Abstract
Background: To investigate relationships between five single nucleotide polymorphisms (SNP) in four maternal genes involved in one carbon metabolism and adverse pregnancy outcomes, including preterm birth (PTB), low birth weight (LBW), and small-for-gestational-age (SGA). Methods: This was a prospective mother and child cohort study in Wuqiang, China. Pregnant women (n = 939) were recruited from Jun 2016 to Oct 2018. Pregnancy outcomes (PTB, LBW, and SGA) were extracted from medical records and other information including age at childbearing, maternal education level, gravidity, parity, pre-pregnancy weight and height was collected by using a structured questionnaire. The maternal serum folate concentration was measured by using Abbott Architect i2000SR chemiluminescence analyzer in the first prenatal care visit. DNA genotyping of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C, methionine synthase reductase (MTRR) A66G, methionine synthase (MTR) A2756G, and thymidylate synthetase (TYMS) rs3819102 was processed by Sequenom MassARRAY iPLEX Platform. Univariate and multivariate logistics regression analysis were used to test the relationships between 5 SNPs and PTB, LBW, SGA. Results: Totally, 849 dyads of women and infants were included in the analysis. The prevalence of PTD, LBW, and SGA were 3.76%, 1.58%, and 5.31% respectively. The homozygote frequencies of MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, and TYMS rs3819102 were 44.2%, 1.4%, 6.7%, 1.3%, and 3.2%, and the alt allele frequencies were 66.1%, 10.8%, 24.9%, 10.5%, and 20.5% respectively. The average serum folate concentration was 11.95 ng/mL and the folate deficiency rate was 0.47%. There were no significant associations between MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 alleles and PTD, LBW, SGA (p > 0.05). Conclusions: In the population with adequate folate status and low prevalence of adverse pregnancy outcomes, MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 alleles may not be related to PTD, LBW, and SGA.
Keywords:
polymorphism; MTHFR; MTRR; MTR; TYMS; preterm delivery; low birth weight; small-for-gestational-age 1. Introduction
Adverse pregnancy outcomes, including preterm birth (PTB), low birth weight (LBW), and small-for-gestational-age (SGA) are major determinants for infant morbidity and mortality. PTB and its complications are the leading causes of death among children under 5 years old [1]. World Health Organization (WHO) estimated that 14.84 million babies were born preterm globally in 2014 and there were the second highest numbers of estimated preterm births in China [2]. LBW was associated with physical and mental development retardation and higher rate of children mortality. According to the estimates from UNICEF and WHO, 20.5 million LBW babies were born globally in 2015, and nearly 8.47 million LBW babies were born in China [3]. SGA posted a huge burden worldwide. In 2010, an estimated 32.4 million infants were born SGA in low and middle income countries [4]. China Nutrition and Health Surveillance on 0–5 years Children and Lactating Women in 2013 reported that the prevalence of PTB and LBW were 9.9% and 3.0%~4.0% in 0~ to 5~ years old, respectively in 2013 [5]. The burden of undernutrition including PTB, LBW, and SGA is heavy globally, especially in developing countries [6,7].
Environmental factors were related to PTB, LBW, and SGA, including behavioral and sociodemographic factors (e.g., maternal education level, gestational age, alcohol, and smoking consumption) and medical and pregnancy conditions (e.g., gestational hypertension, gestational diabetes mellitus) [8,9,10], and genetic factors could be associated to PTB, LBW, and SGA too. Some evidence suggested that genetic factors played an important role in the occurrence of PTB, LBW, and SGA [11,12,13]. As a one-carbon metabolism enzyme, folic acid is critical in DNA and RNA synthesis. High-level folate was associated with lower risks of the above adverse pregnancy outcomes [14,15]. Single nucleotide polymorphisms (SNPs)-related folic acid metabolic enzymes in birth defects had been identified [16]. Recently, both Indian and Japanese studies suggested that folic acid metabolic enzymes-related SNPs may be related to the risks of PTB and LBW [17,18]. However, the existing SNP studies in PTB, LBW, and SGA were limited and the results were inconsistent [19,20,21,22,23,24,25,26,27]. A study showed that the lower level of folate and unfavorable mutations contributed to PTB, and some other outcomes including hyperhomocysteinaemia [28]. Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C, methionine synthase reductase (MTRR) A66G, methionine synthase (MTR) A2756G, thymidylate synthetase (TYMS) rs3819102 were the common target SNPs in the previous studies.
