A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb
Abstract
:1. Introduction
2. Thyme (Thymus vulgaris): An Overview
2.1. Systematic Classification and Distribution
- Kingdom: Plantae
- Subkingdom: Tracheobionta
- Superdivision: Spermatophyta
- Division: Magnoliophyta
- Class: Magnoliopsida
- Subclass: Asteridae
- Order: Lamiales
- Family: Lamiaceae
- Genus: Thymus L.
- Species: Thymus vulgaris L. [16]
2.2. Thyme Botanical Aspects
3. Chemical Composition and Essential Oils of Thyme
4. Thyme Nutritional Value and Health Benefits
5. Applications and Uses of Thyme in Food Industry
6. Biological Activity of Thyme
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Antineoplastic Activity
6.4. Antibacterial Activity
6.5. Antifungal Activity
6.6. Antiviral Activity and Novel Findings in COVID-19
7. Innovative Perspective on Thyme
8. Limitations of the Review
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Heywood, V.H.; Nations, F.; Food and Agriculture Organization of the United Nations. Use and Potential of Wild Plants in Farm Households; Food & Agriculture Org.: Rome, Italy, 1999; ISBN 978-92-5-104151-2. [Google Scholar]
- Shumsky, S.A.; Hickey, G.M.; Pelletier, B.; Johns, T. Understanding the Contribution of Wild Edible Plants to Rural Social-Ecological Resilience in Semi-Arid Kenya. Ecol. Soc. 2014, 19, art34. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Traditionally Used Wild Edible Plants of District Udhampur, J&K, India. J. Ethnobiol. Ethnomed. 2018, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Duguma, H.T. Wild Edible Plant Nutritional Contribution and Consumer Perception in Ethiopia. Int. J. Food Sci. 2020, 2020, 2958623. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L. Edible Wild Plants. In Recent Progress in Medicinal Plants; Chapter: Edible Wild Plants; Majundar, D.K., Govil, J.N., Singh, V.K., Eds.; Sci. Tech Publising LLC: Houston, TX, USA, 2002; Volume 8, Available online: https://www.researchgate.net/publication/270276886_Edible_Wild_Plants (accessed on 17 January 2022).
- Polat, R.; Cakilcioglu, U.; Ulusan, M.D.; Paksoy, M. Survey of Wild Food Plants for Human Consumption in Bingöl (Turkey). Indian J Tradit knowledge. 2017, 16, 378–384. [Google Scholar]
- Batal, M.; Hunter, E. Traditional Lebanese Recipes Based on Wild Plants: An Answer to Diet Simplification? Food Nutr. Bull. 2007, 28, S303–S311. [Google Scholar] [CrossRef] [Green Version]
- Marouf, M.; Batal, M.; Moledor, S.; Talhouk, S.N. Exploring the Practice of Traditional Wild Plant Collection in Lebanon. Food Cult. Soc. 2015, 18, 355–378. [Google Scholar] [CrossRef]
- Baydoun, S.; Chalak, L.; Dalleh, H.; Arnold, N. Ethnopharmacological Survey of Medicinal Plants Used in Traditional Medicine by the Communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015, 173, 139–156. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Jabeen, Q.; Bashir, S.; Hayat, M.; Muhammad, N.; Khan, H.; Khan, S.U.; Rehman, M.S.; Salma, U.; Mazhar, U.; et al. Antihypertensive and Toxicity Studies of Aqueous Methanolic Extract of Mentha longifolia L. J. Anim. Plant Sci. 2013, 23, 1622–1627. [Google Scholar]
- Stahl-Biskup, E.; Venskutonis, R.P. Thyme. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 499–525. ISBN 978-0-85709-039-3. [Google Scholar]
- Jalas, J. Notes on Thymus L. (Labiatae) in Europe. I. Supraspecific Classification and Nomenclature. Bot. J. Linn. Soc. 1971, 64, 199–215. [Google Scholar]
- Morales. The History, Botany and Taxonomy of the Genus Thymus. In Thyme: The Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; Taylor and Francis, Inc.: London, UK, 2002; pp. 1–43. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780203216859-5/history-botany-taxonomy-genus-thymus-ram%C3%B3n-morales (accessed on 18 January 2022).
- Talhouk, S.N.; Fabian, M.; Dagher, R. Landscape Plant Database. Department of Landscape Design & Ecosystem Management, American University of Beirut. Available online: https://landscapeplants.aub.edu.lb/Plants/PlantProfile/%20%20%20%207ccb111d-624a-44e4-8fe0-5c2df080bbb1 (accessed on 6 January 2022).
