Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review
Abstract
1. Introduction
1.1. The Microbiome–Gut–Brain Axis (MGBA)
1.2. Correlation between Gut Microbiome and Mental Disorders
1.3. The Beneficial Effect of Gut Microbiome Modulation on Mental Disorders
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
3. Results and Discussion
- In vitro studies
- Of the 16 in vitro studies that met the inclusion criteria, 12 were performed with colon microorganisms from human fecal samples. Nine of these twelve studies used single fecal samples from either one or several donors, and the remaining three used pooled fecal samples. In the four nonhuman studies, three used fecal samples from different experimental animals (rat, mouse, dog), and one study applied a set of single microbial strains representing major intestinal genera [88].
- A total of 14 of the 16 studies used simple static batch fermentations, preceded in 4 cases by static simulation of upper GI tract digestion [66,168,191,201]. Another two studies applied more sophisticated dynamic digestion models with sequential upper intestinal tract digestion and colonic fermentation [182,193].
- Nine of the sixteen in vitro studies assessed both the microbial composition and metabolite changes during incubation with a herbal material. Of the remaining seven, three assessed only microbiome changes, and four investigated only metabolite profile changes during incubation.
- The metabolites most often studied in vitro were the SCFAs formed by gut microbial metabolization of plant polysaccharides, followed by metabolites derived from polyphenols and triterpenes.
- Microbial community composition changes were most frequently monitored by 16S rRNA gene sequencing (six studies), fluorescence in situ hybridization (FISH) (four studies), or qPCR (three studies). The study with single strains used cultivation-based agar dilution.
- In vivo studies
- Of the 69 in vivo studies that met the inclusion criteria, 11 were clinical, and 58 involved various experimental animal species (34 in mice, 15 in rats, 5 in pigs, and 1 each in rabbits, dogs, C. elegans, and Drosophila).
- The human studies enrolled comparatively small participant numbers, with intervention group sizes ranging from 6 to 38. Different intervention groups (i.e., placebo vs. treatment or different treatments) were compared in only three of these studies, whereas the remaining eight assessed different treatments in a crossover design or compared the effect of a certain treatment on gut microbiota or metabolite profiles in samples taken before and after the intervention. In all studies, fecal samples were collected for assessment of fecal microbiota changes (seven studies), metabolite changes (two), or both (two). Ten of the studies enrolled healthy (in some cases overweight) patients, and one study enrolled participants with type 2 diabetes mellitus. This latter study assessed the effect of a herbal intervention on depression scores and on the GI tract microbiome composition [68], and thus is the only human study that directly investigated a correlation between a mental health condition and the gut microbial community composition.
- Most of the in vivo studies in experimental animals involved mice and rats. In general, the same bacterial phyla occur in rodents and humans, predominantly Bacteroidetes and Firmicutes. The Clostridium superfamily is also widespread in rats and humans, but there are marked differences in the abundance of important genera such as Lactobacillus and Bifidobacterium between humans and rodents [202,203].
- Of these 58 studies, 27 used healthy animals, and 31 relied on different disease models, most commonly obese animals and colitis induced by dextran sodium sulfate (DSS), along with models of diabetes mellitus type 2, hypercholesterolemia, nonalcoholic fatty liver disease, menopause, and colorectal cancer. In five of the studies, the effects of medicinal plants on the gut microbiota in animal models were assessed related to mental health disorders, such as depression-like behavior, anxiety- and depression-like behavior, and memory impairment [42,106,172,173,204]. Changes in the gut microbial community composition were investigated in 33 of these studies, metabolite changes in 4, and both metabolite and microbial community changes in 21, all with fecal samples from the living animals or fecal content or mucosa from different intestinal regions collected after sacrifice.
- The technique most widely used to assess microbiota changes in human and animal studies was 16S rRNA gene sequencing (applied in 43 studies). Other commonly used techniques were qPCR with primers targeting specific bacterial groups or genera, and cultivation-based methods (bacterial plate counting, agar dilution).
- The microbial metabolites most commonly studied were SCFAs, the microbial fermentation products of polysaccharides (determined in 23 in vivo studies). In some of the studies, microbial metabolites of secondary plant metabolites such as ginsenosides [148,150] or phenolic compounds [200] were investigated.
- In the following sections, we group the data on MGBA interactions of herbal drugs into the major secondary metabolites present in these plants.
3.1. Herbal Drugs Rich in Terpenoids
3.1.1. Herbal Drugs Containing Saponins
3.1.2. Essential Oils and Herbs Rich in Essential Oils
3.1.3. Herbal Drugs Containing Other Terpenoids
3.2. Herbal Drugs Rich in Phenolic Constituents
3.2.1. Herbal Drugs Containing Lignans
3.2.2. Herbal Drugs Containing Flavonoids
3.2.3. Herbal Drugs Containing Tannins
3.2.4. Herbal Drugs Containing Other Phenolic Compounds
3.3. Herbal Drugs Rich in Polysaccharides
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Wang, Y.; Wang, P.; Li, Y.; Li, B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr. Neuropharmacol. 2015, 13, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain-gut-microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef]
- Linneberg, A.; Nielsen, N.H.; Madsen, F.; Frølund, L.; Dirksen, A.; Jørgensen, T. Increasing prevalence of specific IgE to aeroallergens in an adult population: Two cross-sectional surveys 8 years apart: The Copenhagen Allergy Study. J. Allergy Clin. Immunol. 2000, 106, 247–252. [Google Scholar] [CrossRef]
- Campbell, A.W. Autoimmunity and the gut. Autoimmune Dis. 2014, 2014, 152428. [Google Scholar] [CrossRef]
- Broussard, J.L.; Devkota, S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Mol. Metab. 2016, 5, 737–742. [Google Scholar] [CrossRef]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut–brain axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Ajiwhen, I.O.; Bisong, S.A. Effect of ethanolic extract of Carpolobia lutea G. Don (polygalaceae) root on learning and memory in CD1 mice. Niger. J. Physiol. Sci. 2013, 28, 141–145. [Google Scholar]
- Zhang, Y.; Cheng, L.; Liu, Y.; Wu, Z.; Weng, P. The Intestinal Microbiota Links Tea Polyphenols with the Regulation of Mood and Sleep to Improve Immunity. Food Rev. Int. 2021, 1–14. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Baretta, I.P.; Felizardo, R.A.; Bimbato, V.F.; dos Santos, M.G.J.; Kassuya, C.A.L.; Junior, A.G.; Da Silva, C.R.; de Oliveira, S.M.; Ferreira, J.; Andreatini, R. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J. Ethnopharmacol. 2012, 140, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Mohan, M.; Kasture, S.; Foddis, C.; Frau, M.A.; Loi, M.C.; Maxia, A. Antidepressant activity of Ceratonia siliqua L. fruit extract, a source of polyphenols. Nat. Prod. Res. 2011, 25, 450–456. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides—Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef]
- Butler, M.I.; Sandhu, K.; Cryan, J.F.; Dinan, T.G. From isoniazid to psychobiotics: The gut microbiome as a new antidepressant target. Br. J. Hosp. Med. 2019, 80, 139–145. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kunugi, H. Gut Microbiota and Pathophysiology of Depressive Disorder. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 11–20. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, J.; Li, X.; Yuan, Y.; Zhang, L.; Hu, W.; Luo, H.; Yu, H.; Zhang, R. Antidepressant Effects of Rosemary Extracts Associate with Anti-inflammatory Effect and Rebalance of Gut Microbiota. Front. Pharmacol. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Ota, M.; Wakabayashi, C.; Noda, T.; Ozawa, H.; Okubo, T.; Kunugi, H. Effects of chronic l-theanine administration in patients with major depressive disorder: An open-label study. Acta Neuropsychiatr. 2017, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- McKernan, D.P.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F. The probiotic Bifidobacterium infantis 35,624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010, 22, 1029-e268. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Phyu, M.P.; Tangpong, J. Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. Biomed Res. Int. 2013, 2013, 186098. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.-L.; Tan, Y.-F.; Li, Y.-H.; Zeng, N.-K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef]
- Adeyemi, O.O.; Akindele, A.J.; Yemitan, O.K.; Aigbe, F.R.; Fagbo, F.I. Anticonvulsant, anxiolytic and sedative activities of the aqueous root extract of Securidaca longepedunculata Fresen. J. Ethnopharmacol. 2010, 130, 191–195. [Google Scholar] [CrossRef]
- Dhama, K.; Tiwari, R.; Chakrabort, S.; Saminathan, M.; Kumar, A.; Karthik, K.; Wani, M.Y.; Amarpal; Singh, S.V.; Rahal, A. Evidence Based Antibacterial Potentials of Medicinal Plants and Herbs Countering Bacterial Pathogens Especially in the Era of Emerging Drug Resistance: An Integrated Update. Int. J. Pharmacol. 2013, 10, 1–43. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Efficacy and tolerability of minocycline for depression: A systematic review and meta-analysis of clinical trials. J. Affect. Disord. 2018, 227, 219–225. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Afrasiabian, F.; Mirabzadeh Ardakani, M.; Rahmani, K.; Azadi, N.A.; Alemohammad, Z.B.; Bidaki, R.; Karimi, M.; Emtiazy, M.; Hashempur, M.H. Aloysia citriodora Palau (lemon verbena) for insomnia patients: A randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother. Res. 2019, 33, 350–359. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Hidalgo-Garcia, L.; Garrido-Mesa, J.; Ruiz-Malagon, A.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Leyva-Jiménez, F.J.; et al. The Beneficial Effects of Lippia Citriodora Extract on Diet-Induced Obesity in Mice Are Associated with Modulation in the Gut Microbiota Composition. Mol. Nutr. Food Res. 2020, 64, e2000005. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Tan, S.-Y.; Mattes, R.D. Effects of almond consumption on the post-lunch dip and long-term cognitive function in energy-restricted overweight and obese adults. Br. J. Nutr. 2017, 117, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Li, Z.; Ortiz, R.M. Almond Snacking for 8 wk Increases Alpha-Diversity of the Gastrointestinal Microbiome and Decreases Bacteroides fragilis Abundance Compared with an Isocaloric Snack in College Freshmen. Curr. Dev. Nutr. 2019, 3, nzz079. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef]
- Liu, C.-H.; Tsai, C.-H.; Li, T.-C.; Yang, Y.-W.; Huang, W.-S.; Lu, M.-K.; Tseng, C.-H.; Huang, H.-C.; Chen, K.-F.; Hsu, T.-S.; et al. Effects of the traditional Chinese herb Astragalus membranaceus in patients with poststroke fatigue: A double-blind, randomized, controlled preliminary study. J. Ethnopharmacol. 2016, 194, 954–962. [Google Scholar] [CrossRef]
- Li, X.-Y.; Shen, L.; Ji, H.-F. Astragalus alters gut-microbiota composition in type 2 diabetes mice: Clues to its pharmacology. Diabetes Metab. Syndr. Obes. 2019, 12, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Watanabe, H.; Murayama, N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients 2019, 11, 1165. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.-S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lu, H.-F.; Chen, J.-C.; Huang, H.-C.; Chen, Y.-H.; Su, Y.-S.; Tung, C.-Y.; Huang, C. Purple-leaf tea (Camellia sinensis L.) ameliorates high-fat diet induced obesity and metabolic disorder through the modulation of the gut microbiota in mice. BMC Complement. Med. Ther. 2020, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Chen, Q.; Luo, L.; Ma, M.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana Ameliorate Intestinal Inflammation and Modulate Gut Microbiota in Dextran Sulfate Sodium-Induced Colitis Mice. Mol. Nutr. Food Res. 2020, 64, 1900943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, L.; Luo, Y.; Zhang, J.; Wang, X.; Sun, K.; Zeng, L. Prebiotic Properties of Green and Dark Tea Contribute to Protective Effects in Chemical-Induced Colitis in Mice: A Fecal Microbiota Transplantation Study. J. Agric. Food Chem. 2020, 68, 6368–6380. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur. J. Intern. Med. 2018, 49, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Assa-Glazer, T.; Gorelick, J.; Sela, N.; Nyska, A.; Bernstein, N.; Madar, Z. Cannabis Extracts Affected Metabolic Syndrome Parameters in Mice Fed High-Fat/Cholesterol Diet. Cannabis Cannabinoid Res. 2020, 5, 202–214. [Google Scholar] [CrossRef]
- Puttarak, P.; Dilokthornsakul, P.; Saokaew, S.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 10646. [Google Scholar] [CrossRef]
- Jana, U.; Sur, T.K.; Maity, L.N.; Debnath, P.K.; Bhattacharyya, D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Med. Coll. J. 2010, 12, 8–11. [Google Scholar] [PubMed]
- Li, H.; Chen, X.; Liu, J.; Chen, M.; Huang, M.; Huang, G.; Chen, X.; Du, Q.; Su, J.; Lin, R. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 2021, 267, 113445. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Iablokov, S.N.; Albayrak, L.; Khanipov, K.; Uchitel, S.; Chopra, D.; Mills, P.J.; Fofanov, Y.; Rodionov, D.A.; et al. 16S rRNA gene profiling and genome reconstruction reveal community metabolic interactions and prebiotic potential of medicinal herbs used in neurodegenerative disease and as nootropics. PLoS ONE 2019, 14, e0213869. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Calapai, F.; Cardia, L.; Inferrera, G.; D’Arena, G.; Di Pietro, M.; Navarra, M.; Gangemi, S.; Ventura Spagnolo, E.; Calapai, G. Clinical Pharmacology of Citrus aurantium and Citrus sinensis for the Treatment of Anxiety. Evid. Based Complement. Alternat. Med. 2018, 2018, 3624094. [Google Scholar] [CrossRef]
- Farshbaf-Khalili, A.; Kamalifard, M.; Namadian, M. Comparison of the effect of lavender and bitter orange on anxiety in postmenopausal women: A triple-blind, randomized, controlled clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Shabanian, G.; Rafieian-Kopaei, M.; Parvin, N.; Saadat, M.; Akhlaghi, M. Citrus aurantium blossom and preoperative anxiety. Rev. Bras. Anestesiol. 2011, 61, 702–712. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Wan, L.; Wang, T.-X.; Jiang, J.-G. Citrus aurantium L. var. amara Engl. inhibited lipid accumulation in 3T3-L1 cells and Caenorhabditis elegans and prevented obesity in high-fat diet-fed mice. Pharmacol. Res. 2019, 147, 104347. [Google Scholar] [CrossRef] [PubMed]
