Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Baseline Evaluation
2.3. Scores and Indexes
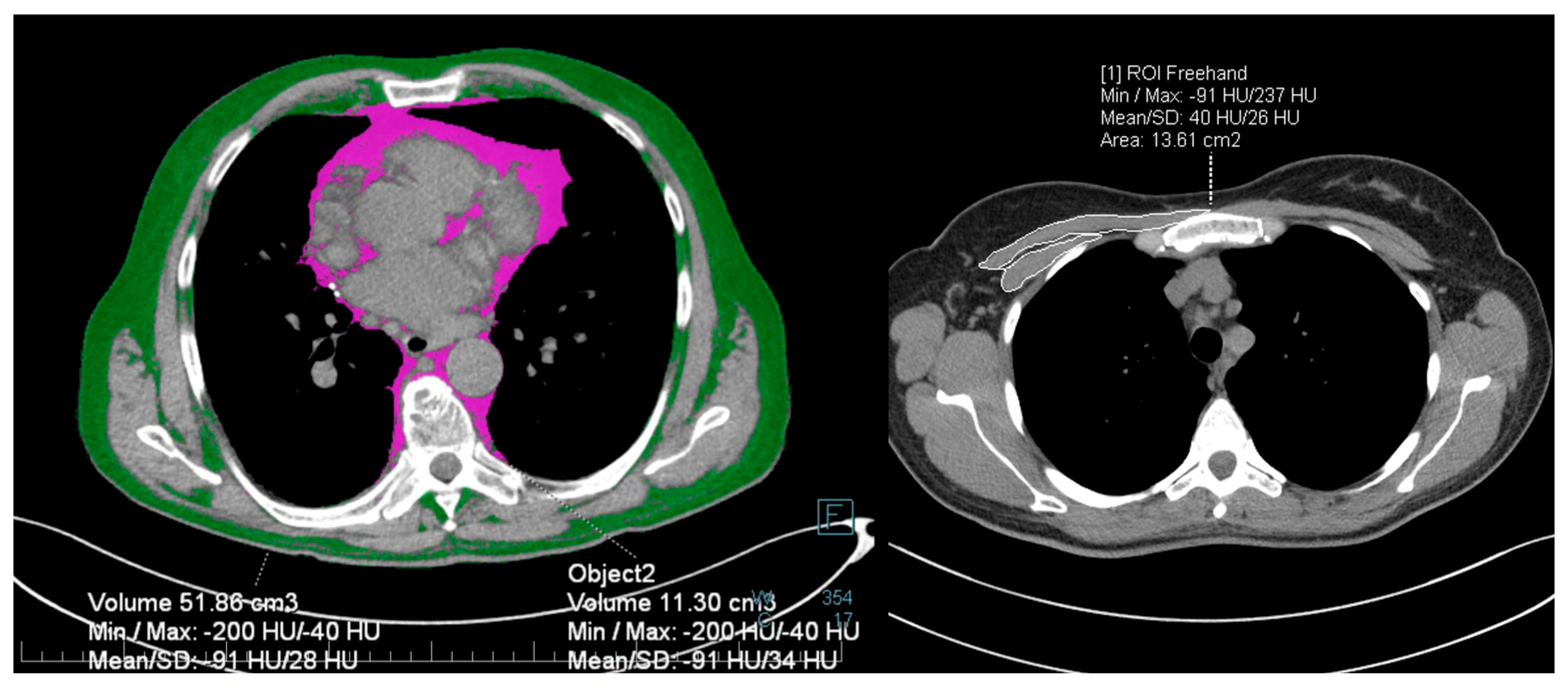
2.4. Imaging
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Taylor, E.H.; Marson, E.J.; Elhadi, M.; Macleod, K.D.M.; Yu, Y.C.; Davids, R.; Boden, R.; Overmeyer, R.C.; Ramakrishnan, R.; Thomson, D.A.; et al. Factors Associated with Mortality in Patients with COVID-19 Admitted to Intensive Care: A Systematic Review and Meta-Analysis. Anaesthesia 2021, 76, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population Risk Factors for Severe Disease and Mortality in COVID-19: A Global Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition and Immunity: Lessons for COVID-19. Nutr. Diabetes 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Chekole, Y.A.; Estifanos, M.B.; Abate, K.H.; Kabthymer, R.H. Prevalence and Outcomes of Malnutrition among Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2021, 43, 174–183. [Google Scholar] [CrossRef]
- Kurtz, A.; Grant, K.; Marano, R.; Arrieta, A.; Grant, K.; Feaster, W.; Steele, C.; Ehwerhemuepha, L. Long-Term Effects of Malnutrition on Severity of COVID-19. Sci. Rep. 2021, 11, 14974. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.; Seguin, P.; Tamion, F.; Pichard, C.; Singer, P. Nutrition of the COVID-19 Patient in the Intensive Care Unit (ICU): A Practical Guidance. Crit. Care 2020, 24, 447. [Google Scholar] [CrossRef]
- Loi, M.; Wang, J.; Ong, C.; Lee, J.H. Nutritional Support of Critically Ill Adults and Children with Acute Respiratory Distress Syndrome: A Clinical Review. Clin. Nutr. ESPEN 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M.; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. Clinical Guidelines—Nutrition Screening, Assessment, and Intervention in Adults. J. Parenter. Enter. Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. [Prognostic Nutritional Index in Gastrointestinal Surgery of Malnourished Cancer Patients] Japanese. Nihon Geka Gekkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Vilar, N.