug, A.; Christophersen, O.A.; Kinabo, J.; Kaunda, W.; Eik, L.O. Use of dried kapenta (Limnothrissa miodon and Stolothrissa tanganicae) and other products based on whole fish for complementing maize-based diets. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2478–2500. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Konyole, S.O.; Onyango-Omolo, S.A.; Kenji, G.M.; Onyango, C.A.; Owino, V.O.; Owuor, B.O.; Estambale, B.B.; Roos, N. Nutrients, functional properties, storage stability and costing of complementary foods enriched with either termites and fish or commercial micronutrients. J. Insects Food Feed. 2015, 1, 149–158. Available online: http://www.wageningenacademic.com/doi/10.3920/JIFF2014.0011 (accessed on 2 February 2018). [CrossRef]
- Bogard, J.R.; Hother, A.L.; Saha, M.; Bose, S.; Kabir, H.; Marks, G.C.; Thilsted, S.H. Inclusion of small indigenous fish improves nutritional quality during the first 1000 days. Food Nutr. Bull. 2015, 36, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Longley, C.; Thilsted, S.H.; Beveridge, M.; Cole, S.; Nyirenda, D.B.; Heck, S.; Hother, A.L. The role of fish in the first 1000 days in Zambia. Inst. Dev. Stud. 2014, 27–35. [Google Scholar]
- Coetzee, O.; Green, R.J.; Masekela, R. A guide to performing skin-prick testing in practice: Tips and tricks of the trade. South Afr. Fam. Pract. 2013, 55, 415–419. [Google Scholar] [CrossRef][Green Version]
- United States Department of Agriculture. Nutritional Needs of Infants Dietary Reference Intakes (DRIs). Infant Nutrition and Feeding. 2009; pp. 11–40. Available online: http://www.nal.usda.gov/wicworks/Topics/FG/Chapter1_NutritionalNeeds.pdf (accessed on 12 February 2018).
- Institute of Medicine of the National Academies. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; Otten, J.J., Hellwig, J.P., Meyers, L.D., Eds.; The National Academies Press: Washington, DC, USA, 2006; Volume 55, 414p. [Google Scholar]
- Food and Nutrition Board; Institute of Medicine, N.A. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances; Food and Nutrition Board: Delhi, India, 2011; pp. 1–8. [Google Scholar] [CrossRef]
- National Food and Nutrition Commission, Republic of Zambia, Zambia Food Composition Tables, 4th ed.; National Food and Nutrition Commision: Lusaka, Zambia, 2009; p. 96p.
- Williams, J.; Walton, S.; Wells, J. Life Study Standard Operating Procedures: Infant Anthropometry. 2016, pp. 5–27. Available online: https://discovery.ucl.ac.uk/id/eprint/1485685/9/Life%20Study%20Infant%20Anthropometry%20Standard%20Operating%20Procedures%201485685.pdf (accessed on 16 February 2018).
- Cape Town Metropole Paediatric Interest Group. Anthropometriy Guidelines Paediatrics. Screening. South Africa. 2009, pp. 19–68. Available online: https://pdf4pro.com/view/anthropometry-guideline-paediatrics-adsa-75db.html (accessed on 28 January 2018).
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Rosemary, K. A Skin Prick Test (SPT) Standard Operating Procedures. 2010, pp. 1–4. Available online: htts://www.bsaci.org/_literature_121183/Paediatric_ski_testing_guidelines (accessed on 22 January 2018).
- Addo, O.Y.; Stein, A.D.; Fall, C.H.; Gigante, D.P.; Guntupalli, A.M.; Horta, B.L.; Kuzawa, C.W.; Lee, N.; Norris, S.A.; Prabhakaran, P.; et al. Maternal height and child growth patterns. J. Pediatr. 2013, 163, 549–554. [Google Scholar] [CrossRef]
- World Health Organisation. Simplified Field Tables Height-for-age GIRLS 5 to 19 Years (z-Scores); World Health Organization: Geneva, Switzerland, 2007; pp. 1–6. Available online: https://www.who.int/growthref/sft_hfa_girls_z_5_19years.pdf (accessed on 10 February 2020).