- Hawrelak, J.A.; Cattley, T.; Myers, S.P. Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern. Med. Rev. 2009, 14, 380–384. [Google Scholar]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron(®) a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016, 13, 195–199. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The Efficacy of Saffron in the Treatment of Mild to Moderate Depression: A Meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Tahmacebi-Pour, N.; Noorbala, A.-A.; Amini, H.; Fallah-Pour, H.; Jamshidi, A.-H.; Khani, M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005, 19, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amr, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondré-Lewis, M.C.; et al. Saffron: The Golden Spice with Therapeutic Properties on Digestive Diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef]
- Lee, M.-S.; Wahlqvist, M.L.; Chou, Y.-C.; Fang, W.-H.; Lee, J.-T.; Kuan, J.-C.; Liu, H.-Y.; Lu, T.-M.; Xiu, L.; Hsu, C.-C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Iablokov, S.N.; Pung, M.A.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Pak, S. Prebiotic Potential of Culinary Spices Used to Support Digestion and Bioabsorption. Evid.-Based Complement. Alternat. Med. 2019, 2019, 8973704. [Google Scholar] [CrossRef]
- Bhavanishankar, T.N.; Murthy, V. Composition of the caecal microflora, faecal bile acids and serum proteins of rats fed turmeric (Curcuma longa L.) and its alcoholic extract. Food Microbiol. 1986, 3, 337–343. [Google Scholar] [CrossRef]
- Tohda, C.; Yang, X.; Matsui, M.; Inada, Y.; Kadomoto, E.; Nakada, S.; Watari, H.; Shibahara, N. Diosgenin-Rich Yam Extract Enhances Cognitive Function: A Placebo-Controlled, Randomized, Double-Blind, Crossover Study of Healthy Adults. Nutrients 2017, 9, 1160. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Si, Y.; Cao, B.; Zhang, Y.; Zheng, X.; Feng, W. Integrated metabolomics and 16S rRNA sequencing to investigate the regulation of Chinese yam on antibiotic-induced intestinal dysbiosis in rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3382–3390. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G.; Brillante, R.; Bernardi, R.; Nascetti, S.; Gaddi, A. Effects of Siberian ginseng (Eleutherococcus senticosus maxim.) on elderly quality of life: A randomized clinical trial. Arch. Gerontol. Geriatr. Suppl. 2004, 38, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.J.; Bentler, S.; Noyes, R.; Hoehns, J.; Logemann, C.; Sinift, S.; Butani, Y.; Wang, W.; Brake, K.; Ernst, M.; et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol. Med. 2004, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G. Adaptogens in mental and behavioral disorders. Psychiatr. Clin. N. Am. 2013, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lauková, A.; Simonová, M.P.; Chrastinová, Ľ.; Plachá, I.; Čobanová, K.; Formelová, Z.; Chrenková, M.; Ondruška, L.; Strompfová, V. Benefits of combinative application of probiotic, enterocin M-producing strain Enterococcus faecium AL41 and Eleutherococcus senticosus in rabbits. Folia Microbiol. 2016, 61, 169–177. [Google Scholar] [CrossRef]
- Singh, S.K.; Barreto, G.E.; Aliev, G.; Echeverria, V. Ginkgo biloba as an Alternative Medicine in the Treatment of Anxiety in Dementia and other Psychiatric Disorders. Curr. Drug Metab. 2017, 18, 112–119. [Google Scholar] [CrossRef]
- Chen, P.; Hei, M.; Kong, L.; Liu, Y.; Yang, Y.; Mu, H.; Zhang, X.; Zhao, S.; Duan, J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019, 10, 8161–8171. [Google Scholar] [CrossRef]
- Tang, D.; Yu, Y.; Zheng, X.; Wu, J.; Li, Y.; Wu, X.; Du, Q.; Yin, X. Comparative investigation of in vitro biotransformation of 14 components in Ginkgo biloba extract in normal, diabetes and diabetic nephropathy rat intestinal bacteria matrix. J. Pharm. Biomed. Anal. 2014, 100, 1–10. [Google Scholar] [CrossRef]
- Albert, A.; Altabre, C.; Baró, F.; Buendía, E.; Cabero, A.; Cancelo, M.J.; Castelo-Branco, C.; Chantre, P.; Duran, M.; Haya, J.; et al. Efficacy and safety of a phytoestrogen preparation derived from Glycine max (L.) Merr in climacteric symptomatology: A multicentric, open, prospective and non-randomized trial. Phytomedicine 2002, 9, 85–92. [Google Scholar] [CrossRef]
- Estrella, R.E.N.; Landa, A.I.; Lafuente, J.V.; Gargiulo, P.A. Effects of antidepressants and soybean association in depressive menopausal women. Acta Pol. Pharm. 2014, 71, 323–327. [Google Scholar]
- Myint, H.; Iwahashi, Y.; Koike, S.; Kobayashi, Y. Effect of soybean husk supplementation on the fecal fermentation metabolites and microbiota of dogs. Anim. Sci. J. 2017, 88, 1730–1736. [Google Scholar] [CrossRef]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Wen, Z.; Zou, P.; Yuan, Y.; Jing, W.; Li, Y.; Zhang, C. Consumption of Black Legumes Glycine soja and Glycine max Lowers Serum Lipids and Alters the Gut Microbiome Profile in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 7367–7375. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.-W.L.; Zidon, T.M.; Welly, R.J.; Park, Y.-M.; Britton, S.L.; Koch, L.G.; Rottinghaus, G.E.; de Godoy, M.R.C.; Padilla, J.; Swanson, K.S.; et al. Soy Improves Cardiometabolic Health and Cecal Microbiota in Female Low-Fit Rats. Sci. Rep. 2017, 7, 9261. [Google Scholar] [CrossRef]
- Choi, E.-K.; Won, Y.H.; Kim, S.-Y.; Noh, S.-O.; Park, S.-H.; Jung, S.-J.; Lee, C.K.; Hwang, B.Y.; Lee, M.K.; Ha, K.-C.; et al. Supplementation with extract of Gynostemma pentaphyllum leaves reduces anxiety in healthy subjects with chronic psychological stress: A randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2019, 52, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Lin, X.; Ma, S.; Ge, S.; Mu, S.; Yang, C.; Shi, S.; Gao, L.; Xu, J.; Bo, T.; et al. Amelioration of hepatic steatosis is associated with modulation of gut microbiota and suppression of hepatic miR-34a in Gynostemma pentaphylla (Thunb.) Makino treated mice. Nutr. Metab. 2018, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Brar, M.S.; Leung, F.C.C.; Hsiao, W.L.W. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 2016, 7, 31226–31242. [Google Scholar] [CrossRef]
- Chen, L.; Tai, W.C.S.; Brar, M.S.; Leung, F.C.C.; Hsiao, W.L.W. Tumor grafting induces changes of gut microbiota in athymic nude mice in the presence and absence of medicinal Gynostemma saponins. PLoS ONE 2015, 10, e0126807. [Google Scholar] [CrossRef]
- Shen, S.-H.; Zhong, T.-Y.; Peng, C.; Fang, J.; Lv, B. Structural modulation of gut microbiota during alleviation of non-alcoholic fatty liver disease with Gynostemma pentaphyllum in rats. BMC Complement. Med. Ther. 2020, 20, 34. [Google Scholar] [CrossRef]
- Liao, W.; Khan, I.; Huang, G.; Chen, S.; Liu, L.; Leong, W.K.; Li, X.A.; Wu, J.; Wendy Hsiao, W.L. Bifidobacterium animalis: The missing link for the cancer-preventive effect of Gynostemma pentaphyllum. Gut Microbes 2020, 13, 1847629. [Google Scholar] [CrossRef]
- Chen, L.; Tai, W.C.; Hsiao, W.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods 2015, 17, 892–902. [Google Scholar] [CrossRef]
- Bian, X.; Liu, X.; Liu, J.; Zhao, Y.; Li, H.; Cai, E.; Li, P.; Gao, Y. Study on antidepressant activity of chiisanoside in mice. Int. Immunopharmacol. 2018, 57, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Christou, A.; Panagiotakos, D.; Stefanaki, C.; Skenderi, K.; Katsana, K.; Tsigos, C. Effects of a hops (Humulus lupulus L.) dry extract supplement on self-reported depression, anxiety and stress levels in apparently healthy young adults: A randomized, placebo-controlled, double-blind, crossover pilot study. Hormones 2017, 16, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Blatchford, P.A.; Parkar, S.G.; Hopkins, W.; Ingram, J.R.; Sutton, K.H. Dose-Dependent Alterations to In Vitro Human Microbiota Composition and Butyrate Inhibition by a Supercritical Carbon Dioxide Hops Extract. Biomolecules 2019, 9, 390. [Google Scholar] [CrossRef]
- Hamm, A.K.; Manter, D.K.; Kirkwood, J.S.; Wolfe, L.M.; Cox-York, K.; Weir, T.L. The Effect of Hops (Humulus lupulus L.) Extract Supplementation on Weight Gain, Adiposity and Intestinal Function in Ovariectomized Mice. Nutrients 2019, 11, 3004. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Tang, Z.; Shi, X.; Song, Z.; Cao, F.; Wei, P.; Li, M.; Li, X.; Jiang, D.; et al. Improvements in estrogen deficiency-induced hypercholesterolemia by Hypericum perforatum L. extract are associated with gut microbiota and related metabolites in ovariectomized (OVX) rats. Biomed. Pharmacother. 2020, 135, 111131. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099. [Google Scholar] [CrossRef]
- Seifritz, E.; Schläfke, S.; Holsboer-Trachsler, E. Beneficial effects of Silexan on sleep are mediated by its anxiolytic effect. J. Psychiatr. Res. 2019, 115, 69–74. [Google Scholar] [CrossRef]
- Barić, H.; Đorđević, V.; Cerovečki, I.; Trkulja, V. Complementary and Alternative Medicine Treatments for Generalized Anxiety Disorder: Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv. Ther. 2018, 35, 261–288. [Google Scholar] [CrossRef]
- Bazrafshan, M.-R.; Jokar, M.; Shokrpour, N.; Delam, H. The effect of lavender herbal tea on the anxiety and depression of the elderly: A randomized clinical trial. Complement. Ther. Med. 2020, 50, 102393. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S. An orally administered lavandula oil preparation (Silexan) for anxiety disorder and related conditions: An evidence based review. Int. J. Psychiatry Clin. Pract. 2013, 17 (Suppl. S1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Anghelescu, I.; Dienel, A. Efficacy of orally administered Silexan in patients with anxiety-related restlessness and disturbed sleep—A randomized, placebo-controlled trial. Eur. Neuropsychopharmacol. 2015, 25, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Paul Hsu, C.-H.; Nance, D.M.; Amagase, H. A meta-analysis of clinical improvements of general well-being by a standardized Lycium barbarum. J. Med. Food 2012, 15, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M.-J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018, 62, e1800535. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Tong-un, T.; Muchimapura, S.; Wannanon, P.; Thukhammee, W.; Anulukanapakorn, K.; Bunjob, M. Evaluation of safety and cognitive enhancing effect of Morus alba leaves extract in healthy older adults. PharmaNutrition 2014, 2, 102. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, J.; Zheng, S.; Liang, F.; Luo, Y.; Huang, K.; Xu, W.; He, X. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. 2019, 10, 4771–4781. [Google Scholar] [CrossRef]
- Cases, J.; Ibarra, A.; Feuillère, N.; Roller, M.; Sukkar, S.G. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med. J. Nutr. Metab. 2011, 4, 211–218. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.J.; Perry, E.K.; Wesnes, K.A.; Scholey, A.B. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef]
- Brochot, A.; Azalbert, V.; Landrier, J.-F.; Tourniaire, F.; Serino, M. A Two-Week Treatment with Plant Extracts Changes Gut Microbiota, Caecum Metabolome, and Markers of Lipid Metabolism in ob/ob Mice. Mol. Nutr. Food Res. 2019, 63, 1900403. [Google Scholar] [CrossRef]
- Geng, J.; Dong, J.; Ni, H.; Lee, M.S.; Wu, T.; Jiang, K.; Wang, G.; Zhou, A.L.; Malouf, R. Ginseng for cognition. Cochrane Database Syst. Rev. 2010, 12, CD007769. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Wei, R.; Xie, X.; Wang, C.; Fan, S.; Zhang, X.; Su, J.; Liu, J.; Jia, W.; et al. Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 2018, 9, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, B.-S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-S.; Balan, P.; Hong, H.-D.; Choi, W.-I.; Cho, C.-W.; Lee, Y.-C.; Moughan, P.J.; Singh, H. Korean ginseng modulates the ileal microbiota and mucin gene expression in the growing rat. Food Funct. 2014, 5, 1506–1512. [Google Scholar] [CrossRef]
- Dong, W.-W.; Xuan, F.-L.; Zhong, F.-L.; Jiang, J.; Wu, S.; Li, D.; Quan, L.-H. Comparative Analysis of the Rats’ Gut Microbiota Composition in Animals with Different Ginsenosides Metabolizing Activity. J. Agric. Food Chem. 2017, 65, 327–337. [Google Scholar] [CrossRef]
- Ossoukhova, A.; Owen, L.; Savage, K.; Meyer, M.; Ibarra, A.; Roller, M.; Pipingas, A.; Wesnes, K.; Scholey, A. Improved working memory performance following administration of a single dose of American ginseng (Panax quinquefolius L.) to healthy middle-age adults. Hum. Psychopharmacol. 2015, 30, 108–122. [Google Scholar] [CrossRef]
- Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 2010, 212, 345–356. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Zhang, C.-F.; Zhang, Q.-H.; Hesse-Fong, J.; Lager, M.; Du, W.; Xu, M.; Yuan, C.-S. Fecal metabolomic dataset of American ginseng-treated DSS mice: Correlation between ginseng enteric inflammation inhibition and its biological signatures. Data Brief 2018, 21, 1403–1408. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Yao, H.; Zhang, C.-F.; Chen, L.; Wan, J.-Y.; Huang, W.-H.; Zeng, J.; Zhang, Q.-H.; Liu, Z.; Yuan, J.; et al. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int. Immunopharmacol. 2018, 64, 246–251. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Wang, C.-Z.; Liu, Z.; Zhang, Q.-H.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Li, P.; Qi, L.-W.; Yuan, C.-S. Determination of American ginseng saponins and their metabolites in human plasma, urine and feces samples by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1015–1016, 62–73. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Wang, C.-Z.; Zhang, Q.-H.; Liu, Z.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Li, P.; Qi, L.-W.; Yuan, C.-S. Significant difference in active metabolite levels of ginseng in humans consuming Asian or Western diet: The link with enteric microbiota. Biomed. Chromatogr. 2017, 31, e3851. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Yu, C.; Wen, X.-D.; Chen, L.; Zhang, C.-F.; Calway, T.; Qiu, Y.; Wang, Y.; Zhang, Z.; Anderson, S.; et al. American Ginseng Attenuates Colitis-Associated Colon Carcinogenesis in Mice: Impact on Gut Microbiota and Metabolomics. Cancer Prev. Res. 2016, 9, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-Y.; Liu, P.; Wang, H.-Y.; Qi, L.-W.; Wang, C.-Z.; Li, P.; Yuan, C.-S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F.; Wesnes, K.A.; Scholey, A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: Comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav. 2004, 79, 401–411. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Campos, M.P.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.A.; Del Giglio, A. Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J. Altern. Complement. Med. 2011, 17, 505–512. [Google Scholar] [CrossRef]
- Kleber Silveira, A.; Moresco, K.S.; Mautone Gomes, H.; Da Silva Morrone, M.; Kich Grun, L.; Pens Gelain, D.; de Mattos Pereira, L.; Giongo, A.; Rodrigues De Oliveira, R.; Fonseca Moreira, J.C. Guarana (Paullinia cupana Mart.) alters gut microbiota and modulates redox status, partially via caffeine in Wistar rats. Phytother. Res. 2018, 32, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; de Miranda Ramos, V.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Da Boit Martinello, K.; Silveira, A.K.; Gomes, H.M.; Rabelo, T.K.; et al. Guarana supplementation attenuated obesity, insulin resistance, and adipokines dysregulation induced by a standardized human Western diet via brown adipose tissue activation. Phytother. Res. 2019, 33, 1394–1403. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, K.Y.; Shin, K.Y.; Won, B.Y.; Jung, H.Y.; Suh, Y.-H. Effects of BT-11 on memory in healthy humans. Neurosci. Lett. 2009, 454, 111–114. [Google Scholar] [CrossRef]