- Dauqan, E.M.A.; Abdullah, A. Medicinal and Functional Values of Thyme (Thymus vulgaris L.) Herb. J. App. Biol. Biotechnol. 2017, 5, 017–022. [Google Scholar] [CrossRef] [Green Version]
- Almanea, A.; El-Aziz, G.S.A.; Ahmed, M.M.M. The Potential Gastrointestinal Health Benefits of Thymus vulgaris Essential Oil: A Review. Biomed. Pharmacol. J. 2019, 12, 1793–1799. [Google Scholar] [CrossRef]
- Jaafari, A.; Mouse, H.A.; Rakib, E.M.; M’barek, L.A.; Tilaoui, M.; Benbakhta, C.; Boulli, A.; Abbad, A.; Zyad, A. Chemical Composition and Antitumor Activity of Different Wild Varieties of Moroccan Thyme. Rev. Bras. Farm. 2007, 17, 477–491. [Google Scholar] [CrossRef] [Green Version]
- Micucci, M.; Protti, M.; Aldini, R.; Frosini, M.; Corazza, I.; Marzetti, C.; Mattioli, L.B.; Tocci, G.; Chiarini, A.; Mercolini, L.; et al. Thymus vulgaris L. Essential Oil Solid Formulation: Chemical Profile and Spasmolytic and Antimicrobial Effects. Biomolecules 2020, 10, 860. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- U.S Department of Agriculture, Agricultural Research Service. Oxygen; Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. Nutrient; Data Laboratory Home Page. 2010. Available online: http://www.ars.usda.gov/nutrientdata/orac (accessed on 31 March 2022).
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Reddy, P.; RaviVital, K.; Varsha, P.; Satyam, S. Review on Thymus vulgaris Traditional Uses and Pharmacological Properties. Med Aromat Plants 2014, 3, 2167-0412. [Google Scholar] [CrossRef] [Green Version]
- Zaborowska, Z.; Przygoński, K.; Bilska, A. Antioxidative Effect of Thyme (Thymus vulgaris) in Sunflower Oil. Acta Sci. Pol. Technol. Aliment. 2012, 11, 283–291. [Google Scholar]
- Iqbal, S.; Haleem, S.; Akhtar, M.; Zia-ul-Haq, M.; Akbar, J. Efficiency of Pomegranate Peel Extracts in Stabilization of Sunflower Oil under Accelerated Conditions. Food Res. Int. 2008, 41, 194–200. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol Bioactivity: A Review Focusing on Practical Applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Sharara, M.S. Antioxidant and Antimicrobial Activity of Thyme and Cinnamon Extracts. Alex. J. Food Sci. Technol. 2012, 9, 39–46. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant Activity and Mechanism of Action of Thymol and Carvacrol in Two Lipid Systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Wisam, S.U.; Nahla, T.K.; Tariq, N.M. Antioxidant Activities of Thyme Extracts. Pak. J. Nutr. 2017, 17, 46–50. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Determination of Antioxidant Potential of Volatile Extracts Isolated from Various Herbs and Spices. J. Agric. Food Chem. 2002, 50, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of Antioxidant Activity, Total Phenols and Phenolic Compounds in Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Marjoram (Origanum majorana L.) Extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Anahi Dandlen, S.; Majdoub, N.; Lyoussi, B.; Raposo, S.; Dulce Antunes, M.; Gomes, V.; Graça Miguel, M. Antioxidant Activity of Thyme Waste Extract in O/W Emulsions. Antioxidants 2019, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant Properties of Thymus vulgaris Oil against Aflatoxin-Induce Oxidative Stress in Male Rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Chao, T.-Y.; Chang, W.-C.; Chang, M.J.; Lee, M.-F. Thymol Reduces Oxidative Stress, Aortic Intimal Thickening, and Inflammation-Related Gene Expression in Hyperlipidemic Rabbits. J. Food Drug Anal. 2016, 24, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Prince, P.S.M. Protective Effects of Thymol on Altered Plasma Lipid Peroxidation and Nonenzymic Antioxidants in Isoproterenol-Induced Myocardial Infarcted Rats. J. Biochem. Mol. Toxicol. 2012, 26, 368–373. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The Antioxidant Properties of Thyme (Thymus zygis L.) Essential Oil: An Inhibitor of Lipid Peroxidation and a Free Radical Scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Gursul, S.; Karabulut, I.; Durmaz, G. Antioxidant Efficacy of Thymol and Carvacrol in Microencapsulated Walnut Oil Triacylglycerols. Food Chem. 2019, 278, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant Activity of PLA/PCL Films Loaded with Thymol and/or Carvacrol Using ScCO2 for Active Food Packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Güvenç, M.; Cellat, M.; Gökçek, İ.; Yavaş, İ.; Yurdagül Özsoy, Ş. Effects of Thymol and Carvacrol on Sperm Quality and Oxidant/Antioxidant Balance in Rats. Arch. Physiol. Biochem. 2019, 125, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A Review on the Beneficial Effect of Thymol on Health and Production of Fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Amer, S.A.; Metwally, A.E.; Ahmed, S.A.A. The Influence of Dietary Supplementation of Cinnamaldehyde and Thymol on the Growth Performance, Immunity and Antioxidant Status of Monosex Nile Tilapia Fingerlings (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 251–256. [Google Scholar] [CrossRef]