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A Tool for Controlling Nutritional Status. First Validation in a Hospital Population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying Critically Ill Patients Who Benefit the Most from Nutrition Therapy: The Development and Initial Validation of a Novel Risk Assessment Tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying Critically-Ill Patients Who Will Benefit Most from Nutritional Therapy: Further Validation of the “Modified NUTRIC” Nutritional Risk Assessment Tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- van Heusden, H.C.; Swartz, J.E.; Chargi, N.; de Jong, P.A.; van Baal, M.C.P.M.; Wegner, I.; de Bree, R. Feasibility of Assessment of Skeletal Muscle Mass on a Single Cross-Sectional Image at the Level of the Fourth Thoracic Vertebra. Eur. J. Radiol. 2021, 142, 109879. [Google Scholar] [CrossRef]
- Schiaffino, S.; Albano, D.; Cozzi, A.; Messina, C.; Arioli, R.; Bna, C.; Bruno, A.; Carbonaro, L.; Carriero, A.; Carriero, S.; et al. CT-Derived Chest Muscle Metrics for Outcome Prediction in Patients with COVID-19. Radiology 2021, 300, E328–E336. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, P.; Miranda-Zazueta, G.; Ortiz-Brizuela, E.; Gonzalez-Lara, M.F.; Tamez-Torres, K.M.; Roman-Montes, C.M.; Diaz-Mejia, B.A.; Perez-Garcia, E.; Villanueva-Reza, M.; Tovar-Mendez, V.H.; et al. Low Thoracic Skeletal Muscle Area Is Not Associated with Negative Outcomes in Patients with COVID-19. Am. J. Phys. Med. Rehabil. 2021, 100, 413–418. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, R.C. A New Method of Classifying Prognostic in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J. APACHE II: A Severity of Disease Classification System. Crit. Care Med. 1985, 13, 812–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT Image Visual Quantitative Evaluation and Clinical Classification of Coronavirus Disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besutti, G.; Pellegrini, M.; Ottone, M.; Cantini, M.; Milic, J.; Bonelli, E.; Dolci, G.; Cassone, G.; Ligabue, G.; Spaggiari, L.; et al. The Impact of Chest CT Body Composition Parameters on Clinical Outcomes in COVID-19 Patients. PLoS ONE 2021, 16, e0251768. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The Prognostic Significance of the Prognostic Nutritional Index in Cancer: A Systematic Review and Meta-Analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Sung, S.H.; Cheng, H.M.; Hsu, P.F.; Guo, C.Y.; Yu, W.C.; Chen, C.H. Prognostic Nutritional Index and the Risk of Mortality in Patients With Acute Heart Failure. J. Am. Heart Assoc. 