Therefore, we aimed to investigate relationships between the five SNPs in four genes (MTHFR, MTRR, MTR, and TYMS) involved in one carbon metabolism, folic acid status, and adverse pregnancy outcomes (PTB, LBW, and SGA) in a Chinese mother and child cohort.
2. Materials and Methods
2.1. Study Subjects
A prospective maternal and child nutrition and health cohort in China was carried out in Wuqiang, China [29]. All pregnant women were recruited in the cohort during Jun 2016 to Oct 2018 at prenatal care center and were followed through delivery in the current study. Inclusion criteria: (1) pregnant women aged 18 to 45 years old, (2) gestational week < 20, (3) singleton pregnancy. Exclusion criteria: pregnant women with history of habitual abortion, diabetes, hypertension, and thrombophilia. Totally, 939 pregnant women were enrolled in the study. The study was approved by the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention Ethical Review Committee, and all study subjects provided written informed consent.
2.2. Data Collection
A structured questionnaire was used to collect maternal sociodemographic information at enrollment, including age at childbearing, maternal education level, gravidity, parity, pre-pregnancy weight and height. Maternal health and pregnancy outcomes including PTB, LBW, and SGA were extracted from pregnancy examination records and delivery medical records. PTB defined childbirth before 37 weeks of pregnancy, and LBW defined birth weight less than 2500 g [30]. SGA defined birthweight less than the tenth percentile at a particular gestational week [31]. The gestational week and birth weight data of baby were extracted from delivery medical records. For those women delivering at non-study hospital, women were asked to recall the delivery information, including gestational age at delivery, gender and birth weight of infant.
2.3. Laboratory Analysis
Venous blood samples were collected in the first prenatal care visit, and serum was separated by centrifugation and stored in −20 ℃ freezer. Serum folate concentration was measured by using Abbott Architect i2000SR chemiluminescence analyzer with folate testing kit (Abbott, Shanghai, China, 40320–40321). Liquichek Immunoassay Plus Control (Bio-Rad Laboratories, Shanghai, China, 40320) was used as quality control. Mean intra-assay and inter-assay coefficient of variations (CV) for serum folate concentration measurements ranged from 4.48% to 7.29% and 6.13% to 11.37% for low level, 2.79% to 3.14% and 4.18 to 4.19% for medium level, 2.59% to 3.16% and 4.23% to 4.24% for high-level, respectively. Serum folate concentration < 2 ng/mL was defined as folate deficiency.
DNA was extracted from 1 milliliter whole blood by using TIANGEN TIANamp Blood DNA Kit (TIANGEN BIOTECH CO., LTD., Beijing, DP349-02). The MTHFR, MTRR, MTR, and TYMS polymorphisms were genotyped by using Sequenom MassARRAY iPLEX Platform (Agena Bioscience, San Diego, CA, USA) [32]. DNA concentration ≥10 ng/ul and volume ≥20 ul are required for the assay. The assay consisted of an initial PCR reaction, followed by single base extension using mass-modifies dideoxynucleotide terminators of an oligonucleotide primer. SNPs were genotyped by using MALDI-TOF mass spectrometry and classified as “A. Conservative”, “B. Moderate”, “C. Aggressive” by the spectrometry automatically or “D” artificially by the lab technician if the spectrometry cannot assign the one into a category defined previously.