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Starcevic, M.; Glamoclija, N.; Djordjevic, V.; Baltic, M.Z. Antioxidative Activity of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare) Essential Oils and Their Effect on Oxidative Stability of Minced Pork Packaged Under Vacuum and Modified Atmosphere. J. Food Sci. 2019, 84, 2467–2474. [Google Scholar] [CrossRef]
- Polednik, K.M.; Koch, A.C.; Felzien, L.K. Effects of Essential Oil from Thymus vulgaris on Viability and Inflammation in Zebrafish Embryos. Zebrafish 2018, 15, 361–371. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the Potential Benefits of Thyme and Its Derived Products for Food Industry and Consumer Health: From Extraction of Value-Added Compounds to the Evaluation of Bioaccessibility, Bioavailability, Anti-Inflammatory, and Antimicrobial Activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of OxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habashy, N.H.; Abu Serie, M.M.; Attia, W.E.; Abdelgaleil, S.A.M. Chemical Characterization, Antioxidant and Anti-Inflammatory Properties of Greek Thymus vulgaris Extracts and Their Possible Synergism with Egyptian Chlorella Vulgaris. J. Funct. Foods 2018, 40, 317–328. [Google Scholar] [CrossRef]
- Golbahari, S.; Abtahi Froushani, S.M. Synergistic Benefits of Nicotine and Thymol in Alleviating Experimental Rheumatoid Arthritis. Life Sci. 2019, 239, 117037. [Google Scholar] [CrossRef] [PubMed]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical Composition and Anti-Inflammatory Activity of Algerian Thymus vulgaris Essential Oil. Nat. Prod. Commun. 2017, 12, 1934578X1701200435. [Google Scholar] [CrossRef] [Green Version]
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-Vitro Anti-Inflammatory Effect of Eucalyptus Globulus and Thymus vulgaris: Nitric Oxide Inhibition in J774A.1 Murine Macrophages. J. Pharm. Pharm. 2004, 56, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a Component of Thyme Oil, Activates PPARalpha and Gamma and Suppresses COX-2 Expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic Effects of Thymol, a Novel Monoterpene Phenol, on Different Types of Cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Timeline: Chemotherapy and the War on Cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Saeed, M.; Ansari, I.A. Molecular Insights on Chemopreventive and Anticancer Potential of Carvacrol: Implications from Solid Carcinomas. J. Food Biochem. 2021, 45, e14010. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Bouhtit, F.; Najar, M.; Moussa Agha, D.; Melki, R.; Najimi, M.; Sadki, K.; Boukhatem, N.; Bron, D.; Meuleman, N.; Hamal, A.; et al. New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules 2021, 26, 410. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Hayakawa, Y.; Pongrakhananon, V. Molecular Mechanisms of Natural Compounds in Cell Death Induction and Sensitization to Chemotherapeutic Drugs in Lung Cancer. Phytother. Res. 2019, 33, 2531–2547. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses: Thymol, Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.; Guimarães, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- PakdemїRlї, A.; Karaca, C.; Sever, T.; Daskin, E.; LeblebїCї, A.; YїĞїTbaşi, T.; Başbinar, Y. Carvacrol Alters Soluble Factors in HCT-116 and HT-29 Cell Lines. Turk. J. Med. Sci. 2020, 50, 271–276. [Google Scholar]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer Activity of Thymol: A Literature-Based Review and Docking Study with Emphasis on Its Anticancer Mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef] [Green Version]
- Al-Menhali, A.; Al-Rumaihi, A.; Al-Mohammed, H.; Al-Mazrooey, H.; Al-Shamlan, M.; AlJassim, M.; Al-Korbi, N.; Eid, A.H. Thymus vulgaris (Thyme) Inhibits Proliferation, Adhesion, Migration, and Invasion of Human Colorectal Cancer Cells. J. Med. Food 2015, 18, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity Screening of Thymus vulgaris L. Essential Oil in Brine Shrimp Nauplii and Cancer Cell Lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus vulgaris Essential Oil Towards Human Oral Cavity Squamous Cell Carcinoma. Anticancer Res. 2011, 31, 81–87. [Google Scholar]
- Günes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Dadak, A. In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules 2020, 25, 3270. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.; Turkez, H.; Tasdemir, S.; Hacımuftuoglu, F. Anticancer, Antioxidant and Cytotoxic Potential of Thymol in Vitro Brain Tumor Cell Model. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 116–122. [Google Scholar] [CrossRef]