2017, 6, 1673. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ren, C.; Jiang, J. The Relationship between Prognostic Nutritional Index and All-Cause Mortality in Critically Ill Patients: A Retrospective Study. Int. J. Gen. Med. 2021, 14, 3619–3626. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.-W.; Wei, X.; Li, F.; Liao, X.-L.; Yuan, H.; Huang, D.; Quin, T.; Geng, H.; Wang, S. Predictive Value of Prognostic Nutritional Index on COVID-19 Severity. Front. Nutr. 2021, 7, 582736. [Google Scholar] [CrossRef]
- Hu, X.; Deng, H.; Wang, Y.; Chen, L.; Gu, X.; Wang, X. Predictive Value of the Prognostic Nutritional Index for the Severity of Coronavirus Disease 2019. Nutrition 2021, 84, 111123. [Google Scholar] [CrossRef]
- Wei, W.; Wu, X.; Jin, C.; Mu, T.; Gu, G.; Min, M.; Mu, S.; Han, Y. Predictive Significance of the Prognostic Nutritional Index (PNI) in Patients with Severe COVID-19. J. Immunol. Res. 2021, 2021, 9917302. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Umegaki, O.; Kadono, N.; Minami, T. Presepsin Values and Prognostic Nutritional Index Predict Mortality in Intensive Care Unit Patients with Sepsis: A Pilot Study. BMC Res. Notes 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Kosovali, B.D.; Kucuk, B.; Soyal, O.B.; Mutlu, N.M. Can Prognostic Nutritional Index Predict Mortality in Intensive Care Patients with COVID-19? Int. J. Clin. Pract. 2021, 75, e14800. [Google Scholar] [CrossRef]
- Sugita, Y.; Miyazaki, T.; Shimada, K.; Shimizu, M.; Kunimoto, M.; Ouchi, S.; Aikawa, T.; Kadoguchi, T.; Kawaguchi, Y.; Shiozawa, T.; et al. Correlation of Nutritional Indices on Admission to the Coronary Intensive Care Unit with the Development of Delirium. Nutrients 2018, 10, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; He, M.; Kang, Y.; Jianguo, X. Controlling Nutritional Status (CONUT) Score Is a Predictive Marker for Patients with Traumatic Brain Injury. Clin. Neurol. Neurosurg. 2020, 195, 105909. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K.; Shimizu, W. The Prognostic Impact of Malnutrition in Patients with Severely Decompensated Acute Heart Failure, as Assessed Using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) Score. Heart Vessel. 2018, 33, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Lai, Q.C.; Duan, Q.; Ge, P.; Ye, L. Nutritional Indices at Admission Are Associated with Mortality Rates of Patients in the Intensive Care Unit. Eur. J. Clin. Nutr. 2021, 76, 557–563. [Google Scholar] [CrossRef]