2.4. Statistical Analysis
All data were exported from the data collection system and imported into SAS 9.4 for statistical description and analysis. The missing values of maternal age at childbearing, education level, pre-pregnancy weight and height were imputed by PROC MI. Data were described as the mean and standard deviation (SD) or median for continuous variables and frequencies (%) for categorical variables. Univariate and multivariate logistics regression analysis were used to test the associations between PTB, LBW, SGA and MTHFR, MTRR, MTR, TYMS polymorphisms. In the multivariate logistics model, maternal education, maternal age at childbearing, pre-pregnancy BMI, serum folate concentration, and gender of baby were adjusted as covariates only if they could alter 10% of the OR value in 5 SNPs respectively.
Sensitive analyses were carried out in women whose SNPs genotyped description of “A. Conservative” or whose delivery information was available only from delivery medical records respectively. In the subgroup of “A. Conservative genotype”, the cases included women with adverse pregnancy outcomes (PTB/LBW/SGA) and the controls included those without those adverse pregnancy outcomes. In the subgroup of “delivery medical records”, the cases included women with adverse pregnancy outcomes (PTB/LBW/SGA) based on medical records and the controls included those without adverse pregnancy outcomes based on medical records.
3. Results
3.1. Demographic Characteristics
Of the 939 pregnant women, 18 women had abortion, induced labor, or dead fetus. After excluding those subjects with miscarriage, still birth, or twin pregnancy, there remained 911 women in the study. Of the 911 women, 849 women had genotyping results and serum folate concentration, and 62 women were excluded because DNA concentration was less than 10 ng/ul (Figure 1).
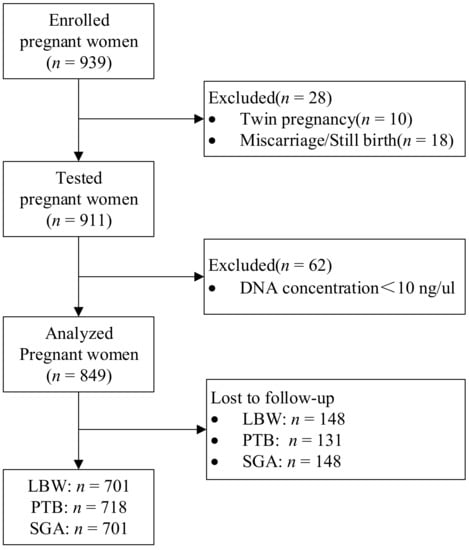
Figure 1.
Flow chart of the study population.
The average maternal age at childbearing in the study was 28.4 years old and gestational age at delivery was 39.0 weeks. About 8.25% pregnant women had college degree. The average pre-pregnancy BMI was 23.67 kg/m2. The average serum folate concentration was 11.95 ng/mL and the folate deficiency rate was 0.47%. Of the 849 pregnant women, 718, 697, and 697 had PTB, LBW, and SGA results respectively. The proportion of results coming from medical records were 74.5% (535/718), 76.8% (535/697), and 76.8 (535/697) respectively. The prevalence of PTD, LBW, and SGA were 3.76%, 1.58%, and 5.31% respectively. The characteristics of study population divided by PTB, LBW, and SGA are shown in Table 1.

Table 1.
Characteristics of study population.
3.2. Genotype Frequencies of SNP
The homozygote frequencies of MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, and TYMS rs3819102 are 44.2%, 1.4%, 6.7%, 1.3%, and 3.2%, and the alt allele frequencies are 66.1%, 10.8%, 24.9%, 10.5%, and 20.5% respectively. The genotype frequencies of MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, and TYMS rs3819102 have no deviation from the Hardy–Weinberg equilibrium (HWE) in all the pregnant women (Table 2).

Table 2.
The frequency of genotypes and alleles of MTHFR, MTRR, MTR, TYMS.