- Hassan, H.F.H.; Mansour, A.M.; Salama, S.A.; El-Sayed, E.-S.M. The Chemopreventive Effect of Thymol against Dimethylhydrazine and/or High Fat Diet-Induced Colon Cancer in Rats: Relevance to NF-ΚB. Life Sci. 2021, 274, 119335. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.-M.; Du, C.-J.; Hu, S.-M.; Chen, J.-X.; Zhang, S.-G.; Zhang, N.; Gao, F.; Li, S.-J.; Mao, X.-W.; et al. Thymol Inhibits Bladder Cancer Cell Proliferation via Inducing Cell Cycle Arrest and Apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-Vitro Evaluation of Apoptotic Effect of OEO and Thymol in 2D and 3D Cell Cultures and the Study of Their Interaction Mode with DNA. Sci. Rep. 2018, 8, 15787. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol Affects Breast Cancer Cells through TRPM7 Mediated Cell Cycle Regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kiziltan, H.S.; Kocyigit, A.; Güler, E.M.; Karataş, E.; Toprak, A. Effects of Natural Phenolic Compound Carvacrol on the Human Gastric Adenocarcinoma (AGS) Cells in Vitro. Anticancer Drugs 2017, 28, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol Inhibits Proliferation and Induces Apoptosis in Human Colon Cancer Cells. Anticancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Kim, S.-Y.; Lee, C. Carvacrol Targets AXL to Inhibit Cell Proliferation and Migration in Non-Small Cell Lung Cancer Cells. Anticancer Res 2018, 38, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol Induces Reactive Oxygen Species (ROS)-Mediated Apoptosis Along with Cell Cycle Arrest at G0/G1 in Human Prostate Cancer Cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus Spp. Plants—Food Applications and Phytopharmacy Properties. Trends Food Sci. Technol. 2019, 85, 287. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M. Antimicrobial Resistance Collaborators Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lancet, T. The Lancet, Null Antimicrobial Resistance: Time to Repurpose the Global Fund. Lancet 2022, 399, 335. [Google Scholar] [CrossRef]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus vulgaris, Thymus Zygis and Thymus Hyemalis Essential Oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris Essential Oil: Chemical Composition and Antimicrobial Activity. J. Med. Life 2014, 7, 56–60. [Google Scholar] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial Activity of Selected Plant Essential Oils against Escherichia Coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Guo, M.; Jin, T.Z.; Arabi, S.A.; Liu, D. Ultrasound Improves the Decontamination Effect of Thyme Essential Oil Nanoemulsions against Escherichia Coli O157: H7 on Cherry Tomatoes. Int. J. Food Microbiol. 2021, 337, 108936. [Google Scholar] [CrossRef] [PubMed]
- Ghrairi, T.; Hani, K. Enhanced Bactericidal Effect of Enterocin A in Combination with Thyme Essential Oils against L. Monocytogenes and E. Coli O157:H7. J. Food Sci. Technol. 2015, 52, 2148–2156. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.Y.; Geornaras, I.; Paik, H.-D.; Kim, K.-T.; Sofos, J.N. Effects of Plant-Derived Extracts, Other Antimicrobials, and Their Combinations against Escherichia Coli O157:H7 in Beef Systems. J. Food Prot. 2015, 78, 1090–1097. [Google Scholar] [CrossRef]
- Selim, S. Antimicrobial Activity of Essential Oils against Vancomycin-Resistant Enterococci (Vre) and Escherichia Coli O157:H7 in Feta Soft Cheese and Minced Beef Meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial Activities of Commercial Essential Oils and Their Components against Food-Borne Pathogens and Food Spoilage Bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Sienkiewicz, M.; Łysakowska, M.; Denys, P.; Kowalczyk, E. The Antimicrobial Activity of Thyme Essential Oil against Multidrug Resistant Clinical Bacterial Strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Kalemba, D.; Wasiela, M. [Sensitivity assessment of thyme and lavender essential oils against clinical strains of Escherichia coli for their resistance]. Med. Dosw. Mikrobiol. 2011, 63, 273–281. [Google Scholar]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Conde-Pérez, K.; Arca-Suárez, J.; Álvarez-Fraga, L.; Pérez, A.; Crecente-Campo, J.; Alonso, M.J.; Bou, G.; et al. Syzygium Aromaticum (Clove) and Thymus Zygis (Thyme) Essential Oils Increase Susceptibility to Colistin in the Nosocomial Pathogens Acinetobacter Baumannii and Klebsiella Pneumoniae. Biomed. Pharm. 2020, 130, 110606. [Google Scholar] [CrossRef] [PubMed]
- Loose, M.; Pilger, E.; Wagenlehner, F. Anti-Bacterial Effects of Essential Oils against Uropathogenic Bacteria. Antibiotics 2020, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Figueira, L.W.; de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Curcuma Longa L. (Turmeric), Rosmarinus Officinalis L. (Rosemary), and Thymus vulgaris L. (Thyme) Extracts Aid Murine Macrophages (RAW 264.7) to Fight Streptococcus Mutans during in Vitro Infection. Arch. Microbiol. 2020, 202, 2269–2277. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus Pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial Activity of Selected Essential Oils against Streptococcus Suis Isolated from Pigs. Microbiologyopen 2018, 7, e00613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Aguiar, F.C.; Solarte, A.L.; Gómez-Gascón, L.; Galán-Relaño, A.; Luque, I.; Tarradas, C.; Rodríguez-Ortega, M.J.; Huerta, B. Antimicrobial Susceptibility of Cinnamon and Red and Common Thyme Essential Oils and Their Main Constituent Compounds against Streptococcus Suis. Lett. Appl. Microbiol. 2022, 74, 63–72. [Google Scholar] [CrossRef]