- Bengelloun, A.K.; Ortega, G.J.; Ancochea, J.; Sanz-Garcia, A.; Rodríguez-Serrano, D.A.; Fernández-Jiménez, G.; Girón, R.; Ávalos, E.; Soriano, J.B.; de Ulíbarri, J.I. Usefulness of the CONUT Index upon Hospital Admission as a Potential Prognostic Indicator of COVID-19 Health Outcomes. Chin. Med. J. 2022, 135, 187–193. [Google Scholar] [CrossRef]
- Song, F.; Ma, H.; Wang, S.; Qin, T.; Xu, Q.; Yuan, H.; Li, F.; Wang, Z.; Liao, Y.; Tan, X.; et al. Nutritional Screening Based on Objective Indices at Admission Predicts In-Hospital Mortality in Patients with COVID-19. Nutr. J. 2021, 20, 46. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Xiang, Y.; Tao, C.; Yu, H.; Huang, J. A Correlation Analysis Between the Nutritional Status and Prognosis of COVID-19 Patients. J. Nutr. Health Aging 2021, 25, 84–93. [Google Scholar] [CrossRef]
- Li, G.; Zhou, C.-L.; Ba, Y.-M.; Wang, Y.-M.; Song, B.; Cheng, X.-B.; Dong, Q.-F.; Wang, L.-L.; You, S.-S. Nutritional Risk and Therapy for Severe and Critical COVID-19 Patients: A Multicenter Retrospective Observational Study. Clin. Nutr. 2021, 40, 2154–2161. [Google Scholar] [CrossRef]
- Liberti, A.; Piacentino, E.; Umbrello, M.; Muttini, S. Comparison between Nutric Score and Modified Nutric Score to Assess ICU Mortality in Critically Ill Patients with COVID-19. Clin. Nutr. ESPEN 2021, 44, 479–482. [Google Scholar] [CrossRef]
- Zhang, P.; He, Z.; Yu, G.; Peng, D.; Feng, Y.; Ling, J.; Wang, Y.; Li, S.; Bian, Y. The Modified NUTRIC Score Can Be Used for Nutritional Risk Assessment as Well as Prognosis Prediction in Critically Ill COVID-19 Patients. Clin. Nutr. 2021, 40, 534–541. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, A.; Kumar, A.; Pattanayak, A.; Singh, K.; Singh, P. Nutric Score as a Predictor of Outcome in COVID-19 ARDS Patients: A Retrospective Observational Study. Indian J. Anaesth. 