- Ochoa, T.J.; Baiocchi, N.; Valdiviezo, G.; Bullon, V.; Campos, M.; Llanos-cuentas, A. Evaluation of the efficacy, safety and acceptability of a fish protein isolate in the nutrition of children under 36 months of age. Public Health Nutr. 2017, 20, 2819–2826. [Google Scholar] [CrossRef][Green Version]
- Rahayu, A.; Yulidasari, F.; Putri, A.O.; Laily, N. Analysis of fish derived protein intake adequacy and cultural factors and its correlation with the occurrence of stunting among children under two years old in martapura riverbanks, Banjar district area. Indian J. Public Health Res. Dev. 2019, 10, 1987–1991. [Google Scholar] [CrossRef]
- Kaimila, Y.; Divala, O.; Agapova, S.E.; Stephenson, K.B.; Thakwalakwa, C.; Trehan, I.; Manary, M.J.; Maleta, K.M. Consumption of animal-source protein is associated with improved height-for-age Z scores in rural malawian children aged 12–36 months. Nutrients 2019, 11, 480. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Lutter, C.K.; Stewart, C.P.; Gallegos Riofrío, C.A.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs in Early Complementary Feeding and Child Growth: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163459. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Caswell, B.; Iannotti, L.; Lutter, C.; Arnold, C.D.; Chipatala, R. The effect of eggs on early child growth in rural Malawi: The Mazira Project randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Domínguez Salas, P.; Alonso, S.; Lannerstad, M.; Muunda, E.M.; Ngwili, N.M.; Omar, A.; Khan, M.; Otobo, E. The Influence of Livestock-Derived Foods on Nutrition during the First 1000 Days of Life Agriculture for Life; ILRI Research Report 44; ILRI: Nairobi, Kenya, 2018. [Google Scholar]
- Delimont, N.M.; Haub, M.D.; Lindshield, B.L. The impact of tannin consumption on iron bioavailability and status: A narrative review. Curr. Dev. Nutr. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; Joshi, N.; Lohani, M.; Singh, R.; Bhatta, N. Head growth of undernourished children in rural Nepal: Association with demographics, health and diet. Paediatr. Int. Child Health 2016, 36, 91–109. [Google Scholar] [CrossRef]
- Herber, C.; Bogler, L.; Subramanian, S.V.; Vollmer, S. Association between milk consumption and child growth for children aged 6–59 months. Sci. Rep. 2020, 10, 2–10. [Google Scholar] [CrossRef]
- Kuehn, A.; Swoboda, I.; Arumugam, K.; Hilger, C.; Hentges, F. Fish allergens at a glance: Variable allergenicity of parvalbumins, the major fish allergens. Front. Immunol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Kang, H.; Kreuels, B.; Adjei, O.; Krumkamp, R.; Small, D.S. The causal effect of malaria on stunting: A Mendelian randomization and matching approach. Int. J. Epidemiol. 2013, 42, 1390–1398. [Google Scholar] [CrossRef]
- Grillenberger, M.; Neumann, C.G.; Murphy, S.P.; Bwibo, N.O.; Weiss, R.E.; Jiang, L.; Hautvast, J.G.; West, C.E. Intake of micronutrients high in animal-source foods is associated with better growth in rural Kenyan school children. Br. J. Nutr. 2006, 95, 379–390. [Google Scholar] [CrossRef]