- Nazeam, J.A.; Ragab, G.M.; El-Gazar, A.A.; El-Mancy, S.S.; Jamil, L.; Fayez, S.M. Topical Nano Clove/Thyme Gel against Genetically Identified Clinical Skin Isolates: In Vivo Targeting Behavioral Alteration and IGF-1/PFOXO-1/PPAR γ Cues. Molecules 2021, 26, 5608. [Google Scholar] [CrossRef]
- Hamed, E.A.; Abdelaty, M.F.; Sorour, H.K.; Elmasry, D.M.A.; Abdelmagid, M.A.; Saleh, M.A.M.; AbdelRahman, M.A.A. A Pilot Study on the Effect of Thyme Microemulsion Compared with Antibiotic as Treatment of Salmonella Enteritidis in Broiler. Vet. Med. Int. 2022, 2022, 3647523. [Google Scholar] [CrossRef]
- Jabraeili, S.; Mirzaei, H.; Anarjan, N.; Javadi, A.; Behnajady, M.A. Nanoliposomal Thyme (Thymus vulgaris) Essential Oil: Effects of Formulation Parameters. Food Sci. Technol. Int. 2021, 10820132211010104. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Arras, G.; Usai, M. Fungitoxic Activity of 12 Essential Oils against Four Postharvest Citrus Pathogens: Chemical Analysis of Thymus capitatus Oil and Its Effect in Subatmospheric Pressure Conditions. J. Food Prot. 2001, 64, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Alshaikh, N.A.; Perveen, K. Susceptibility of Fluconazole-Resistant Candida Albicans to Thyme Essential Oil. Microorganisms 2021, 9, 2454. [Google Scholar] [CrossRef]
- Pereira, R.; Dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida Albicans: Formation, Regulation and Resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris Essential Oil and Thymol Inhibit Biofilms and Interact Synergistically with Antifungal Drugs against Drug Resistant Strains of Candida Albicans and Candida Tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczyńska, A. Effect of Clove and Thyme Essential Oils on Candida Biofilm Formation and the Oil Distribution in Yeast Cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M.; Dąbrowska, M. The Effect of Thyme and Tea Tree Oils on Morphology and Metabolism of Candida Albicans. Acta Biochim. Pol. 2014, 61, 305–310. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-Albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules 2021, 26, 4937. [Google Scholar] [CrossRef]
- Schubert, M.; Spiegel, H.; Schillberg, S.; Nölke, G. Aspergillus-Specific Antibodies—Targets and Applications. Biotechnol. Adv. 2018, 36, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.; Carvajal-Moreno, M.; Correa, B.; Rojo-Callejas, F. Cellular, Physiological and Molecular Approaches to Investigate the Antifungal and Anti-Aflatoxigenic Effects of Thyme Essential Oil on Aspergillus Flavus. Food Chem. 2020, 315, 126096. [Google Scholar] [CrossRef] [PubMed]
- Hlebová, M.; Hleba, L.; Medo, J.; Kováčik, A.; Čuboň, J.; Ivana, C.; Uzsáková, V.; Božik, M.; Klouček, P. Antifungal and Synergistic Activities of Some Selected Essential Oils on the Growth of Significant Indoor Fungi of the Genus Aspergillus. J. Environ. Sci. Health Part A 2021, 56, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Segvić Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal Activity of Thyme (Thymus vulgaris L.) Essential Oil and Thymol against Moulds from Damp Dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) Essential Oils and Their Main Components to Enhance Itraconazole Activity against Azole Susceptible/Not-Susceptible Cryptococcus Neoformans Strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.-W.; Chung, M.-S.; Bai, H.-W.; Chung, B.-Y.; Lee, S. Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus Neoformans. Molecules 2021, 26, 3476. [Google Scholar] [CrossRef]
- Parrish, N.; Fisher, S.L.; Gartling, A.; Craig, D.; Boire, N.; Khuvis, J.; Riedel, S.; Zhang, S. Activity of Various Essential Oils Against Clinical Dermatophytes of Microsporum and Trichophyton. Front. Cell Infect. Microbiol. 2020, 10, 545913. [Google Scholar] [CrossRef]
- Miastkowska, M.; Michalczyk, A.; Figacz, K.; Sikora, E. Nanoformulations as a Modern Form of Biofungicide. J. Environ. Health Sci. Eng. 2020, 18, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral Effect of Aqueous Extracts from Species of the Lamiaceae Family against Herpes Simplex Virus Type 1 and Type 2 in Vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef] [Green Version]
- Toujani, M.M.; Rittà, M.; Civra, A.; Genovese, S.; Epifano, F.; Ghram, A.; Lembo, D.; Donalisio, M. Inhibition of HSV-2 Infection by Pure Compounds from Thymus capitatus Extract In Vitro. Phytother. Res. 2018, 32, 1555–1563. [Google Scholar] [CrossRef]
- Walther, C.; Döring, K.; Schmidtke, M. Comparative in Vitro Analysis of Inhibition of Rhinovirus and Influenza Virus Replication by Mucoactive Secretolytic Agents and Plant Extracts. BMC Complement. Med. 2020, 20, 380. [Google Scholar] [CrossRef] [PubMed]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of Selected Lamiaceae Plants in Anti(Retro)Viral Therapy. Pharm. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon Citratus, and Rosmarinus Officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, e1700436. [Google Scholar] [CrossRef]
- United States Environment Protection Agency. About List N: Disinfectants for Coronavirus (COVID-19). Available online: https://www.epa.gov/coronavirus/about-list-n-disinfectants-coronavirus-covid-19-0 (accessed on 5 March 2022).