2021, 66, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Tu, C.Y.; Chen, C.H.; Wang, Y.T.; Chen, W.C.; Fu, P.K.; Chen, C.M.; Lai, C.C.; Kuo, L.K.; Ku, S.C.; et al. Significance of the Modified Nutric Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients 2022, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poros, B.; Becker-Pennrich, A.S.; Sabel, B.; Stemmler, H.J.; Wassilowsky, D.; Weig, T.; Hinske, L.C.; Zwissler, B.; Ricke, J.; Hoechter, D.J. Anthropometric Analysis of Body Habitus and Outcomes in Critically Ill COVID-19 Patients. Obes. Med. 2021, 25, 100358. [Google Scholar] [CrossRef] [PubMed]
| Score | Abbreviation | Variables/Calculation Formula | Original Reference |
|---|---|---|---|
| Prognostic Nutritional Index | PNI | (10 × serum albumin (g/dL)) + (0.005 × lymphocytes/μL) | Onodera T, et al. [11] |
| Controlling Nutritional Status | CONUT | Point scoring based on serum albumin (g/dL), lymphocyte count/mL, and total cholesterol (mg/dL), ranging from 0–1 (normal), 2–4 (mild), 5–8 (moderate), and 9–12 (severe) | Ignacio de Ulíbarri J, et al. [12] |
| Nutritional Risk in Critically Ill | NUTRIC | Age, APACHE II, SOFA scores, number of comorbidities, days in hospital to ICU admission, and Interleukin-6 | Heyland D, et al. [13] |
| Modified Nutritional Risk in Critically Ill | mNUTRIC | Age, APACHE II, SOFA scores, number of comorbidities, and days in hospital to ICU admission | Rahman A, et al. [14] |
| Variable | Entire Group (n = 90) | Survivors (n = 48) | Deceased (n = 42) | p-Value |
|---|---|---|---|---|
| General data | ||||
| Age (years) | 67 (63.2–67.9) | 62.5 (57.9–64.1) | 72.5 (67.7–73.7) | <0.001 |
| Gender, male (n, %) | 53 (58.9%) | 26 (54.1%) | 27 (64.2%) | 0.334 |
| Charlson Comorbidity Index | 4 (4–5.4) | 3 (2.8–4.6) | 5 (5–6.7) | <0.002 |
| Total hospital stay (days) | 24 (23.8–31.2) | 29.5 (24.4–35.3) | 21.5 (19.8–29.8) | 0.139 |
| Length of ICU stay (days) | 11.1 (11–17.1) | 8 (7.8–16.2) | 14 (12–20.9) | <0.001 |
| Mechanical ventilation (n, %) | 45 (50%) | 8 (16.6%) | 37 (88.0%) | <0.001 |
| PaO2/FiO2 at ICU admission | 116 (112.1–142) | 133.5 (128–165.7) | 87.5 (82.2–126.1) | 0.022 |
| SOFA score at ICU admission | 5 (4.8–6.1) | 4 (3.9–5.3) | 5.5 (5.5–7.1) | 0.079 |
| APACHE II score at ICU admission | 15 (14.1–17.3) | 14 (11.2–15.3) | 17.1 (16.1–21.1) | 0.028 |
| Laboratory work-up | ||||
| Hemoglobin (g/dL) | 13.8 (13–13.9) | 14 (13.3–14.3) | 13.6 (12.4–13.7) | 0.294 |
| White blood cell count (×109/L) | 7.1 (6.8–9.6) | 7.6 (7.6–10.4) | 6.8 (6.7–9.8) | 0.520 |