- Shin, K.Y.; Lee, J.-Y.; Won, B.Y.; Jung, H.Y.; Chang, K.-A.; Koppula, S.; Suh, Y.-H. BT-11 is effective for enhancing cognitive functions in the elderly humans. Neurosci. Lett. 2009, 465, 157–159. [Google Scholar] [CrossRef]
- Feng, G.-F.; Liu, S.; Pi, Z.-F.; Song, F.-R.; Liu, Z.-Q. Comprehensive characterization of in vivo metabolic profile of Polygalae radix based on ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 173–181. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yen, J.-H.; Cheng, Y.-C.; Lin, C.-Y.; Hsieh, C.-T.; Gau, R.-J.; Chiou, S.-J.; Chang, H.-Y. Polygala tenuifolia extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mouse model and affects hepatic transcriptome and gut microbiota profiles. Food Nutr. Res. 2017, 61, 1379861. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.-F.; Liu, S.; Pi, Z.-F.; Song, F.-R.; Liu, Z.-Q. Studies on the chemical and intestinal metabolic profiles of Polygalae Radix by using UHPLC-IT-MS(n) and UHPLC-Q-TOF-MS method coupled with intestinal bacteria incubation model in vitro. J. Pharm. Biomed. Anal. 2018, 148, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Hong, H.; Kim, T.D.; Hong, G.; Lee, S.; Kim, S.; Kim, N.; Jeon, S.D.; Ahn, C.-W.; Kim, H.J.; et al. Efficacy of Polygonatum sibiricum on Mild Insomnia: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chai, Y.; Zhao, M.; Guo, Q.; Bao, Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020, 11, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Cropley, M.; Banks, A.P.; Boyle, J. The Effects of Rhodiola rosea L. Extract on Anxiety, Stress, Cognition and Other Mood Symptoms. Phytother. Res. 2015, 29, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Labachyan, K.E.; Kiani, D.; Sevrioukov, E.A.; Schriner, S.E.; Jafari, M. The impact of Rhodiola rosea on the gut microbial community of Drosophila melanogaster. Gut Pathog. 2018, 10, 12. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS Profile, Gastrointestinal and Gut Microbiota Stability and Antioxidant Activity of Rhodiola rosea Herb Metabolites: A Comparative Study with Subterranean Organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress-Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shan, B.; Zeng, S.; Zhang, J.; Jin, C.; Liao, Z.; Wang, T.; Zeng, Q.; He, H.; Wei, F.; et al. Raw and wine processed Schisandra chinensis attenuate anxiety like behavior via modulating gut microbiota and lipid metabolism pathway. J. Ethnopharmacol. 2021, 266, 113426. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, N.; Liu, B.; Wu, B.; Xiao, F.; He, B.; Jia, Y. Schisandra chinensis ameliorates depressive-like behaviors by regulating microbiota-gut-brain axis via its anti-inflammation activity. Phytother. Res. 2020, 35, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, J.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated With the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 541. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Conway, K.L.; Coyle, K.R.M.; Barton, E.; Smith, L.D.; Esposito, M.; Harvey, C.; Oakes, D.; Hooper, D.R. Efficacy of fenugreek seed extract on men’s psychological and physical health: A randomized placebo-controlled double-blind clinical trial. J. Complement. Integr. Med. 2020, 18, 445–448. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Richard, A.J.; Fernandez-Kim, S.-O.; Ribnicky, D.M.; Salbaum, J.M.; Newman, S.; Carmouche, R.; Stephens, J.M. Fenugreek Counters the Effects of High Fat Diet on Gut Microbiota in Mice: Links to Metabolic Benefit. Sci. Rep. 2020, 10, 1245. [Google Scholar] [CrossRef]
- Zentek, J.; Gärtner, S.; Tedin, L.; Männer, K.; Mader, A.; Vahjen, W. Fenugreek seed affects intestinal microbiota and immunological variables in piglets after weaning. Br. J. Nutr. 2013, 109, 859–866. [Google Scholar] [CrossRef]
- Calapai, G.; Bonina, F.; Bonina, A.; Rizza, L.; Mannucci, C.; Arcoraci, V.; Laganà, G.; Alibrandi, A.; Pollicino, C.; Inferrera, S.; et al. A Randomized, Double-Blinded, Clinical Trial on Effects of a Vitis vinifera Extract on Cognitive Function in Healthy Older Adults. Front. Pharmacol. 2017, 8, 776. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Barroso, E.; van de Wiele, T.; Jiménez-Girón, A.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Huang, J.; Ding, Y.; Pan, Z.; Zhao, Y.; Zhang, R.; Hu, B.; Zeng, X. In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota. Food Funct. 2016, 7, 1959–1967. [Google Scholar] [CrossRef]
- Tebib, K.; Besançon, P.; Rouanet, J.-M. Effects of dietary grape seed tannins on rat cecal fermentation and colonic bacterial enzymes. Nutr. Res. 1996, 16, 105–110. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Grosu, I.A.; Pistol, G.C.; Taranu, I.; Marin, D.E. The Impact of Dietary Grape Seed Meal on Healthy and Aflatoxin B1 Afflicted Microbiota of Pigs after Weaning. Toxins 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Grosu, I.A.; Pistol, G.C.; Marin, D.E.; Cişmileanu, A.; Palade, L.M.; Ţăranu, I. Effects of Dietary Grape Seed Meal Bioactive Compounds on the Colonic Microbiota of Weaned Piglets with Dextran Sodium Sulfate-Induced Colitis Used as an Inflammatory Model. Front. Vet. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Jin, G.; Asou, Y.; Ishiyama, K.; Okawa, A.; Kanno, T.; Niwano, Y. Proanthocyanidin-Rich Grape Seed Extract Modulates Intestinal Microbiota in Ovariectomized Mice. J. Food Sci. 2018, 83, 1149–1152. [Google Scholar] [CrossRef]
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- Mandalari, G.; Chessa, S.; Bisignano, C.; Chan, L.; Carughi, A. The effect of sun-dried raisins (Vitis vinifera L.) on the in vitro composition of the gut microbiota. Food Funct. 2016, 7, 4048–4060. [Google Scholar] [CrossRef] [PubMed]
- Wijayabahu, A.T.; Waugh, S.G.; Ukhanova, M.; Mai, V. Dietary raisin intake has limited effect on gut microbiota composition in adult volunteers. Nutr. J. 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Cueva, C.; Tamargo, A.; Quintela, J.C.; de La Fuente, E.; Walker, A.W.; Moreno-Arribas, M.V.; Bartolomé, B. Application of the dynamic gastrointestinal simulator (simgi®) to assess the impact of probiotic supplementation in the metabolism of grape polyphenols. Food Res. Int. 2020, 129, 108790. [Google Scholar] [CrossRef] [PubMed]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.-F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.J.; Agis-Torres, A.; Hervert-Hernández, D.; Elvira López-Oliva, M.; Muñoz-Martínez, E.; Rotger, R.; Goñi, I. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. J. Food Sci. 2012, 77, H59–H62. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Baldwin, J.; Collins, B.; Wolf, P.G.; Martinez, K.; Shen, W.; Chuang, C.-C.; Zhong, W.; Cooney, P.; Cockrell, C.; Chang, E.; et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 2016, 27, 123–135. [Google Scholar] [CrossRef]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.-C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, 2000149. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Esteban-Fernández, A.; González de Llano, D.; Sanz-Buenhombre, M.; Guadarrana, A.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilánc, C.G.; Martín Gómez, L.; García Bermejo, M.L.; et al. Supplementation with grape pomace in healthy women: Changes in biochemical parameters, gut microbiota and related metabolic biomarkers. J. Funct. Foods 2018, 45, 34–46. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.-H.; Kim, D.-H. Lactobacillus pentosus var. plantarum C29 increases the protective effect of soybean against scopolamine-induced memory impairment in mice. Int. J. Food Sci. Nutr. 2015, 66, 912–918. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Liu, J.; Qi, H.; Li, J.; Chen, J.; Huang, Q.; Liu, Q.; Mi, J.; Li, X. Gut Microbiota: Therapeutic Targets of Ginseng against Multiple Disorders and Ginsenoside Transformation. Front. Cell. Infect. Microbiol. 2022, 12, 853981. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Meng, X.; Zhai, Y.; Zhou, P.; Ye, T.; Wang, Z.; Sun, G.; Sun, X. Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules 2018, 23, 940. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Yang, X.-D.; Yang, Y.-Y.; Ouyang, D.-S.; Yang, G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. Biomed Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng Extract Ameliorates the Negative Physiological Effects of Heat Stress by Supporting Heat Shock Response and Improving Intestinal Barrier Integrity: Evidence from Studies with Heat-Stressed Caco-2 Cells, C. elegans and Growing Broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Li, W.; Yang, L.; Wang, Z.; Ding, L.; Zhou, M. Gut Microbiota: Novel Therapeutic Target of Ginsenosides for the Treatment of Obesity and Its Complications. Front. Pharmacol. 2021, 12, 731288. [Google Scholar] [CrossRef] [PubMed]
- Agarwa, P.; Sharma, B.; Fatima, A.; Jain, S.K. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac. J. Trop. Biomed. 2014, 4, 245–252. [Google Scholar] [CrossRef]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Committee on Herbal Medicinal Products (HMPC): Community Herbal Monograph on Lavandula angustifolia Miller Aetheroleum. EMA/HMPC/143181/2010. 2012. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-lavandula-angustifolia-miller-aetheroleum_en.pdf (accessed on 1 March 2022).
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tocharus, J.; Jamsuwan, S.; Tocharus, C.; Changtam, C.; Suksamrarn, A. Curcuminoid analogs inhibit nitric oxide production from LPS-activated microglial cells. J. Nat. Med. 2012, 66, 400–405. [Google Scholar] [CrossRef]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Galanis, N.; Kaliora, A.C. An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods 2020, 9, 1019. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the Impact of Flavonoids on Symptoms of Depression: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Brobbey Oppong, M.; Qiu, F. Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 2018, 50, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calvo, J.M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Soy isoflavones and their relationship with microflora: Beneficial effects on human health in equol producers. Phytochem. Rev. 2013, 12, 979–1000. [Google Scholar] [CrossRef]
- Ishiwata, N.; Melby, M.K.; Mizuno, S.; Watanabe, S. New equol supplement for relieving menopausal symptoms: Randomized, placebo-controlled trial of Japanese women. Menopause 2009, 16, 141–148. [Google Scholar] [CrossRef]
- Ko, Y.-H.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. 6,7,4′-Trihydroxyisoflavone, a major metabolite of daidzein, improves learning and memory via the cholinergic system and the p-CREB/BDNF signaling pathway in mice. Eur. J. Pharmacol. 2018, 826, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin--are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Oufir, M.; Walter, F.R.; Deli, M.A.; Smiesko, M.; Zabela, V.; Butterweck, V.; Hamburger, M. Validation of UHPLC-MS/MS methods for the determination of kaempferol and its metabolite 4-hydroxyphenyl acetic acid, and application to in vitro blood-brain barrier and intestinal drug permeability studies. J. Pharm. Biomed. Anal. 2016, 128, 264–274. [Google Scholar] [CrossRef]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Martín, N.; Saura-Calixto, F. In vitro digestibility and intestinal fermentation of grape seed and peel. Food Chem. 2005, 90, 281–286. [Google Scholar] [CrossRef][Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood-Brain Barrier Permeability of Green Tea Catechin Metabolites and their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Vini, R.; Azeez, J.M.; Remadevi, V.; Susmi, T.R.; Ayswarya, R.S.; Sujatha, A.S.; Muraleedharan, P.; Lathika, L.M.; Sreeharshan, S. Urolithins: The Colon Microbiota Metabolites as Endocrine Modulators: Prospects and Perspectives. Front. Nutr. 2021, 8, 800990. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Committee on Herbal Medicinal Products (HMPC): Assessment Report on Hypericum perforatum L., Herba. EMA/HMPC/244315/2016. 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-hypericum-perforatum-l-herba_en.pdf (accessed on 1 March 2022).
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Ha Park, J.; Yoo, K.-Y.; Kim, H.; Cho, J.-H.; Lee, J.-C.; Hyeon Ahn, J.; Jin Tae, H.; Chun Yan, B.; Won Kim, D.; Kyu Park, O.; et al. Hydroquinone Strongly Alleviates Focal Ischemic Brain Injury via Blockage of Blood-Brain Barrier Disruption in Rats. Toxicol. Sci. 2016, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- DiPatrizio, N.V. Endocannabinoids in the Gut. Cannabis Cannabinoid Res. 2016, 1, 67–77. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Hoegger, P. Nutrition-derived bioactive metabolites produced by gut microbiota and their potential impact on human health. Nutr. Med. 2013, 1, 1. [Google Scholar]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary Polyphenols in Metabolic and Neurodegenerative Diseases: Molecular Targets in Autophagy and Biological Effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.-B.; Li, S.-L. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; van Rymenant, E.; Grootaert, C.; van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Talbott, J.A.; Stephens, B.J.; Oddou, M.P. Effect of Coordinated Probiotic/Prebiotic/Phytobiotic Supplementation on Microbiome Balance and Psychological Mood State in Healthy Stressed Adults. Funct. Foods Health Dis. 2019, 9, 265. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Willcox, D.C.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). EC: enterochromaffin cell; EEC: enteroendocrine cell; SCFA: short-chain fatty acid; HPA: hypothalamus–pituitary–adrenal; TNF-α: tumor necrosis factor-α; INF-γ: interferon gamma; IL-6: interleukin-6; IL-1: interleukin-1; GABA: gamma-amino butyric acid. ⊕: stimulates/promotes.
inhibition/downregulation). EC: enterochromaffin cell; EEC: enteroendocrine cell; SCFA: short-chain fatty acid; HPA: hypothalamus–pituitary–adrenal; TNF-α: tumor necrosis factor-α; INF-γ: interferon gamma; IL-6: interleukin-6; IL-1: interleukin-1; GABA: gamma-amino butyric acid. ⊕: stimulates/promotes.
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). EC: enterochromaffin cell; EEC: enteroendocrine cell; SCFA: short-chain fatty acid; HPA: hypothalamus–pituitary–adrenal; TNF-α: tumor necrosis factor-α; INF-γ: interferon gamma; IL-6: interleukin-6; IL-1: interleukin-1; GABA: gamma-amino butyric acid. ⊕: stimulates/promotes.
inhibition/downregulation). EC: enterochromaffin cell; EEC: enteroendocrine cell; SCFA: short-chain fatty acid; HPA: hypothalamus–pituitary–adrenal; TNF-α: tumor necrosis factor-α; INF-γ: interferon gamma; IL-6: interleukin-6; IL-1: interleukin-1; GABA: gamma-amino butyric acid. ⊕: stimulates/promotes.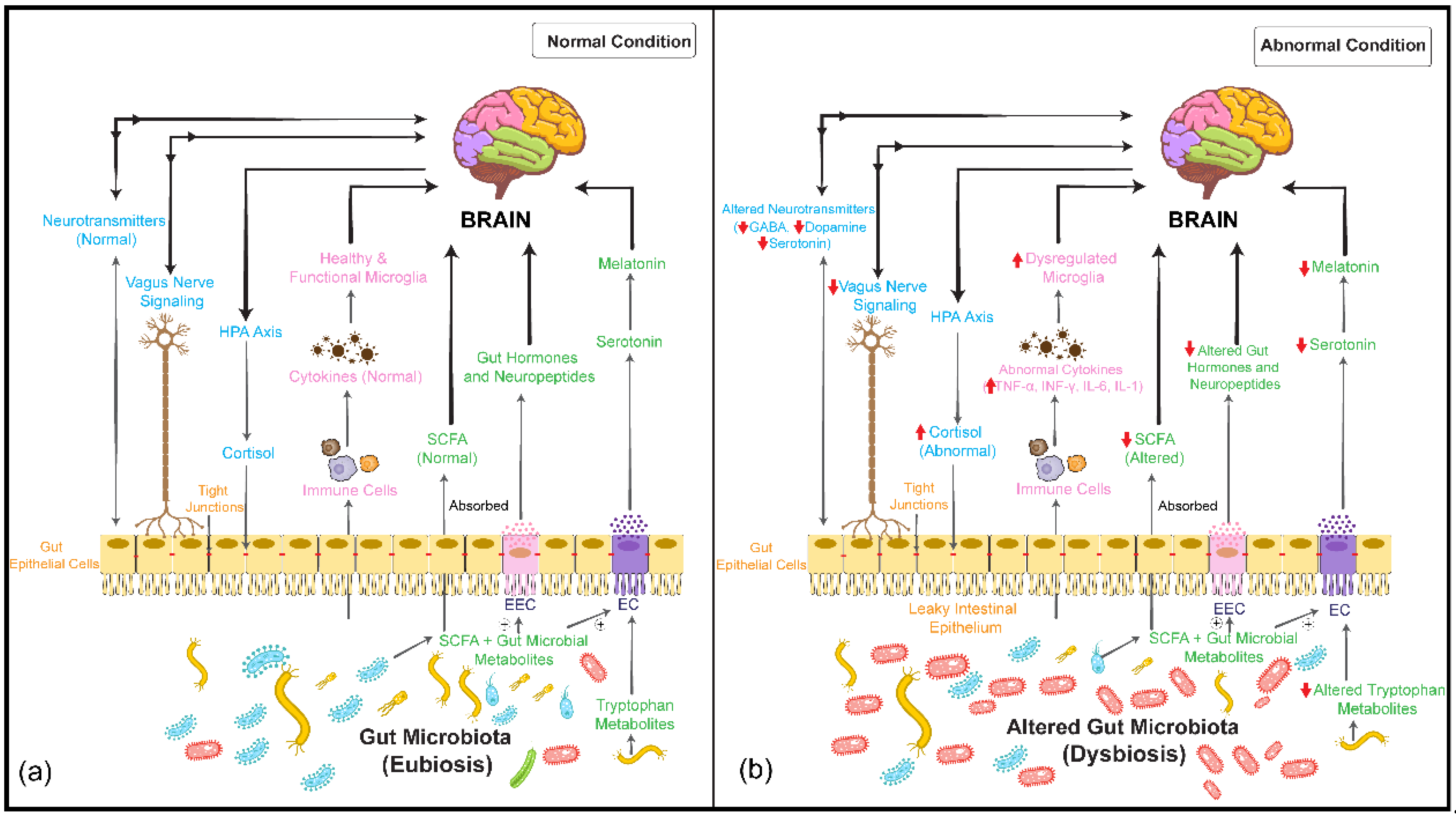

 activation/upregulation,
activation/upregulation,  inhibition/downregulation). TJPs: tight junction proteins; BDNF: brain-derived neurotrophic factor; PI3K: phosphoinositol 3 phosphate; AKT: protein kinase B; IL-1β: interleukin-1β; NF-κB: nuclear factor-κB; PYY: peptide YY; GLP1: glucagon-like peptide 1; ⊕: stimulates/promotes.
inhibition/downregulation). TJPs: tight junction proteins; BDNF: brain-derived neurotrophic factor; PI3K: phosphoinositol 3 phosphate; AKT: protein kinase B; IL-1β: interleukin-1β; NF-κB: nuclear factor-κB; PYY: peptide YY; GLP1: glucagon-like peptide 1; ⊕: stimulates/promotes.