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and Antiviral Effects of Thymus vulgaris Essential Oil on Feline Coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Javed, H.; Meeran, M.F.N.; Jha, N.K.; Ojha, S. Carvacrol, a Plant Metabolite Targeting Viral Protease (Mpro) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19. Front. Plant Sci. 2020, 11, 601335. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational Evaluation of Major Components from Plant Essential Oils as Potent Inhibitors of SARS-CoV-2 Spike Protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A Systematic Review on Ethnopharmacology, Phytochemistry and Pharmacological Aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef]
- Silva, A.S.; Tewari, D.; Sureda, A.; Suntar, I.; Belwal, T.; Battino, M.; Nabavi, S.M.; Nabavi, S.F. The Evidence of Health Benefits and Food Applications of Thymus vulgaris L. Trends Food Sci. Technol. 2021, 117, 218–227. [Google Scholar] [CrossRef]
- Rizwan, B.; Zahur, M.; Azhar, N.; Khalid, S.; Sajid, N.; Qadeer, S. Therapeutic Potential of Thymus vulgaris: A Review. Ann. Res. 2020; 3, 147–161. [Google Scholar] [CrossRef]
- Kuete, V. Chapter 28—Thymus vulgaris. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 599–609. ISBN 978-0-12-809286-6. [Google Scholar]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef] [Green Version]
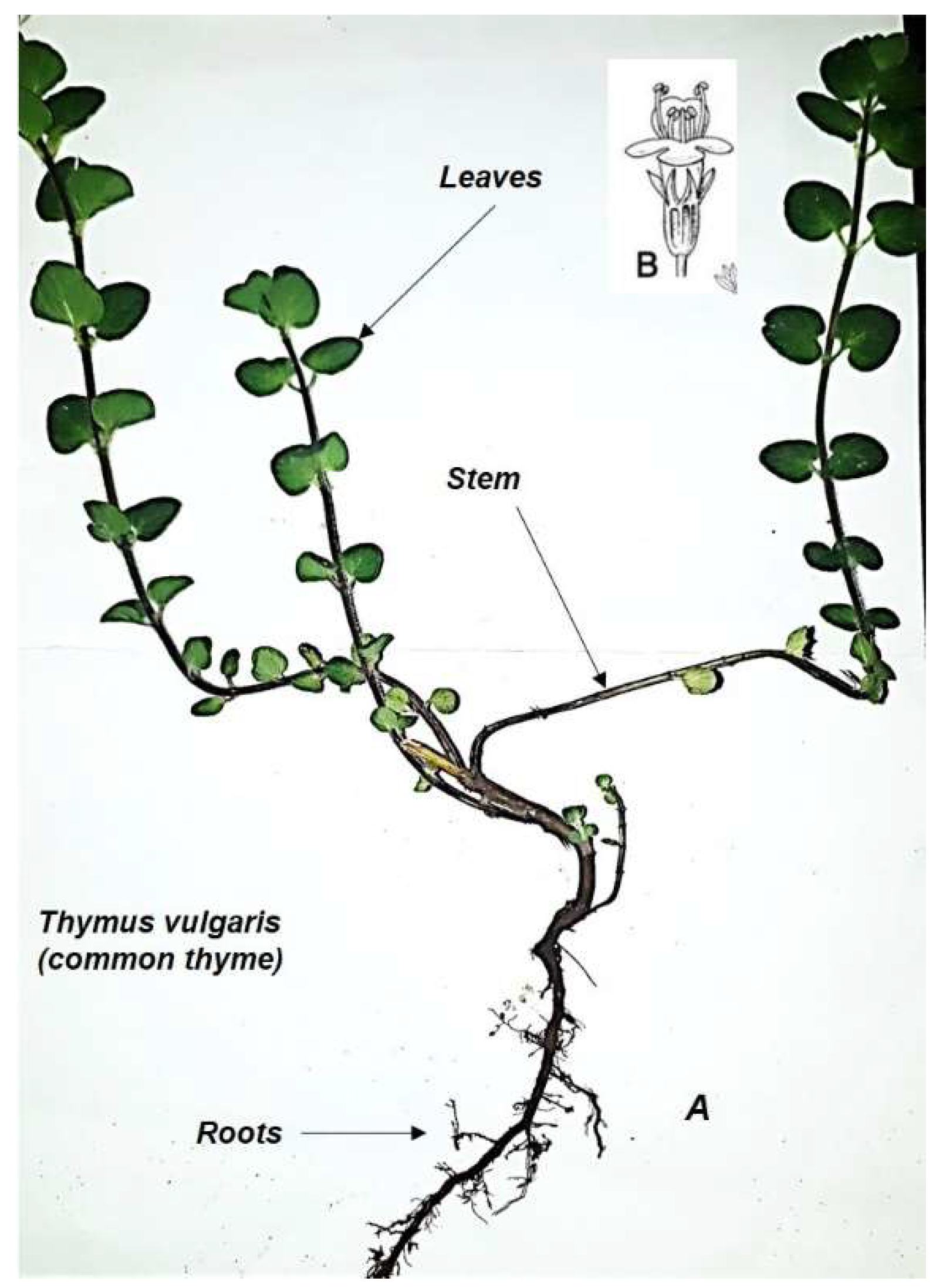
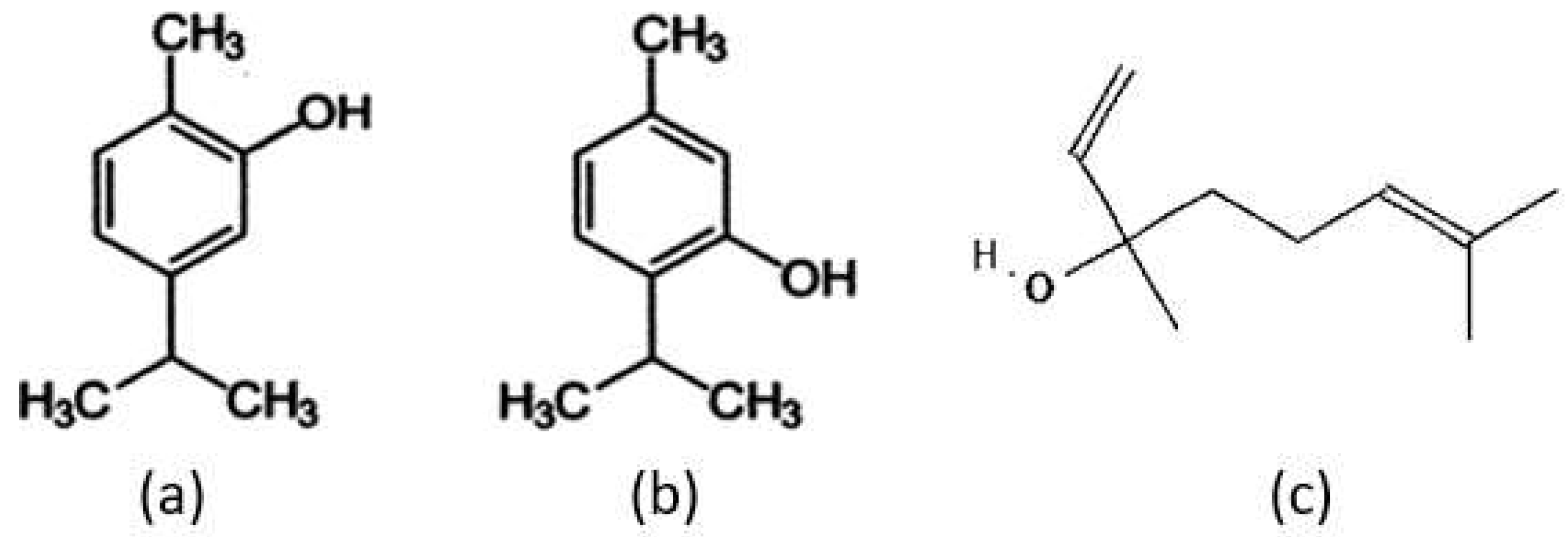
| Component | Formula | Relative Concentration (%) |
|---|---|---|
| 3-Hexanol | C6H12O | 0.10 |
| α-Tujene | C10H16 | 1.52 |
| α-Pinene | C10H16 | 1.31 |
| Camphene | C10H16 | 0.75 |
| Sabinene | C10H16 | 0.84 |
| 3-Otenol | C8H16O | 0.36 |
| 3-Otanone | C8H16O | 0.20 |
| Β-Myrcene | C10H16 | 0.67 |
| 3-Otanol | C8H18O | 0.21 |
| α-Pellandrene | C10H16 | 0.10 |
| δ-3-Carene | C10H16 | 0.11 |
| α-Terpinene | C10H16 | 2.36 |
| ρ-Cymene | C10H14 | 7.61 |
| Sylvestrene | C10H16 | 0.34 |
| 1,8-Cineol | C10H18O | 0.57 |
| cis-Oimene | C10H16 | 0.22 |
| β-Oimene | C10H16 | 0.20 |
| ɤ-Terpinene | C10H16 | 9.50 |
| cis-Sabinene | C10H8O | 0.10 |
| Thymol | C10H14O | 54.26 |
| Carvacrol | C10H14O | 4.42 |
| Octadienoic acid | C18H12O | 0.10 |
| Geranic acid | C10H16O2 | 0.30 |
| Principle | Nutrient Value per 100 g of Fresh Leaves | Percentage of RDA |
|---|---|---|
| Niacin | 1.824 mg | 11% |
| Pantothenic acid | 0.409 mg | 8% |
| Pyridoxine | 0.348 mg | 27% |