| Neutrophil count (×109/L) | 5.8 (5.5–8.2) | 6.2 (6.0–8.8) | 5.4 (5.3–8.3) | 0.131 |
| Lymphocyte count (×109/L) | 0.8 (0.8–1.1) | 0.86 (0.82–1.22) | 0.79 (0.71–1.15) | 0.837 |
| Platelet count (×109/L) | 193 (192–238.2) | 193 (188.2–249.5) | 193.5 (176.8–246.8) | 0.982 |
| C-reactive protein (mg/dL) | 14 (12–16.9) | 15 (119.2–190.1) | 9.6 (9.1–15.6) | 0.599 |
| Procalcitonin (ng/dL) | 0.1 (0.0–3.3) | 0.1 (0.0–3.6) | 0.1 (0.0–3.1) | 0.741 |
| Interleukin-6 (pg/mL) | 23.1 (20–205.2) | 13 (11.1–104.2) | 33.8 (11.2–289.2) | 0.040 |
| Creatinine (mg/dL) | 1.1 (1–1.9) | 1.0 (0.9–1.7) | 1.2 (1.1–2.6) | 0.063 |
| NT-proBNP (pg/mL) | 506 (302.2–4 560.1) | 361.8 (20.6–3062.1) | 830 (760.2–7458.5) | 0.033 |
| Albumin (g/dL) | 2.9 (2.8–3) | 3.1 (3–3.2) | 2.8 (2.6–2.9) | <0.001 |
| Total protein (g/dL) | 5.4 (5.2–5.8) | 5.4 (5.4–5.8) | 5.3 (4.9–6.1) | 0.891 |
| Cholesterol (mg/dL) | 138 (132.6–152) | 145.5 (135.1–159.7) | 134 (120.8–152.2) | 0.551 |
| Triglycerides (mg/dL) | 158 (152–207.2) | 164 (148.7–212.4) | 151.5 (145.5–224.2) | 0.526 |
| Imaging | ||||
| TSS at admission | 14 (12–14.1) | 13 (11.4–14.1) | 14 (11.7–14.7) | 0.899 |
| Peak TSS during hospital stay | 17 (14.7–17.3) | 15 (13.4–16) | 18 (15.4–17.6) | 0.062 |
| Subcutaneous fat (cm3) | 77.9 (70.9–94.5) | 88.2 (80.1–105.5) | 67.2 (66.6–89.6) | 0.062 |
| Intrathoracic fat (cm3) | 9.8 (9.5–11.6) | 10.6 (9.4–12.5) | 9 (8.8–11.5) | 0.131 |
| Total fat (cm3) | 84.5 (88–105.5) | 96.7 (91.9–117.1) | 76.8 (76.1–99.1) | 0.131 |
| Pectoralis muscle area (cm2) | 18.9 (18.1–20.9) | 19.1 (17.5–21.4) | 18.7 (17.6–21.6) | 0.835 |
| Pectoralis muscle density (HU) | 18.5 (16.1–21.2) | 18.5 (15.7–22.8) | 18.5 (14–21.3) | 0.834 |
| Nutritional risk assessment scores | ||||
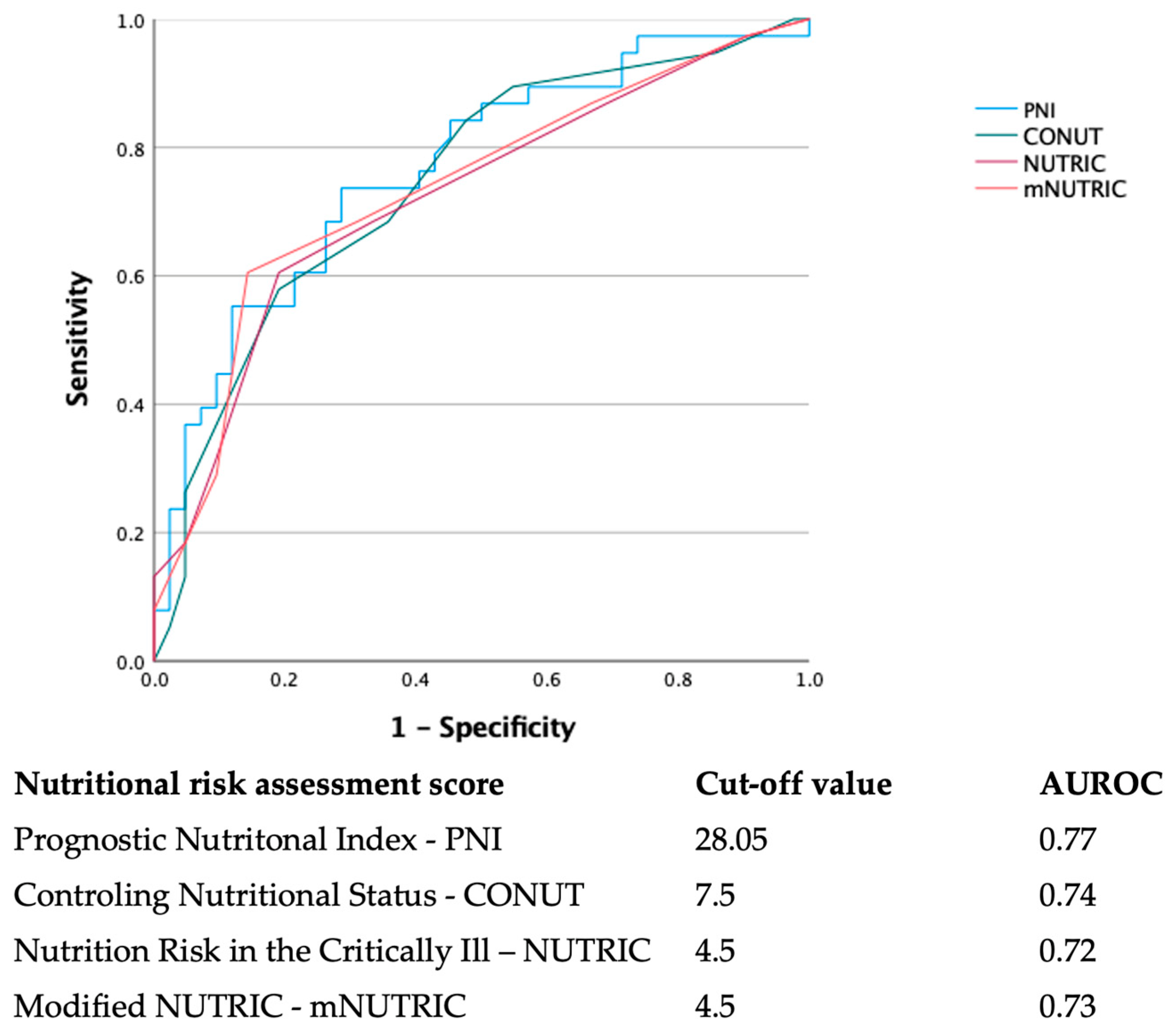
| PNI | 30 (28.5–30.5) | 31.5 (30.2–32.6) | 28 (26–28.9) | <0.001 |
| CONUT | 7 (6.1–7.2) | 5 (4.9–6.3) | 8 (7–8.6) | 0.010 |
| NUTRIC | 3.5 (3.5–4.3) | 3 (2.7–3.7) | 5 (4.1–5.4) | <0.001 |
| mNUTRIC | 3 (2.9–4.2) | 3 (2.7–3.6) | 5 (4–5.2) | <0.001 |
| Mechanical Ventilation | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | 95% Confidence Interval | p-Value | Hazard Ratio | 95% Confidence Interval | p-Value |
| Age (years) | 1.03 | 1.00–1.06 | 0.048 | 1.05 | 1.02–1.09 | <0.001 |
| Charlson Comorbidity Index | 1.03 | 0.95–1.12 | 0.362 | 1.09 | 1.01–1.18 | 0.033 |
