Prognostic Value of Isolated Sarcopenia or Malnutrition–Sarcopenia Syndrome for Clinical Outcomes in Hospitalized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Subjects
2.2. Procedures
2.3. Nutrition Evaluation
2.4. Muscle Strength and Physical Performance Assessment
2.5. Definition of Sarcopenia
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. General Features of Patients
3.2. Association between Sarcopenia and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the international consensus guideline committee. JPEN J. Parenter. Enter. Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. Glim criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; De Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Churilov, I.; Churilov, L.; Macisaac, R.J.; Ekinci, E.I. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos. Int. 2018, 29, 805–812. [Google Scholar] [CrossRef]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Uhl, S.; Siddique, S.M.; McKeever, L.; Bloschichak, A.; D’Anci, K.; Leas, B.; Mull, N.K.; Tsou, A.Y. Malnutrition in Hospitalized Adults: A Systematic Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2021. [CrossRef]
- Bahat, G.; Kilic, C.; Ozkok, S.; Ozturk, S.; Karan, M.A. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin. Nutr. 2021, 40, 2851–2859. [Google Scholar] [CrossRef]
- Huang, P.; Lou, K.; Xu, J.; Huang, W.; Yin, W.; Xiao, M.; Wang, Y.; Ding, M.; Huang, X. Sarcopenia as a risck factor for future hip fracture: A meta-analysis of prospective cohort studies. J. Nutr. Health Aging 2021, 25, 183–188. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia is associated with mortality in adults: A systematic review and meta-analysis. Gerontology 2021, 68, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Chen, D.; Xie, X.H.; Zhang, J.E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Guillen-Solà, A.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Escalada, F.; Duarte, E. Sarcopenia according to the revised European consensus on definition and diagnosis (EWGSOP2) criteria predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. J. Am. Med. Dir. Assoc. 2019, 20, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, R.; Xu, J.; Fang, K.; Abdelrahim, M.; Chang, L. Sarcopenia as a predictor of postoperative risk of complications, mortality and length of stay following gastrointestinal oncological surgery. Ann. R. Coll. Surg. Engl. 2021, 103, 630–637. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Collins, P.; Rattray, M. Identifying and managing malnutrition, frailty and sarcopenia in the community: A narrative review. Nutrients 2021, 13, 2316. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; De Van Der Schueren, M.A.E. Frailty, sarcopenia, and malnutrition frequently (co-) occur in hospitalized older adults: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L.; Wang, H.; Hao, Q.; Dong, B.; Yang, M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci. Rep. 2017, 7, 3171. [Google Scholar] [CrossRef] [Green Version]
- Gümüşsoy, M.; Atmış, V.; Yalçın, A.; Bahşi, R.; Yiğit, S.; Arı, S.; Dokuyan, H.C.; Gözükara, M.G.; Silay, K. Malnutrition-sarcopenia syndrome and all-cause mortality in hospitalized older people. Clin. Nutr. 2021, 40, 5475–5481. [Google Scholar] [CrossRef]