3.3. Associations between SNPs and Adverse Pregnancy Outcomes
No significant associations were found between MTHFR C677T (PTB: OR 1.14, 95% CI 0.34–3.86; SGA: OR 0.71, 95% CI 0.29–1.76), MTHFR A1298C (PTB: OR 0.86, 95% CI 0.32–2.30; LBW: OR 0.37, 95% CI 0.05–2.91; SGA: OR 0.88, 95% CI 0.38–2.03), MTRR A66G (PTB: OR 0.68, 95% CI 0.30–1.54; LBW: OR 0.79, 95% CI 0.23–2.71; SGA: OR 0.66, 95% CI 0.32–1.33), MTR A2756G (PTB: OR 1.82, 95% CI 0.78–4.24; LBW: OR 1.57, 95% CI 0.41–6.01; SGA: OR 1.17, 95% CI 0.52–2.62), TYMS rs3819102 alleles (PTB: OR 0.84, 95% CI 0.37–1.91; LBW: OR 0.37, 95% CI 0.08–1.72; SGA: OR 0.53, 95% CI 0.25–1.14) and either of PTD, LBW, and SGA (p > 0.05). (Table 3, Table 4 and Table 5)

Table 3.
Relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and PTB.

Table 4.
Relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and LBW.

Table 5.
Relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and SGA.
After adjusting for maternal education, maternal age at childbearing, pre-pregnancy BMI, serum folate concentration and gender of baby, the relationships remained non-significant between MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 alleles and PTD, LBW, SGA (p > 0.05).
The results of sensitive analyses were in accordance with the full analysis (Supplementary Tables S1–S6).
4. Discussion
We found that five maternal SNPs in MTHFR, MTRR, MTR, and TYMS were not associated with adverse pregnancy outcomes (PTD, LBW, and SGA) in the current study.
The alt allele frequency of MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 was 66.1%, 10.8%, 24.9%, 10.5%, and 20.5% in the current study, respectively. The frequency of SNP varied greatly among different races. According to the Allele Frequency Aggregator, the alt allele frequency of MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 was 34.0%, 30.4%, 51.7%, 19.1%, 2.6% globally and 38.6%, 21.4%, 27.3%, 10.8%, 23.6% in the East Asian respectively [33,34,35,36,37]. Compared to the East Asian population, the alt allele frequency of MTHFR C677T was higher and MTHFR A1298C lower in the current study. The frequency of other SNPs was similar as East Asian level.
Some studies suggested that the level of maternal serum folate was negatively related to PTB, LBW, and SGA [14,15,38]. In our study, the average serum folate concentration was 11.95 ng/mL and the folate deficiency rate was 0.47%, which may protect from these adverse pregnancy outcomes in the current study.
Two studies showed no significant association between MTHFR C677T, MTHFR A1298C and PTB, SGA in either whites or blacks in the presence of high folate intake after mandatory grain folic fortification in the United State [39,40], even in low folate intake subgroup status, some other factors seemed to affect the association between MTHFR SNPs and adverse pregnancy outcomes, such as racial difference [39]. Nurk’s study found no significant associations between MTHFR C677T and LBW in Norway [41]. Moreover, the Screening for Pregnancy Endpoints (SCOPE) prospective cohort study found no significant gene-nutrient interactions between maternal MTHFR C677T, MTHFR A1298C, and folic acid use, and their association with PTB and SGA [42,43]. The results of the three studies mentioned above were collaborative with our study. However, a case-control study nested in a multicenter cohort found MTHFR C677T was positively associated with PTB and LBW in India, in which folate status was not reported. Extremely, very, and moderately PTB showed higher frequency of MTHFR C677T mutation compared to term delivery cases [20]. A Japanese study suggested that maternal MTHFR C677T, not MTHFR A1298C was independently associated with improvement in infant birth weight in the background of 16.4 nmol/L serum folate [25]. The inconsistence may be related to differences in dietary folate status or mean birth weight. Although hyperhomocysteinemia induced by MTHFR variant genotypes have shown a strong relationship with adverse pregnancy outcomes, high-level intake of folate could reverse the results [44]. In our study, folic acid status was adequate. The results suggested sufficient folic acid status could be crucial to the pregnancy outcomes, including PTB, LBW, and SGA, especially for those with SNPs mutation.