| PaO2/FiO2 at ICU admission | 0.99 | 0.98–1.00 | 0.271 | 0.99 | 0.99–1.00 | 0.621 |
| APACHE II score | 1.08 | 1.03–1.12 | 0.004 | 1.07 | 1.02–1.11 | 0.003 |
| Interleukin-6 (pg/mL) | 1.000 | 0.992–1.000 | 0.918 | 1.000 | 0.991–1.000 | 0.911 |
| NT-proBNP (pg/mL) | 1.000 | 1.000–1.001 | 0.943 | 1.000 | 1.000–1.001 | 0.322 |
| Albumin (g/dL) | 0.96 | 0.90–1.02 | 0.332 | 0.91 | 0.85–0.98 | 0.012 |
| Subcutaneous fat (cm3) | 0.99 | 0.99–1.00 | 0.531 | 0.99 | 0.98–1.00 | 0.094 |
| PNI | 0.96 | 0.90–1.03 | 0.333 | 0.91 | 0.85–0.98 | 0.011 |
| CONUT | 1.05 | 0.94–1.18 | 0.303 | 1.15 | 1.02–1.29 | 0.014 |
| NUTRIC | 1.27 | 1.07–1.51 | <0.001 | 1.31 | 1.10–1.56 | <0.001 |
| mNUTRIC | 1.30 | 1.10–1.54 | <0.001 | 1.37 | 1.15–1.62 | <0.001 |
| Variables | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Scenario 1 | |||
| Charlson Comorbidity Index | 1.04 | 0.95–1.15 | 0.310 |
| APACHE II | 1.06 | 1.02–1.11 | <0.001 |
| PNI | 0.93 | 0.87–0.98 | 0.041 |
| Scenario 2 | |||
| Charlson Comorbidity Index | 1.02 | 0.93–1.13 | 0.571 |
| APACHE II | 1.07 | 1.02–1.12 | <0.001 |
| CONUT | 1.14 | 1.03–1.30 | 0.050 |
| Scenario 3 | |||
| Charlson Comorbidity Index | 1.05 | 0.96–1.16 | 0.221 |
| NUTRIC | 1.28 | 1.07–1.54 | <0.001 |
| Scenario 4 | |||
| Charlson Comorbidity Index | 1.05 | 0.96–1.16 | 0.252 |
| mNUTRIC | 1.33 | 1.12–1.59 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodolea, C.; Nemes, A.; Avram, L.; Craciun, R.; Coman, M.; Ene-Cocis, M.; Ciobanu, C.; Crisan, D. Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19. Nutrients 2022, 14, 2105. https://doi.org/10.3390/nu14102105
Bodolea C, Nemes A, Avram L, Craciun R, Coman M, Ene-Cocis M, Ciobanu C, Crisan D. Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19. Nutrients. 2022; 14(10):2105. https://doi.org/10.3390/nu14102105
Chicago/Turabian StyleBodolea, Constantin, Andrada Nemes, Lucretia Avram, Rares Craciun, Mihaela Coman, Mihaela Ene-Cocis, Cristina Ciobanu, and Dana Crisan. 2022. "Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19" Nutrients 14, no. 10: 2105. https://doi.org/10.3390/nu14102105
APA StyleBodolea, C., Nemes, A., Avram, L., Craciun, R., Coman, M., Ene-Cocis, M., Ciobanu, C., & Crisan, D. (2022). Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19. Nutrients, 14(10), 2105. https://doi.org/10.3390/nu14102105