- Burgel, C.F.; Teixeira, P.P.; Leites, G.M.; Carvalho, G.D.; Modanese, P.V.G.; Rabito, E.I.; Silva, F.M. Concurrent and predictive validity of AND-ASPEN Malnutrition Consensus is satisfactory in hospitalized patients: A longitudinal study. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; Mackenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hall, W.H.; Ramachandran, R.; Narayan, S.; Jani, A.B.; Vijayakumar, S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004, 4, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Silva, T.G.; Bielemann, R.M.; Gonzalez, M.C.; Menezes, A.M.B. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: Results of the COMO VAI? Study. J. Cachexia Sarcopenia Muscle 2015, 7, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gariballa, S.; Alessa, A. Impact of poor muscle strength on clinical and service outcomes of older people during both acute illness and after recovery. BMC Geriatr. 2017, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.; Bae, C.H.; Han, S.J.; Bae, H. Association between length of stay in the intensive care unit and sarcopenia among hemiplegic stroke patients. Ann. Rehabil. Med. 2021, 45, 49–56. [Google Scholar] [CrossRef]
- Attaway, A.; Bellar, A.; Dieye, F.; Wajda, D.; Welch, N.; Dasarathy, S. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J. Am. Geriatr. Soc. 2021, 69, 1815–1825. [Google Scholar] [CrossRef]
- Moore, B.J.; White, S.; Washington, R.; Coenen, N.; Elixhauser, A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Med. Care 2017, 55, 698–705. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Van Ancum, J.M.; Scheerman, K.; Jonkman, N.H.; Smeenk, H.E.; Kruizinga, R.C.; Meskers, C.G.M.; Maier, A.B. Change in muscle strength and muscle mass in older hospitalized patients: A systematic review and meta-analysis. Exp. Gerontol. 2017, 92, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.J.; Buitrago, G.; Rodríguez, N.; Gómez, G.; Sulo, S.; Gómez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2019, 38, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, Y.; Miller, M.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Horwood, C.; Thompson, C. Malnutrition and its association with readmission and death within 7 days and 8-180 days postdischarge in older patients: A prospective observational study. BMJ Open 2017, 7, e018443. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.; Tan, S.; Gao, T.; Ding, W.; Song, Y.; Yang, J.; Li, W.; Yu, W. Sarcopenia associated with 90-day readmission and overall survival after abdominal trauma. Asia Pac. J. Clin. Nutr. 2020, 29, 724–731. [Google Scholar] [CrossRef]
- Chites, V.S.; Teixeira, P.P.; Lima, J.; Burgel, C.F.; Gattermann Pereira, T.; Silva, F.M. Reduced handgrip strength in hospital admission predicts prolonged hospital stay and death but is not accurate to identify malnutrition: A longitudinal study of reduced handgrip strength in hospitalized patients. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1016–1022. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T. The malnutrition overlap syndromes of cachexia and sarcopenia: A malnutrition conundrum. Am. J. Clin. Nutr. 2018, 108, 1157–1158. [Google Scholar] [CrossRef]
- Juby, A.G.; Mager, D.R. A review of nutrition screening tools used to assess the malnutrition-sarcopenia syndrome (MSS) in the older adult. Clin. Nutr. ESPEN 2019, 32, 8–15. [Google Scholar] [CrossRef]
- Sousa, I.M.; Bielemann, R.M.; Gonzalez, M.C.; Da Rocha, I.M.G.; Barbalho, E.R.; De Carvalho, A.L.M.; Dantas, M.A.M.; De Medeiros, G.O.C.; Silva, F.M.; Fayh, A.P.T. Low calf circumference is an independent predictor of mortality in cancer patients: A prospective cohort study. Nutrition 2020, 79, 110816. [Google Scholar] [CrossRef]