MTRR and MTR are the key enzymes in the one-carbon pool by folate, catalyzing the methylation of homocysteine to methionine. The SCOPE study found no significant gene-nutrient interactions between maternal MTRR A66G, MTR A2756G, and folic acid use, and their association with PTB and SGA [42,43], which aligned with our finding of MTRR and MTR SNPs. Tiwari found that MTRR A66G was not related to PTB on the background of high-level folate, but homocysteine concentration did [20]. Another nested case-control study carried out by Engel found that MTRR A66G was positively related to PTB in the white population, but not in the black population regardless of folate intake level [39]. MTRR A66G and MTR A2756G have been proved to be associated with increased homocysteine levels [45,46], and a certain concentration of homocysteine could cause endothelial cell dysfunction and oxidative stress, which is positively related to PTB, LBW, and preeclampsia [47]. Meanwhile, no association was found between MTRR A66G and SGA [39]. However, we found no association between either MTRR or MTR variants associated with PTB, LBW, and SGA in our study.
TYMS catalyzes the methylation of deoxyuridylate to deoxythymidylate, which maintains the dTMP pool critical for DNA replication and repair [48]. There were few studies involving the associations between TYMS rs3819102 gene polymorphism and PTB, LBW, SGA. We first explored the association between TYMS rs3819102 and adverse pregnancy outcomes.
The current study investigated the relationships between five SNPs in four maternal genes involved in one carbon metabolism and adverse pregnancy outcomes, including PTB, LBW, and SGA, and folate status was taken into consideration for the relationship simultaneously. The limitation of this study was the high loss to follow-up rate. Many pregnant women did not follow-up because of delivering at a different hospital, and could not be reached by telephone. However, the results of sensitive analyses were in accordance with the full analysis, which suggested loss to follow-up rate did not affect the results. Moreover, this study did not analyze epigenetics of study population, which could modify the gene expression [49] and the interaction between gene polymorphism and epigenetics may alter the activity of folic acid-related enzymes. However, adequate folic acid status of those pregnant women may maintain similar methylation level of those subjects, which may reduce the impacts of epigenetics.
5. Conclusions
In the population with adequate folic acid status and low prevalence of adverse pregnancy outcomes, MTHFR C677T, MTHFR A1298C, MTRR A66G, MTR A2756G, TYMS rs3819102 alleles may not be related to PTD, LBW, and SGA. It is critical to maintain good folate status for preventing adverse pregnancy outcomes including PTB, LBW, and SGA, even for those with one carbon metabolism enzyme mutations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14102108/s1. Table S1: Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and PTB in women with SNPs genotyped description of “A. Conservative”. Table S2 Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and LBW in women with SNPs genotyped description of “A. Conservative”. Table S3: Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and SGA in women with SNPs genotyped description of “A. Conservative”. Table S4: Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and PTB in women with delivery information only from delivery medical records. Table S5: Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS, and LBW in women with delivery information only from delivery medical records. Table S6: Sensitive analysis-relationship between genotypes of MTHFR, MTRR, MTR, TYMS and SGA in women with delivery information only from delivery medical records.
Author Contributions
Methodology, S.W., Y.D., S.J. and Z.Y.; formal analysis, S.W. and Z.Y.; investigation, Y.D., S.J., Y.B. and X.P.; writing—original draft preparation, S.W.; writing—review and editing, Z.Y.; project administration, Z.Y., C.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Program for Healthcare Reform from the Chinese National Health and Family Planning Commission (A prospective maternal and child nutrition and health cohort in China).
Institutional Review Board Statement
The study was approved by the medical ethics committee of National Institution for Nutrition and Health (formerly National Institute of Nutrition and Food Safety), Chinese Center for Disease Control and Prevention.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated or analyzed during the current study are not publicly available due to the data management requirements of our institution, but are available from the corresponding author on reasonable request.