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). TJPs: tight junction proteins; BDNF: brain-derived neurotrophic factor; PI3K: phosphoinositol 3 phosphate; AKT: protein kinase B; IL-1β: interleukin-1β; NF-κB: nuclear factor-κB; PYY: peptide YY; GLP1: glucagon-like peptide 1; ⊕: stimulates/promotes.
inhibition/downregulation). TJPs: tight junction proteins; BDNF: brain-derived neurotrophic factor; PI3K: phosphoinositol 3 phosphate; AKT: protein kinase B; IL-1β: interleukin-1β; NF-κB: nuclear factor-κB; PYY: peptide YY; GLP1: glucagon-like peptide 1; ⊕: stimulates/promotes.
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). BBB: blood–brain barrier; IDO: indolamine 2,3 dioxygenase; TDO: tryptophan 2,3-dioxygenase; QA: quinolinic acid; PPARγ: peroxisome proliferator-activated receptor gamma; AMPK: 5′AMP-activated protein kinase; ROS: reactive oxygen species; TOR: target of rapamycin; ⊕: stimulates/promotes.
inhibition/downregulation). BBB: blood–brain barrier; IDO: indolamine 2,3 dioxygenase; TDO: tryptophan 2,3-dioxygenase; QA: quinolinic acid; PPARγ: peroxisome proliferator-activated receptor gamma; AMPK: 5′AMP-activated protein kinase; ROS: reactive oxygen species; TOR: target of rapamycin; ⊕: stimulates/promotes.
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). BBB: blood–brain barrier; IDO: indolamine 2,3 dioxygenase; TDO: tryptophan 2,3-dioxygenase; QA: quinolinic acid; PPARγ: peroxisome proliferator-activated receptor gamma; AMPK: 5′AMP-activated protein kinase; ROS: reactive oxygen species; TOR: target of rapamycin; ⊕: stimulates/promotes.
inhibition/downregulation). BBB: blood–brain barrier; IDO: indolamine 2,3 dioxygenase; TDO: tryptophan 2,3-dioxygenase; QA: quinolinic acid; PPARγ: peroxisome proliferator-activated receptor gamma; AMPK: 5′AMP-activated protein kinase; ROS: reactive oxygen species; TOR: target of rapamycin; ⊕: stimulates/promotes.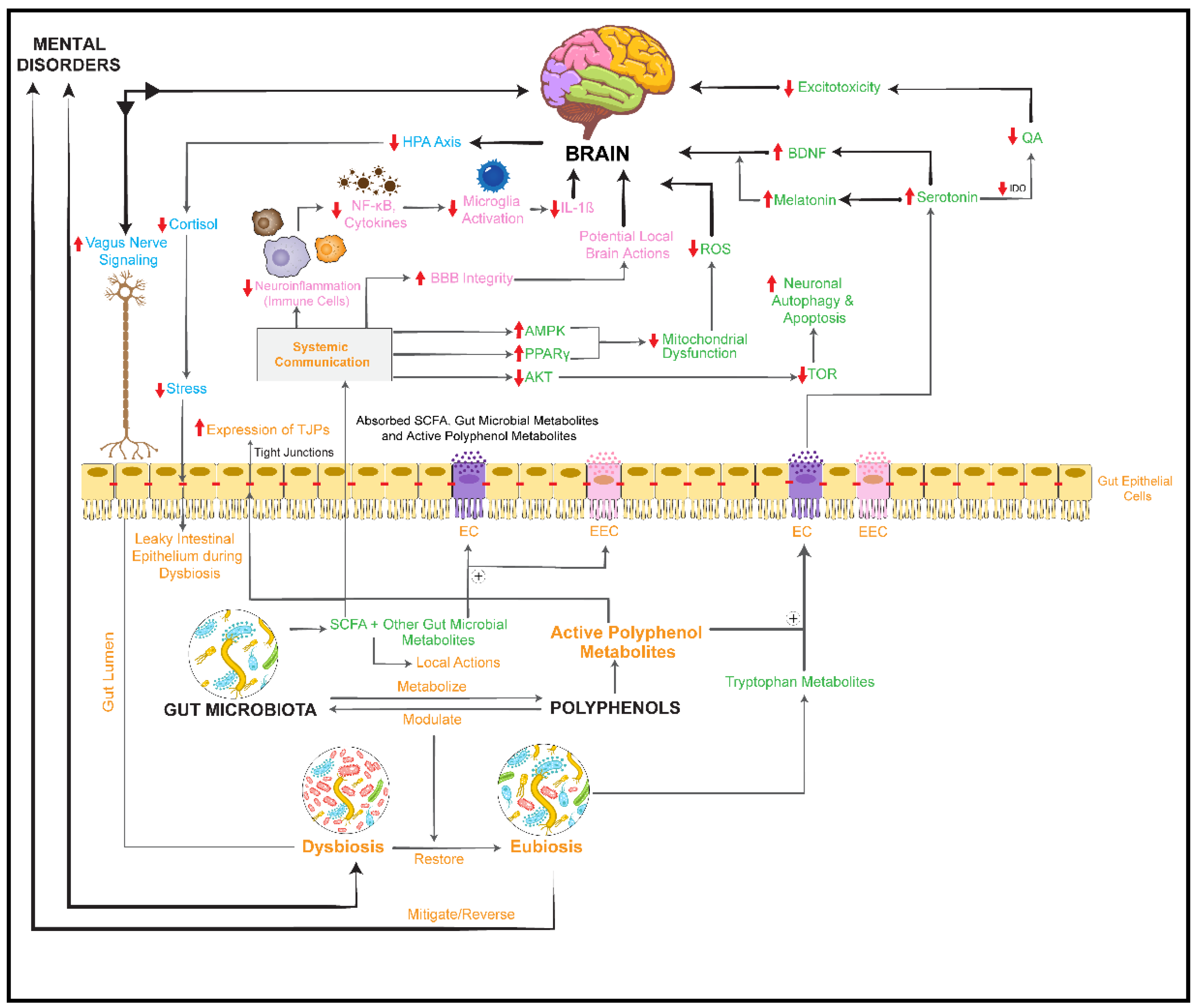
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). HDAC: histone deacetylases; GDNF: glial cell-derived neurotrophic factor; NGF: nerve growth factor. ⊕: stimulates/promotes.
inhibition/downregulation). HDAC: histone deacetylases; GDNF: glial cell-derived neurotrophic factor; NGF: nerve growth factor. ⊕: stimulates/promotes.
 activation/upregulation,
activation/upregulation,  inhibition/downregulation). HDAC: histone deacetylases; GDNF: glial cell-derived neurotrophic factor; NGF: nerve growth factor. ⊕: stimulates/promotes.
inhibition/downregulation). HDAC: histone deacetylases; GDNF: glial cell-derived neurotrophic factor; NGF: nerve growth factor. ⊕: stimulates/promotes.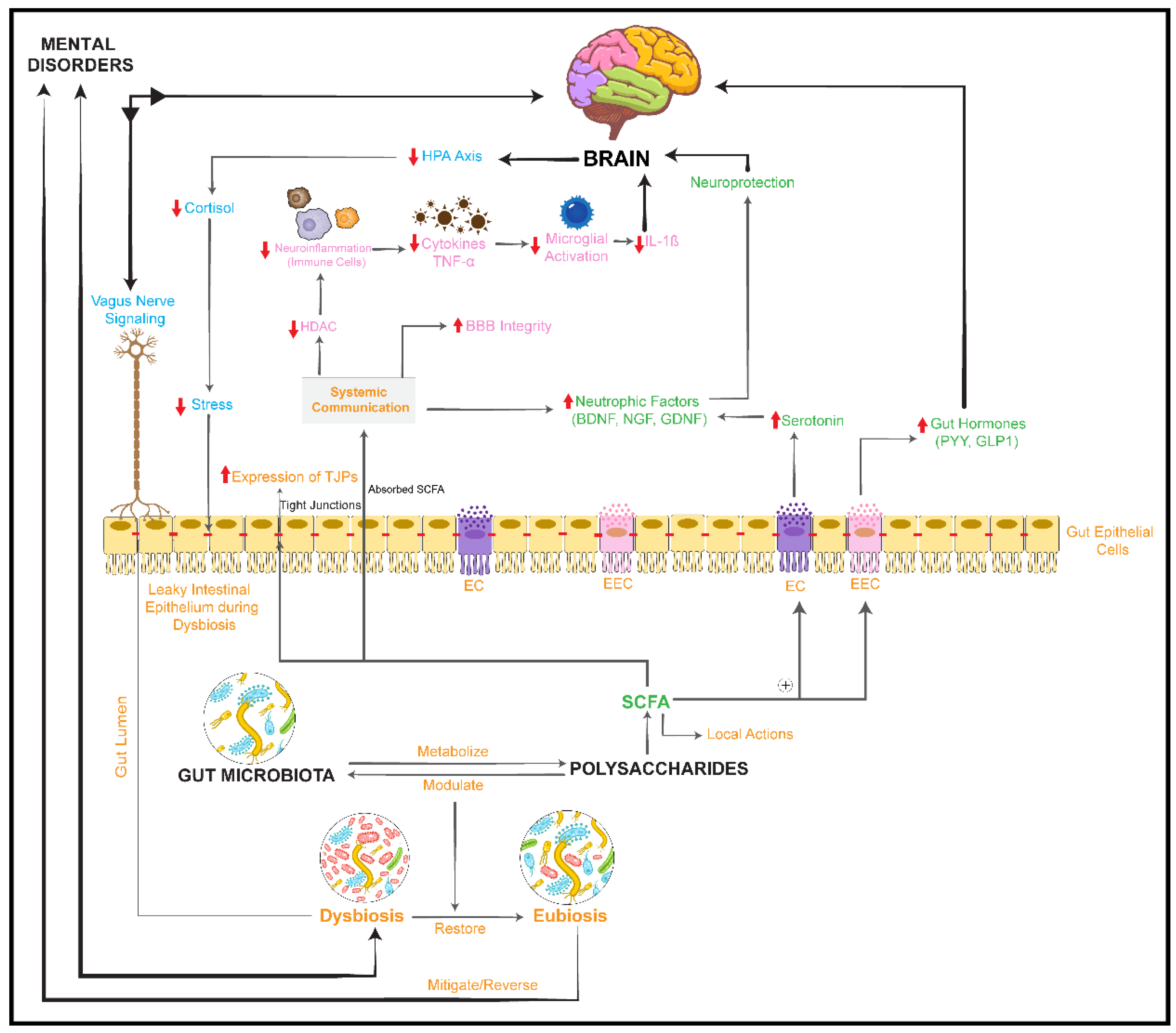
| Botanical Name(s) | Plant Part(s) or Preparation | Common (Local) Name(s) | Dominant Constituent Classes | Application Field in Clinical Studies | Clinical Studies/Reviews | Microbiome Studies |
|---|---|---|---|---|---|---|
| Aloysia citrodora Paláu (syn. Aloysia triphylla (L’Hér.) Kuntze; Verbena triphylla L’Hér.; Lippia citriodora Kunth) | folium | lemon verbena leaf | essential oil, phenolic constituents, iridoids, flavonoids | insomnia | [61] | [62] |
| Amygdalus communis L. (syn. Prunus communis (L.) Arcang.) | semen | almond | lipids, proteins, dietary fiber, polyphenols | cognitive function | [63] | [64,65,66,67,68,69] |
| Astragalus membranaceus (Fisch.) Bunge var. mongholicus (Bge.) Hsiao | radix | membranous milk-vetch root; Huangqi | triterpene saponins, polysaccharides, flavonoids | fatigue | [70] | [71] |
| Camellia sinensis (L.) Kuntze | folium | green tea | methylxanthines, flavonoids, amino acids (theanine) | cognitive function/mood disorders | [72,73] | [74,75,76,77] |
| Cannabis sativa L. | herba | hemp | cannabinoids | insomnia | [78] | [79] |
| Centella asiatica (L.) Urban (syn. Hydrocotyle asiatica L.) | herba | Asiatic pennywort, gotu kola | triterpene saponins | anxiety/mood disorders/cognitive function | [80,81] | [82,83] |
| Citrus aurantium L. ssp. aurantium (syn. Citrus aurantium L. ssp. amara Engl.) | aetheroleum (neroli oil)/flos | bitter orange; orange blossom, Seville orange | essential oil, flavonoids | anxiety | [84,85,86] | [87,88] |
| Crocus sativus L. | stigma | saffron | carotenoids (crocines) | depression/anxiety | [89,90,91,92,93] | [94] |
| Curcuma longa L. (syn. Curcuma domestica Valeton) | rhizoma | turmeric, curcuma, Indian saffron | curcuminoids, essential oil | cognitive function | [95] | [96,97] |
| Dioscorea oppositifolia L. (syn. Dioscorea opposita Thunb.) | rhizoma | Chinese yam | steroid saponins, polysaccharides | cognitive function | [98] | [99,100] |
| Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. (syn. Acanthopanax senticosus) | radix et rhizoma | Eleuthero-coccus (Siberian ginseng) | phenylpropanoids, lignans, triterpene saponins, polysaccharides | fatigue and weakness | [101,102,103] | [104] |
| Ginkgo biloba L. | folium | ginkgo leaf | triterpene lactones, flavonoids | anxiety | [105] | [106,107] |
| Glycine max (L.) Merr. | fructus/hypocotyl (soya bean germ) | soya bean; soya flour; soya testa | isoflavones, saponins, proteins, carbohydrates, lipids | depression/insomnia/anxiety | [108,109] | [110,111,112,113] |
| Gynostemma pentaphyllum (Thunb.) Makino | folium | triterpenoid saponins, sterols, flavonoids | anxiety | [114] | [115,116,117,118,119,120,121] | |
| Humulus lupulus L. | flos | hop strobile | flavonoids, phloroglucinol derivatives, essential oil | depression/stress/anxiety | [122] | [123,124] |
| Hypericum perforatum L. | herba | St. John’s wort | phloroglucinol derivatives (hyperforin), naphthodianthrones (hypericin), flavonoids | depression | [125] | [126] |
| Lavandula angustifolia Mill. (L. officinalis Chaix) | aetheroleum | lavender oil | essential oil | insomnia/anxiety/depression | [127,128,129,130,131,132,133] | [88] |
| Lycium barbarum L. | fructus/fruit juice | GoChi; wolfberry; gouqi; goji berry | polysaccharides, flavonoids, carotenoids | fatigue and weakness/insomnia/stress/depression | [134] | [135] |
| Morus alba L. | folium | mulberry; sang shu | flavonoids | cognitive function | [136] | [137] |
| Melissa officinalis L. | folium | Melissa leaf; lemon balm | essential oil, flavonoids, phenylpropanoids, triterpenes | insomnia/anxiety/mood disorders/cognitive function | [138,139] | [140] |
| Panax ginseng C. A. Meyer. | radix | Korean ginseng; red ginseng | triterpene saponins (ginsenosides), polysaccharides, polyacetylenes | cognitive function | [141] | [120,142,143,144,145] |
| Panax quinquefolius L. | radix | American ginseng | triterpene saponins (ginsenosides) | cognitive function | [146,147] | [148,149,150,151,152,153] |
| Paullinia cupana Kunth ex H.B.K. var sorbilis (Mart.) Ducke (=P. sorbilis C. Mart.) | semen | guarana seed | methylxanthines, tannins, fatty oil | fatigue/cognitive function | [154,155] | [156,157] |
| Polygala tenuifolia Willdenow | radix | Yuan Zhi | triterpene saponins, phenolic glycosides, xanthones | cognitive function | [158,159] | [160,161,162] |
| Polygonatum sibiricum Redoutè | radix | steroidal saponins, polysaccharides | insomnia | [163] | [164] | |
| Rhodiola rosea L. (syn. Sedum roseum (L.) Scop.) | rhizoma et radix | arctic root; roseroot; golden root | phenolic glycosides, essential oil, flavonoids | anxiety/stress/cognitive function/depression | [165,166] | [167,168] |
| Salvia rosmarinus Schleid. (syn. Rosmarinus officinalis L.) | folium/aetheroleum | rosemary | essential oil, rosmarinic acid derivatives | cognitive function/anxiety/depression/insomnia | [169] | [42] |
| Schisandra chinensis Turcz. (Baill.) | fructus et semen | Wu Wei Zi | lignans, essential oil, polysaccharides | fatigue and weakness | [103,170,171] | [172,173,174,175] |
| Trigonella foenum-graecum L. | semen | fenugreek | polysaccharides, alkaloids, saponins, flavonoids | anxiety | [176] | [177,178] |
| Vitis vinifera L. | fructus et semen | grape seeds; grapes | polyphenols (flavonoids, tannins, stilbenoids) | mood disorders/cognitive function | [179,180,181] | [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200] |
| Investigated Plant, Plant Part | Extract, Sample Preparation for Incubation | Preparation of Fecal Samples | Incubation Conditions | Method for Microbiome Analysis | Microbiome Changes | Method for Metabolite Detection | Metabolites | Reference |
|---|---|---|---|---|---|---|---|---|
| Amygdalus communis, semen | blanched finely ground almonds (FG); blanched defatted finely ground almonds (DG) | fecal material from one healthy donor | fecal batch culture after gastric and duodenal digestion (37 °C, pH 6.8, anaerobic; samples were collected over 24 h) | fluorescent in situ hybridization (FISH) with 16S rRNA-targeted probes for Bifidobacterium, Bacteroides, Lactobacillus/Enterococcus spp., Clostridium histolyticum group, Clostridium coccoides-Eubacterium rectale group | increase in Bifidobacterium and E. rectale in FG group; no change in bacterial composition in DG group | SCFA analysis by HPLC with refractive index detector | increase in lactic acid, butyric acid, acetic acid, and propionic acid in FG and DG groups | [65] |
| natural almond skins (NS), blanched almond skins (BS) | fecal material from one healthy donor | fecal batch culture after gastric and duodenal digestion (37 °C, pH 6.8, anaerobic; samples were collected at 0, 4, 8, and 24 h) | FISH with 16S rRNA-targeted probes for Bifidobacterium, Bacteroides, Lactobacillus/Enterococcus spp., Clostridium histolyticum group, Clostridium coccoides-Eubacterium rectale group | increase in Lactobacillus/Enterococcus spp. group, C. coccoides-E. rectale group, and Bifidobacteria in NS and BS group; decrease in C. histolyticum group in NS and BS groups | SCFA analysis by HPLC with refractive index detector | increase in total SCFA, lactic acid, acetic acid, propionic acid, and butyric acid in NS and BS groups | [66] | |
| Centella asiatica, herba | powdered herb | one pooled sample from twelve healthy vegetarian or vegan women and men; 1% herb or 1% glucose | conditions: anaerobic, 37 °C; pH: 7.4 | V3–V4 region of 16S rRNA gene NGS (Illumina); genomic reconstruction of sugar utilization and SCFA pathways | rel. increase: Enterobacteriaceae and Pseudomonadaceae | [83] | ||
| Citrus aurantium ssp. aurantium, aetheroleum | essential oil | twofold dilutions of essential oil (from 2.0% to 0.004% [v/v]) | conditions: 12 bacterial species representing major intestinal genera on selective agars; 24–72 h cultures | agar dilution method | weak antimicrobial effects on Bacteroides fragilis, Clostridium perfringens; no antimicrobial effects on Bifidobacterium, Lactobacillus | - | - | [88] |
| Curcuma longa, rhizoma | powdered rhizome | one pooled sample from twelve healthy vegetarian or vegan women and men; 1% herb | conditions: anaerobic | V3–V4 region of 16S rRNA gene, NGS (Illumina); genome reconstruction of sugar utilization and SCFA pathways | rel. increase at family level: Bacteroidaceae, Desulfovibrionaceae, Rikenellaceae, and Lachnospiraceae rel. increase at genus level: Clostridium spp., Bacteroides spp., Blautia, and Enterobacter spp. rel. increase in propionate- and butyrate-producing taxa rel. decrease in Citrobacter freundii, Enterococcus faecalis, Shigella dysenteriae, and Escherichia coli | [96] | ||
| Ginkgo biloba, folium | extract with ginkgolides, bilobalide, flavonoid glycosides and aglycones (28.1–0.11 µg/mg) | 12 g fresh feces from normal, diabetic, and diabetic nephropathy male Sprague Dawley rats (n = 45) | conditions: anaerobic; 37 °C; reaction mixture taken out at 0.5, 1, 2, 4, 6, 8, 12, 16, 22, 28, 36, and 48 h | - | - | HPLC-MS/MS | all compounds were biotransformed by rat intestinal bacteria; notably different time course of all 14 compounds in feces of diseased compared to normal rats | [107] |
| Glycine max, fructus | soybean husk; 0.9 mg/g total flavonoids | feces from toy poodle dogs (6.5 ± 3.5 months in age, 2.9 ± 0.4 kg in body weight) (n = 3) | conditions: intact soybean husk and enzyme-treated soybean husk; incubated at 39 °C for 24 h | DNA extraction from in vitro cultures; qPCR assay using specific primers | increase: bifidobacteria no effect on total bacteria, total lactobacilli, and E. coli | GC-MS for SCFA analysis and D/L-lactic acid assay kit | increase: total SCFAs, including acetate, propionate, and butyrate acids (p < 0.01) decrease: indole and skatole acids (p < 0.01) no effect on ammonia production | [110] |
| Humulus lupulus, strobile | supercritical CO2 extract mixed with canola oil (extract/oil 2:1); hop bitter acids (α-acids/β-acids 1.73:1); tested range 1.5 mg–750 mg hop extract | mixed inoculum from 10 healthy volunteers | conditions: anaerobic, pH: 6.8; sampling after 2.5, 5, 10, 16, and 24 h | qPCR analyses of total bacteria and key bacterial groups; V3–V4 region of 16S rRNA gene NGS (Illumina) | increase: Proteobacteria, Enterobacteriaceae, Escherichia/Shigella, Enterobacter, Citrobacter, Klebsiella decrease: Lachnospiraceae, Bacteroidetes, Bacteroides, Actinobacteria, Firmicutes, Collinsella, Clostridium, Eubacterium, Desulfovibrio, Bifidobacterium, Lactobacillus, Blautia, Dorea, Veillonella, Coriobacteriaceae; Bacteroides-Prevotella-Porphyromonas group | analyses of SCFA and other organic acids using HPLC/UV-detection | decrease: total organic acids; butyrate clearly decreased at higher hop concentrations | [123] |