| Riboflavin | 0.471 mg | 36% |
| Thiamin | 0.48 mg | 4% |
| Vitamin-A | 4751 IU | 158% |
| Vitamin-C | 160.1 mg | 266% |
| Sodium | 9 mg | 0.5% |
| Potassium | 609 mg | 13% |
| Calcium | 405 mg | 40.5% |
| Iron | 17.45 mg | 218% |
| Magnesium | 160 mg | 40% |
| Manganese | 106 mg | 15% |
| Zinc | 1.81 mg | 16.5% |
| Carotene-β | 2851 mg | - |
| Biological Activity of Thyme | Major Findings | Reference |
|---|---|---|
| Antioxidant | Use of waste thyme extract for preventing the formation of lipid oxidation products in oil in water emulsions, constituted by diverse proportions of wheat and almond oils | [34] |
| Antioxidant efficacy of thymol and carvacrol in microencapsulated walnut oil triacylglycerols | [39] | |
| Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats | [41] | |
| Effectiveness of thymol on the growth performance, antioxidant status of the meat and the immunity of Nile tilapia fingerlings, Oreochromis niloticus | [43] | |
| Anti-inflammatory | Use of unfractionated essential oil from Thymus vulgaris to reduce neutrophil infiltration during an inflammatory response in zebrafish embryos | [45] |
| Effectiveness of thyme extract oils in reducing the production and gene expression of proinflammatory mediators and increasing anti-inflammatory IL-10 cytokine secretion in activated macrophages | [48] | |
| Ability of Greek Thymus vulgaris oil and water extracts to detoxify lipopolysaccharide-induced inflammation | [49] | |
| In vivo anti-inflammatory activities of Thymus vulgaris essential oils by significantly reducing carrageenan-induced paw edema in mice | [52] | |
| Anticancer | Anticancer activities of Thymus vulgaris L. in experimental breast carcinoma in vivo and in vitro | [68] |
| Cytotoxic, genotoxic, apoptotic, and reactive oxygen species (ROS)-generating effects of carvacrol on gastric adenocarcinoma in vitro | [72] | |
| Chemopreventive effect of thymol against dimethylhydrazine and/or high fat diet-induced colon cancer in rats | [74] | |
| Effectiveness of carvacrol in inhibiting cell proliferation and migration in non-small cell lung cancer cells | [80] | |
| Antibacterial | Effectiveness of thyme essential oil against Staphylococcus aureus and Klebsiella pneumoniae | [90] |
| Use of thyme oil nano-emulsions aided by ultrasound to decontaminate the surface of cherry tomatoes against Eschericia coli O157:H7 | [92] | |