- Krebs, N.F.; Mazariegos, M.; Tshefu, A.; Bose, C.; Sami, N.; Chomba, E.; Carlo, W.; Goco, N.; Kindem, M.; Wright, L.L.; et al. Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr. Bull. 2011, 32, 185–191. [Google Scholar] [CrossRef]
- Tang, M. The impact of complementary feeding foods of animal origin on growth and the risk of overweight in infants. Anim. Front. 2019, 9, 5–11. [Google Scholar] [CrossRef]
| Enrolment Group | Year/Month of Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2020 | |
| April | May | June | July | Aug | Sep | Oct | Nov | Dec | Jan | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| 1 (n = 78) | (Baseline) * | Baseline | Exit | |||||||
| 2 (n = 47) | Baseline | Exit | ||||||||
| 3 (n = 51) | Baseline | Exit | ||||||||
| 4 (n = 62) | Baseline | Exit | ||||||||
| Total (n = 238) | ||||||||||
| Nutrients | RDA/AI for 6–12 Month Infants † | Nutrients per 12 g Fish Powder ‡ | % of RDA (Fish Powder) ¥ | Nutrients per 7 g Sorghum Powder | % of RDA (Sorghum Powder) |
|---|---|---|---|---|---|
| Energy (kcal) | 25 | - | 25 | - | |
| 6–12 months | |||||
| Protein (g) | 11.0 | 7.6 | 69.0 | 0.9 | 10.0 |
| Fat (g) | 30.0 | 1.1 | 4.0 | 0.2 | 0.6 |
| Carbohydrates (g) | 95.0 | - | 0.0 | 5.0 | 5.3 |
| EPA3 (g) * | 0.5 | 0.1 | 20.0 | - | - |
| DHA3 (g) ** | 0.5 | 0.1 | 20.0 | - | - |
| Calcium (mg) | 260.0 | 260.0 | 100.0 | 1.4 | 0.5 |
| Iron (mg) | 11.0 | 1.0 | 9.0 | 0.3 | 0.0 |
| Vitamin A (ug) | 500.0 | 34.0 | 13.0 | - | - |
| Nicotinic acid (mg) | 4.0 | 0.7 | 17.5 | 0.2 | 5.0 |
| Zinc (mg) | 3.0 | 1.6 | 52.0 | 0.2 | 7.0 |
| Fish Powder (n = 118) | Sorghum Powder (n = 117) | |||
|---|---|---|---|---|
| Characteristic | n (%) or Mean ± SD | Range | n (%) or Mean ± SD | Range |
| Infants | ||||
| Gender | ||||
| Male | 63 (53.4) | - | 62 (53.0) | - |
| Female | 55 (46.6) | - | 55 (47.0) | - |
| Gestation age (weeks) | 38.4 ± 1.1 | 37.0–44.0 | 38.2 ± 1.0 | 36.0–41.0 |
| Number of low-birth-weight infants § | 1 (0.9) | - | 1 (0.9) | - |
| Age (months) | 6.6 ± 0.5 | 5.9–7.9 | 6.6 ± 0.5 | 6.0–7.9 |
| Infant still being breastfed | 118 (100) | - | 117 (100) | - |
| Age of solid food introduction (months) | 5.2 ± 0.5 | 3.06.0 | 5.1 ± 0.6 | 1.0–6.0 |
| Anthropometry | ||||
| Birth weight (kg) | 3.2 ± 0.4 | 2.1–4.6 | 3.1 ± 0.4 | 2.4–4.0 |
| Weight (kg) | 6.9 ± 0.9 | 5.7–9.9 | 6.7 ± 0.7 | 5.2–9.6 |
| Length (cm) | 62.3 ± 2.7 | 58.6–72 | 61.5 ± 1.7 | 57.3–66.5 |
| Head circumference (cm) | 41.4 ± 1.8 | 39–45.1 | 44.3 ± 34.9 | 39.3–44.9 |
| Mid upper arm circumference (cm) | 13.2 ± 0.6 | 12.5–14.9 | 13.1 ± 0.6 | 11.5–15.5 |
| Anthropometry classifications | ||||
| Stunting † | 88 (74.6) | 96 (82.1) | ||
| Underweight ‡ | 23 (19.5) | - | 27 (23.1) | - |
| Wasting ֏ | 0 (0.0) | 0 (0.0) | - | |
| Overweight | 11 (9.3) | - | 9 (7.7) | - |
| Length-for-age z-score (LAZ) | −2.51 ± 1.12 | −4.4–1.2 | −2.71 ± 0.87 | −4.5–−0.7 |
| Weight-for-age z-score (WAZ) | −1.1 ± 1.0 | −2.7–1.9 | −1.4 ± 0.9 | −3.0–1.2 |
| Weight-for-length z-score (WLZ) | 0.7 ± 0.8 | −1.0–4.4 | 0.6 ± 0.9 | −1.8–4.4 |
| Head-circumference-for-age z-score (HCZ) | −1.4 ± 1.1 | −1.4–1.1 | −1.7 ± 1.0 | −3.4–1.1 |
| Mothers | 118.0 (50.4) | - | 116.0 (49.6) | - |
| Age (years) | 28.71 ± 6.7 | 18.1–45.8 | 29.5 ± 6.3 | 17.4–42.6 |
| Anthropometry | ||||