Acknowledgments
We thank all of the participants in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- UNICEF; WHO. UNICEF-WHO Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lee, A.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef] [Green Version]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef]
- Yang, Z.Y. Report of Chinese National Nutrition and Health Surveillance in (2010–2013): The Nutrition and Health Condition among Chinese Children Aged 0–5; People’s Medical Publishing House: Beijing, China, 2020. [Google Scholar]
- Sheikh, I.A.; Ahmad, E.; Jamal, M.S.; Rehan, M.; Assidi, M.; Tayubi, I.A.; Albasri, S.F.; Bajouh, O.S.; Turki, R.F.; Abuzenadah, A.M.; et al. Spontaneous preterm birth and single nucleotide gene polymorphisms: A recent update. BMC Genom. 2016, 7 (Suppl. 9). [Google Scholar] [CrossRef] [Green Version]
- Dahman, H.A.B. Risk factors associated with preterm birth: A retrospective study in Mukalla Maternity and Childhood Hospital, Hadhramout Coast/Yemen. Sudan J. Paediatr. 2020, 20, 99–110. [Google Scholar] [CrossRef]
- Anil, K.C.; Basel, P.L.; Singh, S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLoS ONE 2020, 15, e0234907. [Google Scholar] [CrossRef]
- Monangi, N.K.; Brockway, H.M.; House, M.; Zhang, G.; Muglia, L. The genetics of preterm birth: Progress and promise. Semin. Perinatol. 2015, 39, 574–583. [Google Scholar] [CrossRef]
- Mallia, T.; Grech, A.; Hili, A.; Calleja-Agius, J.; Pace, N.P. Genetic determinants of low birth weight. Minerva Ginecol. 2017, 69, 631–643. [Google Scholar] [CrossRef]
- Stalman, S.E.; Solanky, N.; Ishida, M.; Alemán-Charlet, C.; Abu-Amero, S.; Alders, M.; Alvizi, L.; Baird, W.; Demetriou, C.; Henneman, P.; et al. Genetic Analyses in Small-for-Gestational-Age Newborns. J. Clin. Endocrinol. Metab. 2018, 103, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Himes, K.P.; Venkataramanan, R.; Chen, J.Y.; Evans, R.W.; Meyer, J.L.; Simhan, H.N. Maternal serum folate species in early pregnancy and risk of preterm birth. Am. J. Clin. Nutr. 2010, 92, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Mao, B.; Wang, M.; Liu, Q.; Yang, L.; Xie, Y.; Wang, Y.; He, X.; Cui, H.; Lin, X.; et al. Folic acid supplementation, dietary folate intake and risk of small for gestational age in China. Public Health Nutr. 2020, 23, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.; Kumar, P.; Yadav, S.K.; Mishra, O.P.; Rai, V. Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: An updated meta-analysis. Metab. Brain Dis. 2015, 30, 7–24. [Google Scholar] [CrossRef]
- Tiwari, D.; Bose, P.D.; Das, S.; Das, C.R.; Datta, R.; Bose, S. MTHFR (C677T) polymorphism and PR (PROGINS) mutation as genetic factors for preterm delivery, fetal death and low birth weight: A Northeast Indian population based study. Meta Gene 2015, 3, 31–42. [Google Scholar] [CrossRef]
- Yila, T.A.; Sasaki, S.; Miyashita, C.; Braimoh, T.S.; Kashino, I.; Kobayashi, S.; Okada, E.; Baba, T.; Yoshioka, E.; Minakami, H.; et al. Effects of maternal 5,10-methylenetetrahydrofolate reductase C677T and A1298C Polymorphisms and tobacco smoking on infant birth weight in a Japanese population. J. Epidemiol. 2012, 22, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Carmichael, S.L.; Shaw, G.M.; Iovannisci, D.M.; Lammer, E. Association between 49 infant gene polymorphisms and preterm delivery. Am. J. Med. Genet. A 2007, 143A, 1990–1996. [Google Scholar] [CrossRef]