| Use of thyme essential oil to increase susceptibility to colistin in Nosocomial Acinetobacter baumannii and K. pneumoniae | [99] | |
| Bacteriostatic and biofilm inhibitory properties of thyme nanogel against genetically identified skin bacterial clinical isolates (Pseudomonas stutzeri, Enterococcus faecium and Bacillus thuringiensis) | [105] | |
| Antifungal | Fungistatic and fungicidal activity of thyme essential oil against Candida albicans | [112] |
| Activity of thyme oil and thymol alone or in combination with antifungal drugs as antibiofilm agents against resistant strains of C. albicans and Candida tropicalis | [114] | |
| Antifungal control of thyme essential oil on Aspergillus flavus and reduction in aflatoxin B1 production, by exerting changes at the molecular level and inducing significant apoptotic-like cell death | [120] | |
| Activity of thyme essential oil against clinical dermatophytes from the two primary genera Microsporum and Trichophyton | [125] | |
| Antiviral | Antiviral activity against herpes simplex virus type 2 (HSV-2) by extracts or essential oil of thyme, via decreasing infectivity of the virus particles | [128] |
| Dose-dependent anti-influenza activity of thyme extract in Madin Darby canine kidney (MDCK) and HeLa Ohio cells | [129] | |
| Active interference with Tat protein in HIV, needed in transcription, by the essential oil of thyme | [131] | |
| Antiviral efficacy of thyme essential oil against feline coronaviruses in vitro, through inhibiting viral replication and reducing viral titer | [133] | |
| Inhibitory effect of thymol and carvacrol on the spike protein of SARS-CoV2 | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoudi Halat, D.; Krayem, M.; Khaled, S.; Younes, S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients 2022, 14, 2104. https://doi.org/10.3390/nu14102104
Hammoudi Halat D, Krayem M, Khaled S, Younes S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients. 2022; 14(10):2104. https://doi.org/10.3390/nu14102104
Chicago/Turabian StyleHammoudi Halat, Dalal, Maha Krayem, Sanaa Khaled, and Samar Younes. 2022. "A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb" Nutrients 14, no. 10: 2104. https://doi.org/10.3390/nu14102104
APA StyleHammoudi Halat, D., Krayem, M., Khaled, S., & Younes, S. (2022). A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients, 14(10), 2104. https://doi.org/10.3390/nu14102104