| Weight (kg) | 49.9 ± 3.6 | 40.5–61.2 | 49.7 ± 3.6 | 39.5–63.0 |
| Height (m) | 1.5 ± 0.1 | 1.4–1.6 | 1.5 ± 0.0 | 1.4–1.7 |
| BMI (kg/m2) ¥ | 22.6 ± 1.5 | 18.1–26.7 | 22.5 ± 1.5 | 17.9–26.9 |
| Maternal stunting (%) † | 71 (60.2) | - | 70 (60.3) | - |
| Number of births | 3.3 ± 1.9 | 1.0–8.0 | 3.6 ± 1.8 | 1.0–8.0 |
| Family size | 5.4 ± 1.9 | 3.0–10.0 | 5.6 ± 1.9 | 3.0–10.0 |
| Marital status | ||||
| Single | 5.0 (4.0) | - | 3.0 (2.6) | - |
| Married | 110.0 (93.0) | - | 113.0 (97.4) | - |
| Divorced | 3.0 (3.0) | - | 0.0 (0) | - |
| Education level | - | - | ||
| No formal education | 18.0 (15.3) | - | 12.0 (10.4) | - |
| Primary level | 64.0 (54.2) | - | 85.0 (73.2) | - |
| Secondary level | 36.0 (30.5) | - | 19.0 (16.4) | - |
| Fish Powder (n = 118) | Sorghum Powder (n = 116) ‡ | |
|---|---|---|
| Characteristic | n (%) | n (%) |
| Occupation | ||
| Housewife | 112.0 (94.9) | 109.0 (93.9) |
| Farmer | 0.0 (0.0) | 3.0 (2.6) |
| Own business | 2.0 (1.7) | 1.0 (0.9) |
| Formal employment | 1.0 (0.8) | 0 |
| Stays with parents | 3.0 (2.5) | 3.0 (2.6) |
| Source of income | ||
| Salaried job | 0.0 (0) | 3.0 (2.6) |
| Husband | 109.0 (92.4) | 108.0 (93.1) |
| Own business | 5.0 (4.2) | 2.0 (1.7) |
| Parents | 4.0 (3.4) | 3.0 (2.3) |
| Monthly Income | ||
| Below K500 ($50) | 117.0 (99.2) | 114.0 (98.3) |
| K500–K99 ($50–$99) | 1.0 (0.8) | 2.0 (1.7) |
| Type of fuel | ||
| Electricity | 0.0 (0) | 1.0 (0.9) |
| Solar | 11.0 (9.0) | 8.0 (6.9) |
| Charcoal | 1.0 (1.0) | 0.0 (0.0) |
| Firewood | 106.0 (90.0) | 107.0 (92.2) |
| Domestic animal ownership | ||
| Chickens | 38.0 (32.2) | 46 (39.7) |
| Goats | 10.0 (8.5) | 10 (8.6) |
| Pigs | 2.0 (1.7) | 2 (1.7) |
| None | 68.0 (57.6) | 58 (50.0) |
| Garden ownership | ||
| Green leafy vegetables | 4.0 (3.4) | 3 (2.6) |
| Red vegetables(e.g., tomatoes) | 1.0 (0.9) | 0 (0.0) |
| None | 113.0 (95.7) | 113 (97.4) |
| Source of drinking water | ||
| Mono pump | 34.0 (28.8) | 29.0 (25.0) |
| Shallow well | 83.0 (70.3) | 86.0 (74.1) |
| Lake Bangweulu | 1.0 (0.9) | 1.0 (0.9) |
| Type of toilet | ||
| Pit latrine | 118.0 (100.0) | 117.0 (100.0) |
| Fish Powder | Sorghum Powder | Intervention Effect ‡ | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 118) | End Point (n = 100) | Baseline (n = 117) | End Point (n = 86) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean Difference2 (95% CI) | p-Value | |
| n (%) | n (%) | n (%) | n (%) | |||
| LAZ † | −2.51 ± 1.12 | −1.76 ± 1.09 | −2.71 ± 0.87 | −3.12 ± 0.84 | 1.26 (0.94–1.57) | <0.001 |
| WAZ | −1.09 ± 1.06 | 0.10 ± 0.66 | −1.25 ± 0.89 | −0.95 ± 0.68 | 0.95 (0.6–1.23) | <0.001 |
| Stunting | 88 (74.6) | 49 (49) | 96 (82.1) | 78 (90.1) | ||
| Fish Powder | Sorghum Powder | Intervention Effect † | ||||
|---|---|---|---|---|---|---|
| Month of Follow-Up | n | LAZ Mean ± SD | n | LAZ Mean ± SD | Mean Difference ‡ 95% CI | p-Value |
| Follow-up month 1 | 115 | −2.13 ± 1.18 | 111 | −2.93 ± 0.93 | 0.63 (0.40–0.87) | <0.001 |
| Follow-up month 2 | 112 | −2.35 ± 1.17 | 110 | −2.79 ± 1.13 | 0.26 (0.02–0.50) | 0.032 |
| Follow-up month 3 | 110 | −1.99 ± 1.16 | 104 | −3.16 ± 0.90 | 1.02 (0.77–1.28) | <0.001 |