| Lavandula angustifolia, aetheroleum | essential oil | twofold dilutions of essential oil (from 2.0% to 0.004% [v/v]) | conditions: 12 bacterial species representing major intestinal genera on selective agars; 24–72 h cultures | agar dilution method | antimicrobial effects (Bacteroides fragilis, Candida albicans, Clostridium perfringens); no impact on beneficial species | - | - | [88] |
| Panax quinquefolius, radix | ethanolic extract (70%) | 6 fecal samples from healthy adult volunteers | conditions: anaerobic, 37 °C; sampling after 24 h incubation | - | - | HPLC/Q-TOF-MS | ginsenoside Rb1 metabolized to compound K and ginsenoside Rg3 | [149] |
| ethanolic extract (70%) | one fresh fecal sample from a healthy Chinese man (28 years old) | conditions: anaerobic, 37 °C; sampling after 24 h incubation | - | - | HPLC/Q-TOF-MS | 25 identified metabolites: 13 metabolites were undoubtedly assigned, 12 were tentatively assigned; the 3 most abundant metabolites: 20S-ginsenoside Rg3, ginsenoside F2, and compound K; main metabolic pathways: deglycosylation (stepwise cleavage of sugar moieties), dehydration | [153] | |
| Polygala tenuifolia, radix | ethanolic extract (75%) | rat intestinal bacteria with Radix Polygala extract (final concentration of 0.02 g/mL), control, and blank samples | conditions: anaerobic; 37 °C; sampling after 0, 2, 8, 24, 48, 72, or 96 h | V4 region of bacterial 16S rRNA gene, NGS (Illumina); 3 replicates of PCR reactions combined | Bacteroides rel. increase more than 60% | UHPLC-IT-MSn and UHPLC-Q-TOF MS | 44 detected metabolites: 25 triterpene saponin metabolites (formed by deglycosylation, deacetylation); 16 oligosaccharide ester metabolites; 3 xanthone C-glycoside metabolites | [162] |
| Rhodiola rosea, radix | Methanolic extract (70%) | 1 g of human feces in 10 mL of brain heart infusion medium | static upper GI tract digestion, followed by incubation of intestinal phase non-dialyzed retentate in fecal slurries of healthy donors (anaerobic, 37 °C, 48 h) | HPLC-DAD | main metabolites: cinnamyl alcohol, tyrosol, hydroquinone | [168] | ||
| Vitis vinifera, fructus | red grape polyphenol extract (653 mg gallic acid equivalents (GAE)/g) | fecal samples from two healthy females | dynamic simulator of the GI tract (simgi®); extract with or without probiotic supplementation (Lactobacillus plantarum CLC-17: 2 × 1010 CFU/day); five periods: microbiota stabilization (14 days), extract (800 mg) acute feeding (8 days), probiotic implantation (7 days), extract (800 mg) acute-feeding during probiotic supplementation (8 days), washout (8 days) | 16S rRNA gene, NGS (Illumina); bacteria plate counting and qPCR of Lactobacillus spp. | increase in Enterobacteriaceae by extract feeding; decrease in Enterobacteriaceae after probiotic implantation; no changes in bacterial diversity after probiotic implantation | targeted analysis of phenolic compounds by UHPLC-ESI-MS/MS and of ammonium ions by ammonium test | increase in phenolic metabolites (benzoic acids) after probiotic implantation; no change in ammonium production | [193] |
| sun-dried raisins | fecal sample from one healthy volunteer | upper gastrointestinal digestion followed by fecal batch culture fermentation (37 °C, anaerobic, 24 h) | bacteria plate counting; V4 region of 16S rRNA gene, NGS (Illumina) | sequencing: rel. increase in Proteobacteria, Actinobacteria, and Roseburia ssp. rel. decrease in Bacteroidetes, Ruminococcus, and Faecalibacterium prausnitzii; plate counting: increase in Bifidobacteria and Lactobacilli | SCFA analysis by HPLC-RID | increase in total SCFAs, lactic acid, acetic acid, propionic acid, and butyric acid | [191] | |
| Vitis vinifera, semen | grape seed polyphenol extract (80% ethanol; 23.5 mg GAE/g) | fecal samples from three healthy volunteers (one female, two males, ages 25–30) | conditions: 37 °C, anaerobic; samples were taken at 0, 12, 24, and 36 h | FISH targeting specific regions of 16S rRNA for total bacteria, Bifidobacterium spp., Lactobacillus-Enterococcus group, Bacteroides-Prevotella group, Clostridium histolyticum group, Eubacterium-Clostridium group, and Atopobium cluster | increase in Bifidobacterium spp. and Lactobacillus-Enterococcus group; decrease in Bacteroides-Prevotella and Clostridium histolyticum; no change in total bacteria, Eubacterium-Clostridium group, and Atopobium cluster | SCFA analysis by HPLC | increase in acetic acid, propionic acid, and butyric acid | [183] |
| grape seed extract (GSE; 629 mg GAE/g) | in vitro cultured microbiota with a reproducible human microbial community representative of in vivo conditions | in vitro simulator of the gastrointestinal tract SHIME®: ascending colon (AC) and descending colon (DC) compartments; conditions: 37 °C, anaerobic, 48 h; samples were taken at 0, 6, 24, and 48 h | qPCR, specific primers for total bacteria, Lactobacillus, Bifidobacterium, Bacteroides, Prevotella, Enterobacteriaceae, Blautia coccoides-Eubacterium rectale group, Clostridium leptum, and Ruminococcus | decrease in all analyzed bacterial groups | SCFA and branched-chain fatty acid (BCFA) analysis by GC-FID; phenolic metabolites by UHPLC-ESI-MS/MS | increase in acetic acid, propionic acid, butyric acid, and total SCFAs and BCFAs in AC; no significant change in SCFAs and BCFAs in DC; steady release of phenylacetic and phenylpropionic acids up to 48 h; formation of flavan-3-ol metabolites | [182] |
| Investigated Plant, Plant Part | Extract, Sample Preparation | Animal or Study Groups (n = Number of Analyzed Individuals) | Animal Species, Volunteers | Conditions | Method for Microbiome Analysis | Microbiome Changes | Method for Metabolite Detection | Metabolites | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Aloysia citrodora, folium | ethanolic extract (25%) (LCE) | 6 groups: control diet (CD); CD + LCE (25 mg/kg); control high-fat diet (HFD); HFD + LCE (1 mg/kg); HFD + LCE (10 mg/kg); HFD + LCE (25 mg/kg) (n = 10 mice per group) | male C57BL/6J mice (7–9 weeks old) | treated for 6 weeks; colonic luminal contents collected | V4–V5 region of 16S rRNA gene, NGS (Illumina) | LCE reduced the enhanced Firmicutes/Bacteroidetes ratio and relative abundance of Bacilli in HFD mice; reversed reduced Bacteroidia, Erysipelotrichia, Cytophaga, and Akkermansia relative abundances in HFD mice | - | - | [62] |
| Amygdalus communis, semen | almonds | 2 groups: low-fat diet (LFD) (n = 23); almond-based low-carbohydrate diet (a-LCD); 56 g almonds/day (n = 22) | patients with type 2 diabetes mellitus (71.98 ± 5.63 years) | treated for 3 months; fecal samples collected | V4–V5 region of 16S rRNA, gene sequencing (Illumina) | a-LCD: rel. decrease in Bacteroidetes and Bacteroides; rel. decrease in Ruminococcus, Eubacterium, and Roseburia | - | - | [68] |
| whole, dry-roasted almonds | 2 groups: almond group (57 g/day) (n = 38); cracker group (77.5 g/day of graham crackers) (n = 35) | female and male young adults (BMI 18–41 kg/m2; 18–19 years) | treated for 8 weeks; fecal samples collected at baseline and after 8 weeks | V4–V5 region of 16S rRNA, gene sequencing (Illumina) | increase in alpha diversity in the almond group compared to the cracker group rel. decrease in Bacteroides fragilis | - | - | [67] | |
| almonds | three groups: almonds, 0 g/day; 42 g/day; 84 g/day; n = 18 | healthy adults (10 male, 8 female) | 3 feeding periods of 18 days separated by a 2-week washout period; fecal sample collection on first and last days of each feeding period | 16S rRNA gene, NGS (454 pyrosequencing), targeting universal primers 27F and 533R; qPCR with specific primers for Bifidobacteria, lactic acid bacteria, and Eubacteria | decrease in lactic acid bacteria by almond consumption; no change in Bifidobacteria by almond consumption | - | - | [69] | |
| natural almonds; roasted almonds; almond butter | 5 periods: 0 g/day of almonds (control diet) (n = 18); 42 g/day of whole, natural almonds (n = 17); 42 g/day of whole, roasted almonds (n = 18); 42 g/day of roasted, chopped almonds (n = 15); 42 g/day of almond butter (n = 18) | female and male volunteers (BMI 29.7 + 4.4 kg/m2; 56.7 + 10.2 years) | 5 diet periods of 3 weeks, separated by 1-week non-controlled diet breaks; fecal sample collection at the end of each diet treatment period | V4 region of 16S rRNA gene, NGS (Illumina) | rel. decrease in Actinobacteria, Bifidobacterium, and Parabacteroides by almond consumption; rel. increase in Lachnospira, Roseburia, and Oscillospira by chopped almond diet; rel. increase in Lachnospira by whole, roasted almond diet; increase in Dialister by whole, natural almond diet | - | - | [64] | |
| Astragalus membranaceus, radix | fine powder (70% astragalan, 10% total saponins) | two groups: control (0.5% CMC-Na buffer), astragalus (1 g/kg bwd) (n = 5 per group) | BKS.Cg-Dock7m +/+ Leprdb/Nju mice (5 weeks old) | treated for 15 days, fresh feces collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) microbial function prediction (PICRUst, KEGG, STAMP) | composition analysis: rel. increased (significant): Oscillibacter; LEfSe: inhibited growth: Clostridium cluster XI; increased growth: Lactobacillus and Bifidobacterium | - | - | [71] |
| Camellia sinensis, folium | water extracts of green tea (GTWE); black tea (BTWE); oolong tea (OTWE) | 5 groups: LFD, 9.4% of calories from fat; HFD, 40% of calories from fat; HFD + 1% GTWE; HFD + 1% BTWE; HFD + 1% OTWE (n = 12 per group) | male C57BL/6J mice (7 weeks old) | treated for 28 weeks; fecal samples were collected at week 28 | V3–V4 region of 16S rRNA gene, NGS (Illumina) | increase in microbial richness in all tea groups; rel. decrease in Rikenellaceae, Desulfovibrionaceae, Alistipes, and Rikenella in GTWE group; rel. increase in Lachnospiraceae_NK4A136_group, Acetatifactor, and Ruminiclostridium_9 in GTWE group | SCFA analysis by GC | increase in total SCFAs, propionic acid, and valeric acid | [74] |
| purple-leaf tea leaf powder (PLT) | 4 groups: normal diet (ND); HFD; HFD-1% PLT; HFD-3% PLT (n = 8 per group) | male C57BL/6J mice (5 weeks old) | treated for 10 weeks, fecal samples were collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) | HFD-PLT groups compared to HFD group: rel. increase in microbial richness; decrease in Firmicutes/Bacteroidetes ratio; rel. increase in Ruminococcaceae | - | - | [75] | |
| water extracts from: green tea (GTE); black tea (BTE); yellow tea (YTE); oolong tea (OTE); white tea (WTE); dark tea (DTE); hawk tea (HTE) | 9 groups: healthy group; DSS group; GTE + DSS group; WTE + DSS group; YTE + DSS group; OTE + DSS group; BTE + DSS group; DTE + DSS group; HTE + DSS group; (n = 6 per group) | Kunming female mice (7–8 weeks old) | treated for 14 days; fecal samples were collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) | in GTE group: increase in microbial diversity; rel. decrease in Bacteroides, Oscillibacter, Mucispirillum, Helicobacter, and Brachyspira; rel. increase in Bifidobacterium and Ruminococcaceae_UCG-014 | SCFA analysis by HPLC | increase in acetic acid, propionic acid, and butyric acid | [76] | |
| green tea water extract (GTE); dark tea water extract (DTE) | 3 groups of healthy mice: normal group; GTE (5 mg/kg) group; DTE (5 mg/kg) group | female C57BL/6 mice (7–8 weeks old) | treated for 4 weeks; fecal samples were collected after 4 weeks | V3–V4 region of 16S rRNA gene, NGS (Illumina) | bacterial community richness and diversity unchanged in healthy mice; healthy GTE group: rel. increase in Lactococcus, Akkermansia, Lactobacillus intestinalis, Alistipes, and Parabacteroides distasonis; rel. decrease in Turicibacter, Romboutsia, Allobaculum, Ileibacterium, and Muribaculum | - | - | [77] | |
| Cannabis sativa, herba | inflorescence extracts (99.9% ethanol): cannabidiol (CBD)-rich CN1 extract; tetrahydrocannabinol (THC)-rich CN2 extract; CN6 extract (CBD/THC ca. 1:1) | 5 groups: ND; high-fat + 1% cholesterol + 0.5% cholate diet (HFCD); HFCD diet + CN1 (HFCD+CN1); HFCD diet + CN2 (HFCD+CN2); HFCD diet + CN6 (HFCD+CN6) (n = 8 per group) | male C57BL/6J mice (7–8 weeks old) | treated for 6 weeks, 5 mg/kg BW of extract administered every 3 days; cecal contents were collected after sacrifice | V3–V4 region of 16S rRNA gene, NGS (Illumina) | rel. decrease in Bacteroidetes and decrease in Bacteroidetes/Firmicutes ratio in HFCD+CN1 group compared to HFCD group; no significant microbiota changes in HFCD+CN2 and HFCD + CN56 | - | - | [79] |
| Centella asiatica, herba | ethanolic extract (75%) | 6 groups: control, model group (DSS-induced colitis), DSS+5-aminosalicyclic acid, DSS+C. asiatica (100, 200, and 400 mg/kg) (n = 8 per group) | male Balb/c mice (22–24 g, 8 weeks old) | treated for 7 days, cecum contents collected after sacrifice | V4 region of 16S rRNA gene NGS (Illumina) | DSS+C. asiatica (400 mg/kg): rel. increase: Firmicutes; rel. decrease: Proteobacteria, Helicobacter, Jeotgalicoccus, and Staphylococcus | - | - | [82] |
| Citrus aurantium ssp. aurantium, flos | ethanolic extract (85%) partitioned to ethyl acetate subextract (EA) | 6 groups: control ND; model control HFD; HFD+ low, middle, and high citrus ethyl acetate (LEA (50 mg/kg), MEA (100 mg/kg), HEA (200 mg/kg)); HFD+simvastatin (n = 8 mice per group) | male C57BL/6 mice (weighing 16–17 g, 4 weeks old) | treated for 12 weeks; fresh fecal pellets collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) | HEA increased microbiota diversity and richness; decreased Firmicutes/Bacteroidetes ratio; rel. decrease Erysipelotrichaceae and others rel. increase: Bifidobacteria and others | - | - | [87] |