- Hwang, I.W.; Kang, Y.D.; Kwon, B.N.; Hong, J.H.; Han, S.H.; Kim, J.S.; Park, J.W.; Jin, H.J. Genetic variations of MTHFR gene and their association with preterm birth in Korean women. Medicina 2017, 53, 380–385. [Google Scholar] [CrossRef]
- Wang, B.J.; Liu, M.J.; Wang, Y.; Dai, J.R.; Tao, J.Y.; Wang, S.N.; Zhong, N.; Chen, Y. Association between SNPs in genes involved in folate metabolism and preterm birth risk. Genet. Mol. Res. 2015, 14, 850–859. [Google Scholar] [CrossRef]
- Nan, Y.; Li, H. MTHFR genetic polymorphism increases the risk of preterm delivery. Int. J. Clin. Exp. Pathol. 2015, 8, 7397–7402. [Google Scholar]
- Lykke, J.A.; Bare, L.A.; Olsen, J.; Lagier, R.; Arellano, A.R.; Tong, C.; Paidas, M.J.; Langhoff-Roos, J. Thrombophilias and adverse pregnancy outcomes: Results from the Danish National Birth Cohort. J. Thromb. Haemost. 2012, 10, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, L.M.; Laivuori, H.; Rautanen, A.; Kaaja, R.; Kere, J.; Krusius, T.; Rasi, V.; Paunio, M. Factor V Leiden as a risk factor for preterm birth—A population-based nested case-control study. J. Thromb. Haemost. 2011, 9, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, Y.; Dai, J.; Wang, B.; Liu, M.; Wang, Y.; Tao, J.; Li, H. Methylenetetrahydrofolate reductase polymorphisms at 3′-untranslated region are associated with susceptibility to preterm birth. Transl. Pediatr. 2015, 4, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, N.; Ataç, F.B.; Verdi, H.; Cetintaş, S.; Gürakan, B.; Haberal, A. Relationship between small-for-gestational age births and maternal thrombophilic mutations. Thromb. Res. 2008, 122, 175–178. [Google Scholar] [CrossRef]
- Sukla, K.K.; Tiwari, P.K.; Kumar, A.; Raman, R. Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an Indian cohort: Association of homocysteine, its metabolic pathway genes and micronutrients as risk factors. PLoS ONE 2013, 8, e71587. [Google Scholar] [CrossRef]
- Kramer, M.S.; Goulet, L.; Lydon, J.; Séguin, L.; McNamara, H.; Dassa, C.; Platt, R.W.; Chen, M.F.; Gauthier, H.; Genest, J.; et al. Socio-economic disparities in preterm birth: Causal pathways and mechanisms. Paediatr. Perinat. Epidemiol. 2001, 15 (Suppl. 2), 104–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Duan, Y.; Yang, J.; Li, J.; Li, F.; Zhou, P.; Liu, C.; Zhao, Y.; Gu, X.; Yuan, C.; et al. Cohort profile: The Taicang and Wuqiang mother-child cohort study (TAWS) in China. BMJ Open 2022. accepted. [Google Scholar]
- WHO. WHO: Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths, Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar]
- Mikolajczyk, R.T.; Zhang, J.; Betran, A.P.; Souza, J.P.; Mori, R.; Gülmezoglu, A.M.; Merialdi, M. A global reference for fetal-weight and birthweight percentiles. Lancet 2011, 377, 1855–1861. [Google Scholar] [CrossRef]
- Gabriel, S.; Ziaugra, L.; Tabbaa, D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009, 60, 2.12.1–2.12.18. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1801133 (accessed on 2 April 2021).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1801131 (accessed on 2 April 2021).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1801394 (accessed on 2 April 2021).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs1805087 (accessed on 2 April 2021).