| Follow-up month 4 | 108 | −1.88 ± 1.11 | 100 | −3.15 ±.0.87 | 1.14 (0.87–1.41) | <0.001 |
| Follow-up month 5 | 103 | −1.78 ± 1.11 | 90 | −3.13 ± 0.85 | 1.24 (0.95–1.53) | <0.001 |
| Follow-up month 6 | 100 | −1.76 ± 1.09 | 86 | −3.12 ± 0.84 | 1.26 (0.94–1.58) | <0.001 |
| Fish Powder | Sorghum Powder | Intervention Effect † | ||||
|---|---|---|---|---|---|---|
| Month of Follow-Up | n | LAZ Mean ± SD | n | LAZ Mean ± SD | Mean Difference ‡ 95% CL | p-Value |
| Follow-up month 1 | 78 | −2.37 ± 0.96 | 73 | −2.84 ± 0.92 | 0.47 (0.19–0.76) | <0.001 |
| Follow-up month 2 | 75 | −2.56 ± 1.06 | 72 | −2.72 ± 1.19 | 0.16 (−0.12–0.46) | 0.268 |
| Follow-up month 3 | 74 | −2.22 ± 0.93 | 66 | −3.02 ± 0.87 | 0.84 (0.53–1.16) | <0.001 |
| Follow-up month 4 | 72 | −2.12 ± 0.87 | 64 | −3.05 ± 0.88 | 0.98 (0.65–1.31) | <0.001 |
| Follow-up month 5 | 69 | −2.06 ± 0.85 | 58 | −3.04 ± 0.84 | 1.07 (0.72–1.42) | <0.001 |
| Follow-up month 6 | 66 | −2.04 ± 0.83 | 57 | −3.06 ± 0.84 | 1.09 (0.71–1.47) | <0.001 |
| Fish Powder (n = 118) | Sorghum Powder (n = 117) | Total (n = 235) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Total number of illnesses | 53 (22.6) | ||
| Type of illnesses † | |||
| Diarrhoea | 22 (18.6) | 29 (25.6) | 51 (21.7) |
| Vomiting | 12 (10.2) | 12 (10.3) | 24 (10.2) |
| Coughing | 8 (6.7) | 16 (13.7) | 24(10.2) |
| Malaria | 3 (2.5) | 15 (12.8) | 18 (7.7) |
| Skin rash | 0 (0) | 2 (1.7) | 2 (0.9) |
| Total number of illnesses | 25 (21.2) | 28 (23.9) | 53 (22.6) |
| Had no illness | 93 (78.8) | 89 (76.1) | 182 (77.4) |
| Fish Powder Group | Sorghum Powder Group | |
|---|---|---|
| Variable | n (%) | n (%) |
| Baseline | 118 | 117 (100) |
| Consumption of fish | 0 (0.0) | 0 (0.0) |
| Consumption of meat and poultry | 1 (0.8) | 1 (0.9) |
| Consumption of dairy | 1 (0.8) | 1 (0.9) |
| Dietary follow-up month 2 | 112 | 110 |
| Consumption of fish | 112 (100) | 9 (8.2) |
| Consumption of meat and poultry | 3 (2.7) | 1 (0.9) |
| Consumption of dairy | 1 (0.9) | 0 (0.0) |
| Dietary follow-up month 4 | 108 | 100 |
| Consumption of fish | 108 (100) | 3 (3.0) |
| Consumption of meat and poultry | 5 (4.6) | 2 (2.0) |
| Consumption of dairy | 0 | 0 |
| Dietary follow-up month 6 | 100 | 86 |
| Consumption of fish | 100 (100) | 9 (10.5) |
| Consumption of meat and poultry | 6 (6.0) | 3 (3.5) |
| Consumption of dairy | 0 (0.0) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chipili, G.; Van Graan, A.; Lombard, C.J.; Van Niekerk, E. The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial. Nutrients 2022, 14, 2191. https://doi.org/10.3390/nu14112191
Chipili G, Van Graan A, Lombard CJ, Van Niekerk E. The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial. Nutrients. 2022; 14(11):2191. https://doi.org/10.3390/nu14112191
Chicago/Turabian StyleChipili, Given, Averalda Van Graan, Carl J. Lombard, and Evette Van Niekerk. 2022. "The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial" Nutrients 14, no. 11: 2191. https://doi.org/10.3390/nu14112191
APA StyleChipili, G., Van Graan, A., Lombard, C. J., & Van Niekerk, E. (2022). The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial. Nutrients, 14(11), 2191. https://doi.org/10.3390/nu14112191