| Crocus sativus, stigma | saffron (not defined) | two groups: control (water), saffron in drinking water (120 mg/day) (n = 10 per group) | rats (not defined) | treated for 4 weeks; stool samples collected before and after 4 weeks | 16S rRNA gene NGS (Illumina) using universal bacterial primers | strong rel. reduction: Cyanobacteria, Proteobacteria less strong rel. decrease: Bacteroidetes, Firmicutes rel. increase: Spirochaetes, Tenericutes, Candidatus saccharri | - | - | [94] |
| Curcuma longa, rhizoma | turmeric powder (2.5% curcumin); alcoholic turmeric extract containing curcumin and turmeric oil fraction | three groups: control diet (CD); CD + 100 mg turmeric powder; CD + 20 mg turmeric extract (n = 10 rats per group) | male Wistar albino rats (21 days old; ≈32 g) | five animals of each group killed after 3 months, others after 2 years; cecal contents collected after sacrifice | agar dilution (0.1% peptone for aerobes; sterile mineral solution for anaerobes) | significant decrease after 3-month treatment: total aerobes, Lactobacilli significant increase after 3-month treatment: total anaerobes, Clostridium perfringens, and coliforms significant decrease after 2-year treatment: coliforms | - | - | [97] |
| Dioscorea oppositifolia, rhizoma | dried Chinese yam powder (CY) | five groups: normal control (NC) group (water); model control (MC) group (antibiotic-associated diarrhea, AAD); low-dosage (CL) group (AAD + 4.28 g/kg BW CY suspension); medium-dosage (CM) group (AAD + 8.56 g/kg BW CY suspension); high-dosage (CH) group (25.68 g/kg BW CY suspension) (n = 10 per group) | male Balb/c mice (7 weeks old) | days 1–5: MC, CL, CM, and CH groups: ampicillin (22.4 g/kg BW, two times per day); days 6–15: water for MC group, CY for CL, CM, and CH groups; fecal samples were collected | bacterial counting, specific agar plates for Bifido-bacteria, lactobacilli, Enterococcus, and Clostridium perfringens; denatured gradient gel electrophoresis (DGGE) and V3 region 16S rRNA gene sequen-cing of DGGE target bands | increase in Bifidobacteria and Lactobacilli in CH group; decrease in Enterococcus in CH group and Clostridium perfringens in CL, CM, and CH groups; increase in Bacteroides spp. and Clostridium spp. in CL, CM, and CH groups | SCFA analysis by GC-FID | increase in total SCFAs in CL, CM, and CH groups | [99] |
| Chinese yam extract (hot water) (CY) | three groups: NC; antibiotic group (A; 50 mg/kg BW imipenem/cilastatin Na); CY group (ADR; 50 mg/kg BW imipenem/cilastatin Na + 3.4 g/kg BW CY) (n = 6 per group) | SPF-grade male Wistar rats (100 ± 10 g) | treated for 21 days; fecal samples were collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) | ADR group: increase in microbial diversity reduced by antibiotic; rel. increase in Lachnospiraceae, Ruminococcaceae, Clostridiales, and Firmicutes; rel. decrease in Blautia, Prevotella, and Eisenbergiella | metabolic profile analysis by UPLC-Q-TOF/MS | CY administration returned fecal sample metabolite profile to normal | [100] | |
| Eleutherococcus senticosus, plant part not specified | ethanolic extract (EE) | four groups: control, EE (30 g/100 kg), Enterococcus faecium AL41 (EFAL41), EFAL41 + EE (n = 24 rabbits in each group) | post-weaned rabbits (Hyplus breed) (5 weeks old) | treated for 42 days; fecal sampling on day 0/1 (start of experiment), day 21, and day 42; on days 21 and 42, 3 animals per group were sacrificed | agar dilution methods on specified agars for enterococci, EFAL41, coagulase-negative and coagulase-positive staphylococci, Clostridium difficile, coliforms, pseudomonads | EE group: reduction in: coagulase-negative staphylococci and Clostridia on day 21 | cecal lactic acid and SCFA analysis using GC (days 21 and 42, 3 animals per group were sacrificed) | different concentrations of propionic acid in all experimental groups in comparison to control on day 42 | [104] |
| Ginkgo biloba, folium | polysaccharide-rich water extract (GPS) | stage 1–4 groups: control; unpredictable chronic mild stress mice (UCMS); UCMS + GPS (300 mg/kg BW); UCMS + paroxetine (30 mg/kg BW), (n = 10 per group); stage 2 fecal microbiota transplant (2 groups): mixed antibiotics, oral gavage of fecal samples from donor mice (UCMS-FMT or GPS-FMT) (n = 8 per group) Lactobacillusreuteri treatment (3 groups): control; UCMS; UCMS + oral gavage of L. reuteri (n = 8 per group) | male SPF BALB/c mice (3–4 weeks old) | treated for 4 weeks, fresh feces collected; behavioral experiment after 30 days of GPS/paroxetin treatment, FMT, or L. reuteri treatment | V3–V4 region of 16S rRNA gene, NGS (pyrosequencing) | antidepressant effect in forced swimming test in UCMS-GPS group vs. UCMS group, and in GPS-FMT group vs. UCMS-FMT group; GPS reversed gut dysbiosis induced by UCMS; 113 differential OTUs between UCMS-GPMS and UCMS groups | - | - | [106] |
| Glycine max, fructus | legume powder; isoflavone content in Glycine soja (HFG) 788.77 µg/g, in Glycine max (HFB) 139.72 µg/g | four groups: control (normal chow; NCD); standard HFD; HFD with 20% HFG; HFD with 20% HFB (n = 12 mice per group) | male C57BL/6J mice (7 weeks old, 18–20 g) | treated for 11 weeks; fresh feces collected in the last week in the morning | V3–V4 region of 16S rRNA gene, NGS (Illumina) | reversal of HFD-induced gut microbiota changes in HFB and HFG rel. increase: Bacteroidetes, Proteobacteria, Allobaculum, Parasutterella, Anaerotruncus, Helicobacter, Alistipes; rel. decrease: Verrucomicrobia, Akkermansia | analysis of fecal SCFA content by HPLC/PDA detector | total SCFA and acid concentrations reduced in HFD group, but elevated in HFG- and HFB- supplemented groups; acetic and propionic acids and total SCFAs higher in HFG than in HFB | [112] |
| soybean husk with 0.9 mg/g total flavonoids | two groups: cellulose powder (10 g) or soybean husk powder (5.6% of total diet) (n = 4 per group) | healthy Shiba dogs (7–48 months in age and 7.5 ± 1.7 kg in body weight) | treated for 7 days; feces collected on morning and evening of days 6 and 7 | qPCR assay using specific primers | increase: total lactobacilli, Clostridium cluster IV, Faecalibacterium prausnitzii, Clostridium cluster XIVa, Bacteroides-Prevotella-Porphyromonas group; decrease: Clostridium cluster XI | analysis of SCFA by GC-MS; D/L-lactic acid assay | increase: total SCFAs, acetic, butyric, and lactic acids (p < 0.05) decrease: indole and skatole | [110] | |
| soy (590 mg/isoflavones kg diet (genistein and daidzein equivalents)) | 4 groups: OVX + soy; SHM + soy; OVX + soy-free (control); SHM + soy-free (control) (n = 10 rats per group) | female rats bred for low-running capacity, either ovariectomized (OVX) or sham-operated (SHM) (27 weeks old) | treated for 28 weeks; cecal digesta samples collected | V3–V4 region of 16S rRNA gene, NGS (Illumina) | OVX + soy and SHM + soy: rel. increase: Bacteroidetes, Prevotella, Lachnospiraceae, Dorea, Phascolarctobacterium, rc4-4, Sutterella rel. decrease: Firmicutes, Coprococcus, SMB53, Clostridiaceae, Desulfovibrionaceae, Adlercreutzia, Bifidobacterium CF231, Desulfovibrio, Roseburia, Treponema, Peptostreptococcaceae; lower Firmicutes/Bacteroidetes ratio (p < 0.001) | - | - | [113] | |
| Gynostemma pentaphyllum, folium | Gynostemma pentaphyllum saponins (GpS) | 3 FMT donor groups: GpS treatment (Apc+GpS 300 mg/kg BW); non-treatment (Apc-GpS); wild-type (WT) control (C57BL/6J mice—GpS, B6 group) 4 FMT groups: control group (no FMT), B6 FMT, Apc-GpS FMT, and Apc+GpS FMT (n = 8 per group) | male C57BL/6J (WT) and ApcMin/+ (colon cancer model) mice (4–6 weeks) | treated for 8 weeks; at the end of week 4, fresh feces collected every 3 days from FMT donors; FMT groups received transplants every 3rd day for 4 consecutive weeks | enterobacterial repetitive intergenic consensus (ERIC)-PCR and qPCR with taxon-specific 16S rRNA gene primers | Apc/GpS FMT group: significant increase in Bacteroides, Bacteroidetes/Firmicutes ratio, beneficial bacteria such as Bacteroides, Bifidobacterium, Lactobacillus, Clostridium Cluster IV, and Faecalibacterium prausnitzii | [119] | ||
| Gynostemma pentaphyllum saponins (GpS); 50 mg/mL in 0.5% carboxymethyl cellulose | four groups: nonxenograft-control, nonxenograft-GpS (n = 6 per group); xenograft-control and xenograft-GpS; (750 mg/kg BW; n = 7 per group) | athymic nude mice (BALB/c-nu/nu); xenograft performed by injecting 106 R6/GFP-ras-transformed cells into the flank (7 to 8 weeks old) | treated for 12 days; animal feces collected from each mouse for two consecutive hours on day 0 (before xenograft), and day 5 and day 10 after GpS treatment | ERIC-PCR; 3 fecal samples randomly picked from each experimental group on day 10 for further 16S rRNA gene NGS (454 pyrosequencing) | GpS induced alteration in microbiota in xenograft, but not in nonxenograft mice; Clostridium cocleatum and Bacteroides acidifaciens rel. increase by GpS treatment in xenograft and nonxenograft mice | - | - | [117] | |
| Gynostemma pentaphyllum saponins (GpS); 50 mg/mL in 0.5% carboxymethyl cellulose | three groups: WT-control, WT-GpS, ApcMin/+-control, ApcMin/+-GpS; 500 mg/kg (n = 12 mice per group) | heterozygous male ApcMin/+ (C57BL/6J-ApcMin/+) and female WT C57BL/6J mice (6 weeks of age) | treated for 8 weeks; fecal samples collected from for two consecutive hours before treatment and weekly after treatment | ERIC-PCR; 5 fecal samples randomly picked from each experimental group on week 8 for further 16S rRNA gene NGS (454 pyrosequencing) | GpS rel. increase: Bacteroides acidifaciens, Bifidobacterium pseudolongum, Clostridium cocleatum, Lactobacillus intestinalis, Parabacteroides distasonis, Streptococcus thermophilus, and Bacteroidetes/Firmicutes ratio GpS rel. decrease: Acinetobacter lwoffii and sulfate-reducing bacteria | - | - | [116] | |
| Gynostemma pentaphyllum saponins, saponin content 85% (GpS) | 2 groups: control group (water), GpS group (500 mg GS/kg BW 1× per day) (n = 10 per group) | male C57BL/6 mice (8 weeks old) | treated for 15 days; feces collected for 2 consecutive hours on days 0, 5, 10, and 15 upon treatment | ERIC-PCR; qPCR with primers targeting 16S rRNA gene of specific bacterial groups | GpS group vs. control: increased: Bacteroidetes, Bacteroidetes/Firmicutes ratio, Bacteroides spp., Lactobacillus spp., Faecalibacterium prausnitzii decreased: Firmicutes | - | - | [120] | |
| Gynostemma pentaphyllum (GP) decocted twice with 4 L water (2 g/mL) | 6 groups: control, model group (HFD-induced nonalcoholic fatty liver disease, NAFLD), NAFLD + positive control (22.8 mg/kg DLPC), NAFLD + GP, 6 g/kg BW (GPH), NAFLD+ GP, 3 g/kg BW (GPM); NAFLD + GP, 1.5 g/kg BW (GPL) (n = 10 per group) | male adult Sprague Dawley rats (180–220 g) | rats fed with chow diet or HFD for 8 weeks; from week 5, treated for 4 weeks; cecum, contents collected after sacrifice | V3–V4 region of 16S rRNA gene; V4 and V9 regions of 18S rRNA gene, NGS (Illumina); PCR of ITS1 and ITS2 regions | GP treatment shifted microbiota composition towards that of healthy control; GP decreased Firmicutes/Bacteroidetes ratio to a value comparable to healthy control; GP rel. increase: Lactococcus; GP rel. decrease: pathogenic bacteria, including Ruminococcus spp. | - | - | [118] | |
| 100 g G. pentaphyllum dry herb boiled in water (1.25 g/mL) (GP) | 3 groups: control (chow diet + water), model group (HFD-induced NAFLD + water), GP treatment group (HFD-induced NAFLD + GP; 11.7 g/kg BW (12 mL GP/kg BW) | male C57BL/6J mice (6 weeks old) | feeding with chow diet or HFD for 28 weeks; treatment from week 13 on; 6 animals per group picked for feces collection (once per day on 3 consecutive days) | V3–V4 region of 16S rRNA gene, NGS (Illumina) | GP restored reduced gut microbial diversity and microbial shifts induced by HFD: rel. decrease in the enhanced Firmicutes levels including genera Eubacterium, Blautia, Clostridium, and Lactobacillus; rel. increase in the reduced Parasutterella levels | - | - | [115] | |
| Humulus lupulus, strobile | hop extract suspended in sesame oil; hop extract (HE) (5.1 mg/g 8-prenylnaringenin, 6.3 mg/g xanthohumol), 400 mg/kg BW | 5 groups: OVX placebo (sesame seed oil, n = 11), OVX plus HE (n = 11), OVX plus 17β-estradiol (n = 9), SHAM placebo (sesame seed oil, n = 10), SHAM plus HE (n = 8) | female C57BL/6 retired breeder mice (7 months old); ovariectomized (OVX) or sham-operated (SHAM) | duration: 12 weeks surgery after week 2; treatment started 4–7 days post-surgery; fecal samples from week 10 (SCFAs), cecal contents (microbiota analysis) | V3–V4 region of 16S rRNA gene, NGS (Illumina) | no influence on total bacterial abundances; rel. decrease Akkermansia muciniphila in SHAM plus HE group compared to SHAM placebo and OVX plus 17β-estradiol group; no reduction in OVX plus HE group | SCFA analyses using GC-FID | no significant differences in fecal SCFA levels among groups | [124] |