- Trussardi Fayh, A.P.; De Sousa, I.M. Comparison of revised EWGSOP2 criteria of sarcopenia in patients with cancer using different parameters of muscle mass. PLoS ONE 2021, 16, e0257446. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Mehrnezhad, A.; Razaviarab, N.; Barbosa-Silva, T.G.; Heymsfield, S.B. Calf circumference: Cutoff values from the NHANES 1999–2006. Am. J. Clin. Nutr. 2021, 113, 1679–1687. [Google Scholar] [CrossRef]
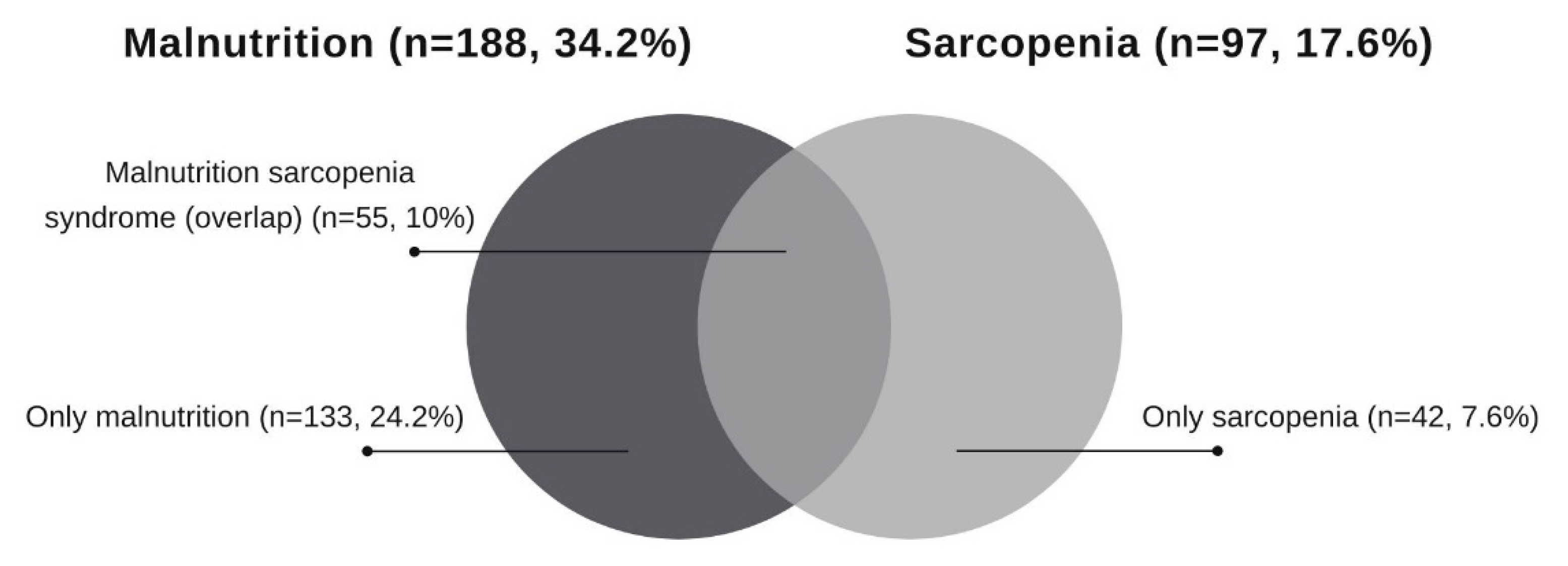
| Non-Sarcopenic Patients (n = 453) | Sarcopenic Patients (n = 97) | p Value | |
|---|---|---|---|
| Age (years) | 54.7 ± 14.6 | 58.0 ± 15.9 | 0.0491 |
| Males | 243 (53.9%) | 48 (49.5%) | 0.501 2 |
| White ethnicity | 352 (77.7%) | 73 (75.3%) | 0.698 2 |
| Cancer diagnosis | 249 (55.0%) | 46 (47.4%) | 0.215 2 |
| Surgical procedure | 328 (72.4%) | 63 (64.9%) | 0.178 2 |
| BMI (kg/m2) | 28.6 ± 5.2 | 22.1 ± 3.4 | <0.001 1 |
| Malnutrition (SGA B or C) | 133 (29.4%) | 55 (56.7%) | <0.001 2 |
| HGS (kg) | 29.2 ± 10.5 | 22.2 ± 8.0 | <0.001 1 |
| CC (cm) | 37.7 ± 3.7 | 31.0 ± 2.3 | <0.001 1 |
| TUG (seconds) | 12.0 ± 4.7 | 13.9 ± 3.6 | 0.229 1 |
| CCI age adjusted | 4.0 (2.0–6.0) | 4.0 (2.5–7.5) | 0.066 3 |
| LOS (days) | 10.0 (4.5–17.0) | 10.0 (4.5–24.5) | 0.033 3 |
| In-hospital death | 4 (0.9%) | 8 (8.2%) | <0.001 4 |
| Hospital readmission * | 145 (33.2%) | 31 (37.3%) | 0.542 2 |
| Six-month death * | 26 (5.9%) | 15 (18.1%) | <0.001 2 |
| Independent Variable | Sarcopenia | Malnutrition | ||
|---|---|---|---|---|
| Dependent Variable | OR 1/HR 2 (95% CI) | p Value | OR 1/HR 2 (95% CI) | p Value |
| Prolonged LOS (>10 days) 1 | 1.23 (0.77–1.97) | 0.382 | 2.27 (1.57–3.28) | <0.001 |
| In-hospital death 2 | 3.95 (1.11–13.91) | 0.034 | 3.62 (1.09–11.95) | 0.035 |
| Readmission within six months 1 | 1.09 (0.66–1.80) | 0.741 | 1.27 (0.87–1.84) | 0.224 |
| Death within six months 1 | 3.25 (1.56–6.62) | 0.001 | 3.42 (1.82–6.45) | <0.001 |
| Predictors | Prolonged LOS (>10 Days) | In-Hospital Death | ||
| OR (95% CI) 1 | p Value | HR (95% CI) 2 | p Value | |
| Without malnutrition or sarcopenia | Ref. | - | Ref. | - |
| With malnutrition or sarcopenia | 1.70 (1.15–2.51) | 0.008 | 2.71 (0.41–17.83) | 0.300 |
| Malnutrition–sarcopenia syndrome | 2.73 (1.42–5.25) | 0.003 | 4.95 (0.98–25.17) | 0.054 |
| Readmission within Six Months | Death within Six Months | |||
| OR (95% CI) 1 | p Value | OR (95% CI) 1 | p Value | |
| Without malnutrition or sarcopenia | Ref. | - | Ref. | - |
| With malnutrition or sarcopenia | 2.69 (1.24–5.86) | <0.001 | 1.64 (0.84–3.20) | 0.147 |
| Malnutrition–sarcopenia syndrome | 7.64 (3.06–19.06) | <0.001 | 1.15 (1.08–1.21) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, I.M.; Burgel, C.F.; Silva, F.M.; Fayh, A.P.T. Prognostic Value of Isolated Sarcopenia or Malnutrition–Sarcopenia Syndrome for Clinical Outcomes in Hospitalized Patients. Nutrients 2022, 14, 2207. https://doi.org/10.3390/nu14112207
Sousa IM, Burgel CF, Silva FM, Fayh APT. Prognostic Value of Isolated Sarcopenia or Malnutrition–Sarcopenia Syndrome for Clinical Outcomes in Hospitalized Patients. Nutrients. 2022; 14(11):2207. https://doi.org/10.3390/nu14112207
Chicago/Turabian StyleSousa, Iasmin Matias, Camila Ferri Burgel, Flávia Moraes Silva, and Ana Paula Trussardi Fayh. 2022. "Prognostic Value of Isolated Sarcopenia or Malnutrition–Sarcopenia Syndrome for Clinical Outcomes in Hospitalized Patients" Nutrients 14, no. 11: 2207. https://doi.org/10.3390/nu14112207
APA StyleSousa, I. M., Burgel, C. F., Silva, F. M., & Fayh, A. P. T. (2022). Prognostic Value of Isolated Sarcopenia or Malnutrition–Sarcopenia Syndrome for Clinical Outcomes in Hospitalized Patients. Nutrients, 14(11), 2207. https://doi.org/10.3390/nu14112207