- Available online: https://www.ncbi.nlm.nih.gov/snp/rs3819102 (accessed on 2 April 2021).
- Bergen, N.E.; Jaddoe, V.W.; Timmermans, S.; Hofman, A.; Lindemans, J.; Russcher, H.; Raat, H.; Steegers-Theunissen, R.P.M.; Steegers, E.A.P. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: The Generation R Study. BJOG 2012, 119, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.M.; Olshan, A.F.; Siega-Riz, A.M.; Savitz, D.A.; Chanock, S.J. Polymorphisms in folate metabolizing genes and risk for spontaneous preterm and small-for-gestational age birth. Am. J. Obstet. Gynecol. 2006, 195, 1231.e1–1231.e11. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Holzman, C.B.; Senagore, P.K.; Reuss, M.L.; Pathak, D.R.; Friderici, K.H.; Jernigan, K.; Fisher, R. Polymorphisms in thrombophilia and renin-angiotensin system pathways, preterm delivery, and evidence of placental hemorrhage. Am. J. Obstet. Gynecol. 2009, 201, 317.e1–317.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurk, E.; Tell, G.S.; Refsum, H.; Ueland, P.M.; Vollset, S.E. Associations between maternal methylenetetrahydrofolate reductase polymorphisms and adverse outcomes of pregnancy: The Hordaland Homocysteine Study. Am. J. Med. 2004, 117, 26–31. [Google Scholar] [CrossRef]
- Bulloch, R.E.; Wall, C.R.; McCowan, L.M.E.; Taylor, R.S.; Roberts, C.T.; Thompson, J.M.D. The Effect of Interactions between Folic Acid Supplementation and One Carbon Metabolism Gene Variants on Small-for-Gestational-Age Births in the Screening for Pregnancy Endpoints (SCOPE) Cohort Study. Nutrients 2020, 12, 1677. [Google Scholar] [CrossRef]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal folate one-carbon metabolism and pregnancy outcomes. Matern. Child Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef]
- Li, W.X.; Cheng, F.; Zhang, A.J.; Dai, S.X.; Li, G.H.; Lv, W.W.; Zhou, T.; Zhang, Q.; Zhang, H.; Zhang, T.; et al. Folate Deficiency and Gene Polymorphisms of MTHFR, MTR and MTRR Elevate the Hyperhomocysteinemia Risk. Clin. Lab. 2017, 63, 523–533. [Google Scholar] [CrossRef]
- Gaughan, D.J.; Kluijtmans, L.A.; Barbaux, S.; McMaster, D.; Young, I.S.; Yarnell, J.W.; Evans, A.; Whitehead, A.S. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis 2001, 157, 451–456. [Google Scholar] [CrossRef]
- Barbosa, P.R.; Stabler, S.P.; Machado, A.L.; Braga, R.C.; Hirata, R.D.; Hirata, M.H.; Sampaio-Neto, L.F.; Allen, R.H.; Guerra-Shinohara, E.M. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur. J. Clin. Nutr. 2008, 62, 1010–1021. [Google Scholar] [CrossRef]
- Liu, C.; Luo, D.; Wang, Q.; Ma, Y.; Ping, L.; Wu, T.; Tang, J.; Peng, D.; Zhao, P. Serum homocysteine and folate concentrations in early pregnancy and subsequent events of adverse pregnancy outcome: The Sichuan Homocysteine study. BMC Pregnancy Childbirth 2020, 20, 176. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Bigler, J.; Bostick, R.; Fosdick, L.; Potter, J.D. Thymidylate synthase promoter polymorphism, interaction with folate intake, and risk of colorectal adenomas. Cancer Res. 2002, 62, 3361–3364. [Google Scholar] [PubMed]
- Naselli, F.; Catanzaro, I.; Bellavia, D.; Perez, A.; Sposito, L.; Caradonna, F. Role and importance of polymorphisms with respect to DNA methylation for the expression of CYP2E1 enzyme. Gene 2014, 536, 29–39. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).