| Hypericum perforatum L., herba | H. perforatum extract (8.94% total flavonoids, 0.026% hyperoside, 0.323% hypericin) (HP) | 3 groups: OVX group; OVX-HP group (extract 300 mg/kg BW HP); sham group (n = 8 per group) | female Sprague Dawley rats (260–300 g, 6–8 weeks old) | treated for 12 weeks; feces were collected for 3 days before the end of the experiment | V3–V4 region of 16S rRNA gene, NGS (Illumina) | HP group: increased Firmicutes/Bacteroidetes ratio; rel. increase Firmicutes and Verrucomicrobia; rel. decrease Bacteroidetes, Elusimicrobia, and Gemmatimonadetes | SCFA analysis by GC-FID | HP group: increased acetic acid, propionic acid, butyric acid, valeric acid, and hexanoic acid | [126] |
| Lycium barbarum L., fructus | goji berry powder | 2 groups: standard rodent diet (Con); Con diet + 1% goji (n = 7 per group) | male IL-10-deficient mice (6 weeks old) | treated for 10 weeks; fecal samples (colonic contents) were collected at necropsy | V4 region of 16S rRNA gene, NGS (Illumina) | goji group: increase in Firmicutes/Bacteroidetes ratio; rel. increase in Actinobacteria, Bifidobacteriaceae, Lachnospiraceae, Ruminococcaceae, Bifidobacterium, Clostridium XVIII, Roseburia sp., Clostridium leptum, and Faecalibacterium prausnitzii; rel. decrease in Peptostreptococcaceae | SCFA analysis by GC-FID | increase in butyric acid and isovaleric acid | [135] |
| Melissa officinalis, folium | lemon balm water extract (LB) (2.76 mg rosmarinic acid/100 mg dried raw material) | 2 groups: control (water); LB group (LB dissolved in water, 500 mg LB/day/mouse) (n = 5 per group) | C57Bl/6J male ob/ob mice (12 weeks old) | treated for two weeks; gut (fecal) microbiome analyzed before and after treatment | V3–V4 region of 16S rRNA gene, NGS (Illumina) | LB group: increase: Chao-1 diversity index and Porphyromonadaceae | metabolomic analysis of cecum content for SCFAs and other metabolites | significantly higher levels of butyrate, propionate, and ethanol; significantly lower level of lactate | [140] |
| Morus alba L., folium | dried and powdered mulberry leaves | three groups: control group, LFD, 10% calories from fat; HFD, 60% calories from fat; mulberry group (M + HFD; HFD plus 20% M) (n = 6 per group) | male C57BL/6J mice (15–20 g, 4 weeks old) | 8 weeks until weight difference between HFD and LFD is ca. 20%; treated for 13 weeks; feces collected after adaptation, HFD-induced obese model construction, and at the end | V3–V4 region of 16S rRNA gene, NGS (Illumina) | increase in Bacteroidetes/Firmicutes ratio; rel. decrease in Firmicutes and Proteobacteria; rel. increase in Bacteroidetes and Akkermansia | - | - | [137] |
| Panax ginseng, radix | red and white Korean ginseng powder (WG, RG) | three groups: control (basal diet), WG group (7.0% w/w of diet WG), RG group (7.0% w/w of diet RG) (n = 10 per group) | Sprague Dawley male rats | treated for 21 days, postmortem: ileum contents (anterior to the ileocecal valve) collected | qPCR with primers for all bacteria, Lactobacillus, Bifidobacterium, Escherichia coli, Clostridium cluster I, Bacteroides-Prevotella-Porphyromonas group | RG and WG groups: significantly higher number of total bacteria (p = 0.014) and Lactobacillus strains (p = 0.018) | - | - | [144] |
| freeze-dried granulated Panax ginseng extracts g | Panax ginseng extract (4 g two times/day), no placebo group (n = 10 women) | women aged 40–60 years and body mass index ≥ 25 kg/m2 | 8-week clinical trial, fresh human stools collected on the 1st visit day (week 0) and the last day (week 8) | V1–V3 region of 16S rRNA gene, NGS (454 pyrosequencing) | rel. abundance of Anaerostipes decreased after ginseng intake; subgroup analyses with effective (EWG) and ineffective weight loss groups (IWG): increased in EWG: rel. abundance of Anaerostipes and Eubacterium_g5; increased in IWG: Lactobacillus; rel. abundance of Bifidobacterium, Escherichia, and Clostridium_g23 in EWG significantly lower than in IWG | [143] | |||
| ethanolic extract (80%) (PGE) | PGE (100 mg total saponins/kg BW) (n = 60 rats), no control group | male Sprague Dawley rats (7 weeks old, weight: 220 ± 20 g) | treated for 12 h; colonic content samples collected | V1–V3 region of 16S rRNA gene, NGS (Illumina) | subgroup with low-efficiency metabolism (LEM) and high-efficiency metabolism (HEM): rel. abundance of Alcaligenaceae, Coriobacteriaceae, Bifidobacteriaceae, S24-7, Erysipelotrichaceae, Peptostreptococcaceae, and Campylobacteraceae significantly higher in HEM; Lachnospiraceae, Prevotellaceae, Porphyromonadaceae, Defluviitaleaceae, Lactobacillaceae, and Veillonellaceae significantly lower in HEM | LC-MS/MS (MRM mode, precursor-product ion pairs) | protopanaxadiol-type ginsenosides: selective elimination of the C-20 and C3- terminal sugar moieties to compound K, or of the C-20 sugar chain to ginsenoside Rg3; protopanaxatriol-type ginsenosides: C-20 and C-6 sugar moieties hydrolyzed to protopanaxatriol | [145] | |
| ginseng extract (not defined) | 2 groups: control (distilled water), ginseng extract (100 mg/kg; n = 9 per group) | male Wistar rats (34 weeks with 300 g) | treated for 34 weeks, intestinal (cecum, ileum) contents collected after sacrifice | V3 region of 16S rRNA gene, NGS (pyrosequencing with the GS FLX platform) | rel. increase in ginseng group: Bifidobacterium, Lactobacillus, Methylobacteriaceae, and Parasutterella | untargeted GC-TOFMS | ginseng group: 25 significantly changed metabolites from cecum and 35 from ileum; upregulated: amino acids, arachidonic acid, polyamines, and organic acids; downregulated: linoelaidic acid, palmtelaidic acid, oleic acid, and glycerol | [142] | |
| ginseng saponin extract (80% saponins) (GS); red ginseng saponin extract (80% saponins (RGS)) | 3 groups: control group (water); GS group (500 mg GS/kg BW 1× per day); RGS group (500 mg RGS/kg BW 1× per day) (n = 10 per group) | male C57BL/6 mice (8 weeks old) | treated for 15 days; feces collected for 2 consecutive hours on days 0, 5, 10, and 15 upon treatment | ERIC-PCR; qPCR with primers targeting 16S rRNA gene of specific bacterial groups | GS group vs. control: increased: Lactobacillus RGS group vs. control: increased: Bifidobacterium, Clostridium Cluster IV | [120] | |||
| Panax quinquefolius, radix | ethanolic extract (70%) PQE | 2 groups: drinking water; metronidazole-supplemented drinking water; after 7 days, mice received PQE (30 mg/kg/day) (n = 3 per group) | male C57BL6 mice (6–8 weeks) | treated for 3 days, fecal samples collected | - | - | HPLC/TOF-MS | compound K detected in feces from mice treated with no antibiotic; undetectable in feces of metronidazole- pretreated mice | [148] |
| air-dried American ginseng powder | 1 group: 2 g American ginseng powder per day for 7 days (n = 6); no control | healthy male volunteers (ages 18–45 years) | day 1 (control) and day 7: feces samples collected | - | - | LC-Q-TOF-MS | 16 metabolites in feces: compound K major metabolite; Rk1 and Rg5, Rk3 and Rh4, Rg6 and F4 produced via dehydration | [150] | |
| air-dried American ginseng powder | 1 group: 2 g American ginseng powder in capsules per day for 7 days (n = 6), no control | healthy male volunteers (ages 18–45 years); three on Asian diet and three on Western diet | day 1 (control) and day 7: feces samples collected | - | - | LC-Q-TOF-MS | higher relative abundance in Asian diet subjects: ginsenoside Rb1; higher relative abundance in Western diet subjects: compound K, ginsenoside Rh2 | [151] | |
| ethanolic extract (70%) AGE | 4 groups: control, azoxymethane/DSS-induced colitis model group, AGE low dose (15 mg/kg/day), AGE high dose (30 mg/kg/day) (n = 10 per group) | male A/J mice (6 weeks old with 18–22 g) | treated from day 1 to week 13; fecal samples collected during weeks 1, 2, 5, 8, and 13 | terminal-restriction fragment length polymorphism (T-RFLP) with broad-range primers for bacterial domain, followed by 16S rRNA gene NGS Illumina) | AGE vs. model group: increased rel. levels of Firmicutes, decreased rel. levels of Bacteroidetes and Verrucomicrobia | untargeted GC/TOF-MS | major metabolites: compound K, ginsenoside Rg3, and protopanaxadiol | [152] | |
| Paullinia cupana, semen | guarana seed powder | 3 groups: guarana (0.021 g/kg); caffeine (0.0007 g/kg); saline (1.0 mL/kg) (n = 10 per group) | male Wistar rats (250–300 g) | treated for 21 days; fecal samples were collected | 16S rRNA gene, NGS (Ion PGM System) | rel. decrease in Bacteroidetes and Prevotella, rel. increase in cyanobacteria in guarana group compared to caffeine and saline group; decrease in Lactobacillus in caffeine and guarana group | - | - | [156] |
| guarana seed powder (Gua) | 4 groups: control diet (low-fat, CD); CD + 0.5% Gua; Western diet (WD; high fat); WD + 0.5% Gua (n = 12 per group) | male Wistar rats (8 weeks old) | treated for 18 weeks; fecal samples were collected during week 16 | V1–V3 region of 16S rRNA gene, NGS (Illumina) | WD + 0.5% Gua compared to WD: increase in Butyricicoccus and Streptococcus, decrease in Holdemania | - | - | [157] | |
| Polygala tenuifolia, radix | ethanolic extract (75%) RPE | 3 groups: control (saline), 0.5 h group, and 1.5 h group (both RPE 2 g/kg) (n = 6 per group) | male Sprague Dawley rats (200 ± 20 g) | treated for 6 days | - | - | targeted UHPLC-Q-TOF-MS | feces of RPE groups: 44 native RPE constituents (3 xanthones, 1 sucrose ester, 9 oligoesters, 33 saponins), and 29 metabolites | [160] |
| water extract (100 g radix polygalae powder refluxed at 100 °C with 1 L water) PGW | 3 groups: normal diet (ND; n = 8), HFD control (HFD-C), HFD- polygala group (HFD-PGW) (PGW dissolved in distilled water orally once daily, dose not given) (n = 10 per group) | male ICR mice (4 weeks old) | treated for 5 weeks after model construction, fecal samples collected after 5 weeks treatment | V3–V4 region of 16S rRNA gene, NGS (Illumina) | HFD-PGW group vs. HFD-C group: reduced Bacteroidetes/Firmicutes ratio in HFD-C group mitigated in HFD-PGW group; rel. increase: Proteobacteria, Bacteroidaceae, Rikenellaceae, S24-7, Desulfovibrionaceae, Enterobacteriaceae; rel. decrease: Deferribacteres, Lachnospiraceae, Ruminococcaceae, Peptococcaceae | - | - | [161] | |
| Polygonatum sibiricum, radix | ethanolic extract (70%) with a saponin yield of 3.07 ± 0.02 mg/g (PSS) | 6 groups: non-diabetic control, diabetic model control (DMC, HFD-streptozotocin induced), metformin-positive control group (MPC), LPT (1 g/kg PSS), MPT (1.5 g/kg PDD), HPT (2 g/kg PSS) | male ICR mice (6 weeks, weight 20 ± 1.5 g) | treated for 5 weeks, fecal samples were collected during week 5 | agar plate counting using fecal bacteria selective agars | LPT, MPT, HPT groups vs. DMC group: number of probiotics in the feces increased significantly (p < 0.01), especially Bifidobacterium; the number of harmful bacteria (Enterococcus, Enterobacteriaceae) decreased | - | - | [164] |
| Rhodiola rosea, radix | root extract (SHR-5) | two groups: control group (yeast solution); SHR-5 group (25 mg/mL SHR-5 + yeast solution) | Oregon-R flies | treated throughout the lifespan of the flies; flies were homogenized in PBS for microbiome analyses | V6–V8 region of 16S rRNA gene, NGS (Illumina); bacterial growth plates | SHR-5 group: increase in Acetobacter; decrease in Lactobacillales; SHR-5 decreased the total culturable bacterial load of the fly gut while increasing the overall quantifiable bacterial load | - | - | [167] |
| Salvia rosmarinus, folium | rosemary extract (RE) containing 60% carnosic acid | 3 groups: control; chronic restraint stress (CRS) group; CRS + RE (100 mg/kg) (n = 12 per group) | male adult ICR mice | treated for 21 days; fecal samples collected (timepoint not indicated) | V1–V3 region of 16S rRNA gene, NGS (Illumina) | CRS+RE group: reversed intestinal microbiota composition of CRS group; rel. increase Firmicutes and Lactobacillus; rel. decrease Bacteroidetes and Proteobacteria | - | - | [42] |
| Schisandra chinensis, fructus | total ethanolic extract (95%) (SCE), lignan fraction (SCL), polysaccharide fraction (SCPS), volatile oil (SCVO) | 6 groups: control, lipopolysaccharide (LPS)-induced inflammation, SCE (1.2 g/kg) + LPS, SCL (500 mg/kg BW) + LPS, SCPS (300 mg/kg) + LPS, SCVO (150 mg/kg BW) + LPS (n = 10 per group) | C57BL/6 mice (18–22 g) | treated for 14 days; fecal samples collected after behavioral tests | V3–V4 region of 16S rRNA gene, NGS (Illumina) | SCE and SCL-treated group: LPS-induced increase in Bacteroidetes and decrease in Firmicutes alleviated rel. increase: Lactobacillus; rel. decrease: Bacteroides | SCFA analysis by GC-MSTQ8040 | SCE and SCL-treated group: increased levels of butyric acid and propionic acid | [173] |
| dried, powdered fruits (SC); wine- processed fruits (WSC); main SC and WSC constituent: lignans | 4 groups: control (0.9% saline); chronic unpredictable stress procedure (CUSP) group; CUSP + SC (280 mg/kg BW); CUSP + WSC (280 mg/kg BW) (n = 6 per group) | male Sprague Dawley rats (180–220 g) | treated for 5 weeks; fresh fecal samples collected on day 30 | V3–V4 region of 16S rRNA gene, NGS; (Illumina) | CUSP+SC/WSC vs. CUSP: increased rel. abundance of Lachnospiraceae; rel. decrease in Bacteroides | lactate analysis in the intestine by ELISA | reduction: D- and L-lactate | [172] | |
| water extract (SCW) | two groups: placebo (n = 15); SCW (n = 13) 2 pouches in a day, equivalent to 6.7 g of dried S. chinensis fruits | female obese volunteers BMI ≥ 25 kg/m2 | feces samples collected at the beginning and the end of treatment | denaturing gradient gel electrophoresis; qPCR with specific primers | SCF group vs. placebo: increase: Akkermansia, Roseburia, Bacteroides, Prevotella, Bifidobacterium; decrease: Ruminococcus | - | [174] | ||
| S. chinensis polysaccharide extract (total carbohydrate content: 94.9%) (SCP) | 4 groups: normal control (saline), model group (DSS-induced colitis), DSS+ positive control (salazosulfapyridine), DSS + SCP (8.0 g/kg BW) (n = 8 per group) | male C57BL/6J mice (20 ± 2 g, 8–10 weeks old) | treated for 3 weeks | 16S rRNA gene, NGS (Illumina) | SCP vs. DSS group: Firmicutes, Proteobacteria, and Bacteroidetes returned to normal relative abundances; rel. increase: Alloprevotella, Saccharibacteria, Bacteroidetes Bacteroidales_S24_7_group family; rel. decrease: Anaerotruncus, Firmicutes | SCFA analysis by GC-MS | SCP vs. DSS group: recovery/increase in propionic acid, butyric acid, valeric acid | [175] | |
| Trigonella foenum-graecum, semen | ground seeds (2% of the diet by weight) (FS) | 4 groups: HFD; HFD + FG; control diet (CD); CD + FG (n = 20 per group) | male C57BL/6J mice (9 weeks old) | treated for 16 weeks; fecal samples collected after euthanasia | V4 region of 16S rRNA gene, NGS (Illumina) | CD ± FS and HFD ± FS: shifts in alpha and beta diversity compared to non-FS groups; diversity and significantly increased alpha diversity; FS mitigated dysbiotic effects of HFD | - | - | [177] |
| fenugreek seeds (28% galactomannan and 0.672% apigenin-7-glycoside) FS | 2 groups: control (n = 11); FS (n = 10, 1.5 g fenugreek seeds/kg BW) | male castrated piglets (Duroc × Piétrain; 8.26 kg) | treated for 28 days; stomach, distal jejunum, ileum, cecum, and colon contents removed after sacrifice | qPCR with specific primers | increase: Lactobacillus group, L. johnsonii, Clostridium cluster I, L. reuteri decrease: Escherichia/Hafnia/Shigella group Clostridium cluster YIV remained stable | lactate (HPLC), SCFAs (GC-FID) | FS vs. control group: increased colonic butyric acid levels; increased L-lactic acid levels in the small intestinal digesta | [178] | |
| Vitis vinifera, fructus | lyophilized table grape mixture of red-, green-, and black-seeded and seedless grapes (G) | 5 groups: low fat (LF; 10% of energy from fat); high fat (HF; 34% of energy from fat) plus 3% G (w/w; HF-3G); HF plus 3% sugar (w/w; HF-3S); HF plus 5% G (HF-5G); HF plus 5% sugar (HF-5S) (n = 10 per group) | male C57BL/6J mice (4 weeks old) | treated for 11 weeks; colonic mucosa and digesta from duodenum, jejunum, cecum, proximal and distal colon collected after sacrifice | qPCR with primers targeting 16S rRNA gene of specific bacterial genera; V3–V4 region of 16S rRNA, Illumina sequencing | decreased alpha diversity in HF-5G and HF-5S group compared to HF-3G group; increase in Allobaculum in LF and HF-3G group; tendency to increase in Akkermansia muciniphila in HF-3G and HF-5G group; decrease in Desulfobacter spp. in HF-3G group | - | - | [197] |
| phenolic compound-rich grape pomace extract (70% ethanol; 920 mg/g phenolic compounds) (PC) | 5 groups: PC 2.5 (2.5 mg/kgBW/d); PC 5 (5 mg/kg BW/d); PC 10 (10 mg/kg BW/d); PC 20 (20 mg/kg/d); control group (0.1% DMSO) (n = 6 per group) | male adult Wistar rats (2 months old) | treated for 14 months; fecal samples collected at baseline, and after 6 and 14 months of treatment | qPCR with primers targeting 16S rRNA gene of specific bacterial genera and universal primer for total bacteria | increase in Bifidobacterium in PC 2.5 and PC 5 groups after 6 and 14 months compared to control and young rats; PC (all groups) abolished increase in Clostridium (cluster 1) after 14 months occurring in control | - | - | [194] | |
| grape antioxidant dietary fiber (GADF) | 2 groups: control diet; GADF diet (50 g/kg) (n = 10 per group) | male Wistar rats (body weight of 215 ± 2 g) | treated for 4 weeks; cecal content collected after sacrifice | qPCR with primers targeting 16S rRNA gene of specific bacterial genera | GADF group: increase: Lactobacillus spp. decrease: Bifidobacterium spp. | - | - | [195] | |
| grape seed and grape marc meal extract (GSGME) | 3 groups: control group (basal diet BD); GSGME group (BD with 1% GSGME) (n = 16 per group) | crossbreed pigs (5 weeks old) | treated for 4 weeks; fecal samples collected after sacrifice | qPCR with primers targeting 16S rRNA gene of specific bacterial genera | decrease in Streptococcus in GSGME group | volatile fatty acid analysis by GC with FI detector | Decrease in acetic acid, propionic acid, and valeric acid in GSGME group | [196] | |
| grape extract (GE) | 3 groups: standard diet (LFD, 3.85 kcal g−1, 10% energy from fat); high-fat +high-fructose diet (HFFD, 4.73 kcal g−1, 22% fructose + 22% lard); HFFD + 1% w/w GE diet (HFFD + GE) (n = 12 per group) | male C57BL/6Cnc mice (4 weeks old) | treated for 13 weeks; fecal samples were collected after sacrifice | V3–V4 region of 16S rRNA gene, NGS | GE group: increased gut microbiota diversity, Firmicutes/Bacteroidetes ratio, rel. increase in Verrucomicrobia, Bifidobacteria, Akkermansia, Clostridia; rel. decrease in Bacteroidetes, Proteobacteria, Desulfovibrio, and Bacteroides | - | - | [199] | |
| lyophilized table grape mixture (red-, green-, and black-seeded and seedless) (GP); extractable polyphenol-rich fraction (EP) (180 mg/g total phenolics); nonextractable, polyphenol-poor fraction (NEP) (10.5 mg/g total phenolics) | 6 groups: low fat (LF; 10% of energy from fat); high fat (HF; 44% of energy from fat); HF plus extractable polyphenol-rich fraction (HF-EP); HF plus nonextractable, polyphenol-poor fraction (HF-NEP); HF plus extractable and nonextractable polyphenol fraction (HF-EP + NEP); HF plus 5% powdered grapes (HF-GP) (n = 10 per group) | male C57BL/6J mice (4 weeks old) | treated for 16 weeks; cecal mucosa and digesta samples collected after sacrifice | V4–V5 region of 16S rRNA gene, NGS (Illumina) of cecal mucosa samples | HF-GP vs. HF control: rel. increase in microbiota diversity compared to HF control group HF-EP vs. HF-control: rel. increase in Lachnospiraceae HF-NEP vs. HF-control: rel. increase in Coprococcus HF-EP+NEP vs. HF-control: rel. increase in Lachnospiraceae and Coprococcus; rel. decrease in Ruminococcus and Mogibacteriaceae | SCFA analysis in cecal digesta by GC-MS-MS | HF-GP vs. HF-EP + NEP group: increase in the SCFAs acetate, propionate, and butyrate HF-EP + NEP vs. HF control group: decrease in cecal acetate | [198] | |
| sun-dried raisins | 1 group: three servings per day of 28.3 g raisins (90 cal, 2 g dietary fiber) (n = 13) | healthy volunteers (ages 18–59 years) | treated for 2 weeks; fecal samples collected before the start of raisin consumption, on day 7 and day 14 | V1–V2 region of 16S rRNA gene, NGS (Illumina) | weeks 1 and 2 vs. day 0: rel. increase in Ruminococcaceae; Faecalibacterium prausnitzii, and Bacteroidetes longum rel. decrease in Bifidobacterium spp., Klebsiella spp., Prevotella spp. | - | - | [192] | |
| red grape pomace (GP) extract (Eminol®) | 1 group: two capsules of GP extract per day (1400 mg GP/day) (n = 10) | healthy female volunteers (ages 25–65 years; BMI < 25 kg/m2) | treated for 21 days; fecal samples collected after washout period, on day 14 and on day 21 of GP consumption | qPCR with primers targeting specific bacterial genera | no change in the intestinal microbiota composition | phenolic metabolite analysis by UPLC-ESI-MS/MS; short- and medium-chain fatty acid analysis by SPME-GCMS | day 0 vs. day 7 or 14: SCFA: increase in total SCFAs and propionic acid (14 and 21 days); increase in acetic acid (14 days) MCFA: decrease in pentanoic, hexanoic, and octanoic acids; fecal phenolic metabolites: increase in 3-(4′-hydroxyphenyl)-propionic acid | [200] | |
| Vitis vinifera, semen | grape seed tannins: monomer fraction (GSM); polymer fraction (GSP) | 3 groups: control group (standard diet), GSM group (standard diet + GSM 71 mg/kg diet), GSP (standard diet + GSP, 71 mg/kg diet) (n = 6 per group) | male Sprague Dawley rats (145 g) | treated for 12 weeks; cecal contents were collected after sacrifice | - | - | cecal volatile fatty acid (SCFA) analysis by GC | GSP vs. control: increase in total VFAs, acetate, propionate, and butyrate GMP vs. control: increase in acetate, decrease in butyrate | [184] |
| grape seed extract (GSE) | 1 group: standard diet (SD, 2 kg per day), treatment diet (SD plus 1% w/w GSE) (n = 6) | crossbred female pigs (130–150 kg) | duration 12 days; SD for 3 days, SD+GSE for 6 days, post-treatment SD for 3 days; fecal samples collected daily | V3–V4 region of 16S rRNA gene NGS (Illumina) | before vs. during GSE: increase in Lachnospiraceae, unclassified Clostridales, Lactobacillus, and Ruminococcus | phenolic metabolite analysis by HPLC-MS | before vs. during GSE: increase in 4-hydroxyphenylvaleric acid and 3-hydroxybenzoic acid | [185] | |
| grape seed meal (GSM) | 4 groups: control group (standard diet, SD); AFB1 group (SD + 320 µg/kg aflatoxin B1, AFB1); GSM group (SD+ 8% GSM); AFB1 + GSM group (SD + 32 µg/kg AFB1 + 8% GSM) (n = 6 per group) | healthy weaned crossbred TOPIGS-40 hybrid piglets (9.13 ± 0.03 kg) | treated for 30 days; colon contents collected after sacrifice | V3–V4 region of 16S rRNA gene NGS | GS vs. control: rel. increase in Bacteroidetes, Proteobacteria, Prevotella, Megasphaera, Clostridiales, and Anaerovibrio; rel. decrease in Firmicutes, Lactobacillus, and Lachnospiraceae | - | - | [186] | |
| grape seed meal (GSM) | 4 groups: control group (standard diet, SD); DSS colitis group (SD + DSS 1 g/kg BW); GSM group (SD + 8% GSM); DSS+GSM group (SD + 8% GSM + DSS 1 g/kg BW) (n = 5–6 per group) | weaned crossbred TOPIGS-40 hybrid piglets (9.13 ± 0.03 kg) | treated for 30 days; descending colon contents collected after sacrifice | V3–V4 region of 16S rRNA gene NGS (Illumina) | rel. increase in Proteobacteria and rel. decrease in Lactobacillus in DSS, GSM, and DSS + GMS group; rel. increase in Megasphaera and Anaerovibrio in GSM and DSS+GSM groups; rel. decrease in Roseburia in GSM and DSS + GSM groups | SCFA analysis by GC-FID | increase in butyric acid and valeric acid, and decrease in acetic acid by GSM | [187] | |
| GSE Leucoselect® (proanthocyanidin content >80%) | 3 groups: sham-operated group (standard diet, SD); OVX group (SD); OVX + GSE group (GSE diet, 10 g GSE/5 kg diet) (n = 5 per group) | female C57BL/6J mice (7 weeks old) | treated for 8 weeks; fecal samples were collected 8 weeks after surgery | qPCR with group-specific primers targeting 16S rRNA of total bacteria, Firmicutes, and Bacteroidetes | OVX + GSE vs. OVX group: increase in Bacteroidetes; decrease in Firmicutes and Firmicutes/Bacteroidetes ratio | - | - | [188] | |
| GSE Vitaflavan® (procyanidin content 75.6%) | 4 groups: control LFD (10% kcal from fat, CD); HFD (45% kcal from fat); HFD + 0.07 g GSE/4057 kcal (HF10); HFD + 0.70 g GSE/4057 kcal (HF100) (n = 8 per group) | male C57BL/6J mice (9 weeks old) | treated for 16 weeks; small intestine, cecum, and colonic tissue collected after sacrifice | V4 region of 16S rRNA gene NGS (Illumina) of mucosal-adherent metabolically active bacteria (results converted to 16S cDNA values; HF 100 group not analyzed) | HF10 group vs. HFD: small intestine: decrease in Firmicutes, Bacteroides-Prevotella spp., and Parabacteroides spp.; increase in Bacteroidetes and Bifidobacterium spp. | - | - | [189] | |
| proanthocyanidin-rich GSE | 1 group, 3 treatments: 0.5 g GSE/day (0.19 g/day/subject as proanthocyanidin); 0.5 g green tea extract/day; 0.5 g champignon extract/day | 9 healthy male adults (ages 37–42 years) | duration 10 weeks; 6 periods: 14-day washout period, three 14-day administration periods interrupted by two 14-day washout periods; fecal samples collected on days 0, 2, 7, and 14 of administration | bacterial plate counting | GSE, day 14 vs. day 0: increase in Bifidobacterium; tendency to decrease in Enterobacteriaceae | fecal putrefactive product analysis by GC; ammonium analysis by HPLC | GSE, day 14 vs. day 0: tendency to decrease in skatol, indole, 4-ethylphenol, p-cresol, phenol, and ammonia after grape seed extract administration | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pferschy-Wenzig, E.-M.; Pausan, M.R.; Ardjomand-Woelkart, K.; Röck, S.; Ammar, R.M.; Kelber, O.; Moissl-Eichinger, C.; Bauer, R. Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients 2022, 14, 2111. https://doi.org/10.3390/nu14102111
Pferschy-Wenzig E-M, Pausan MR, Ardjomand-Woelkart K, Röck S, Ammar RM, Kelber O, Moissl-Eichinger C, Bauer R. Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients. 2022; 14(10):2111. https://doi.org/10.3390/nu14102111
Chicago/Turabian StylePferschy-Wenzig, Eva-Maria, Manuela R. Pausan, Karin Ardjomand-Woelkart, Stefanie Röck, Ramy M. Ammar, Olaf Kelber, Christine Moissl-Eichinger, and Rudolf Bauer. 2022. "Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review" Nutrients 14, no. 10: 2111. https://doi.org/10.3390/nu14102111
APA StylePferschy-Wenzig, E.-M., Pausan, M. R., Ardjomand-Woelkart, K., Röck, S., Ammar, R. M., Kelber, O., Moissl-Eichinger, C., & Bauer, R. (2022). Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients, 14(10), 2111. https://doi.org/10.3390/nu14102111








