Abstract
Military veterans often have numerous physical and mental health conditions and can face unique challenges to intervention and management. Dietary interventions can improve the outcomes in many health conditions. This study aimed to evaluate the scope of health conditions targeted with dietary interventions and the effectiveness of these interventions for improving health-related outcomes in veterans. A systematic literature review was performed following PRISMA guidelines to identify and evaluate studies related to veterans and dietary interventions. Five electronic databases were searched, identifying 2669 references. Following screening, 35 studies were evaluated, and 18 were related to a US national veteran weight-loss program. The included studies were critically appraised, and the findings were narratively synthesized. Study designs ranged from randomised controlled trials to cohort studies and were predominantly U.S. based. The intervention durations ranged from one to 24 months. The mean subject age ranged from 39.0 to 69.7 years, with often predominantly male participants, and the mean body mass index ranged from 26.4 to 42.9 kg/m2. Most dietary interventions for veterans were implemented in populations with overweight/obesity or chronic disease and involved single dietary interventions or dietary components of holistic lifestyle interventions. The most common primary outcome of interest was weight loss. The success of dietary interventions was generally moderate, and barriers included poor compliance, mental health conditions and large drop-out rates. The findings from this review illustrate the need for further refinement of dietary and lifestyle interventions for the management of veterans with chronic health conditions.
1. Introduction
Military veterans often experience numerous health issues, such as mental health conditions, insomnia, obesity, chronic diseases and chronic pain [1]. It has been reported that over a third of older veterans have at least three comorbid conditions [2]. The incidence of psychological conditions and risk of physical comorbidities, such as heart disease and hypertension, osteoarthritis, diabetes, chronic pain and lung disease, is greater in the military population than in the general population [3].
The medical management of these complex presentations is challenging. Previous research has evaluated several types of interventions to improve the mental and physical health of military veterans, and these commonly include psychology-based interventions and pharmacological interventions. These interventions have shown mixed results, with some studies showing benefits and others showing limited effects on health in veterans [4,5]. There is great scope for exploring additional approaches to holistic management of the complex individual.
Dietary interventions have been advocated to improve outcomes in numerous health conditions. Various diet interventions have shown to be generally effective (although with some mixed results) for improving outcomes and reducing risk factors for, inter alia, obesity [6], pain conditions [7,8], anxiety and depression [9], insomnia [10], cardiovascular disease [11], diabetes [12] and certain cancers [13]. Concurrently addressing complex health conditions in veterans with dietary management as a component of a multidisciplinary approach appears logical as a safe and beneficial approach and contributes to improving quality of life.
Attrition rates in various dietary intervention studies can often be high [14], and many different strategies for delivery and implementation have been adopted for dietary interventions in the effort to improve adherence [15]. Poor health behaviours, such as tobacco use, physical inactivity, poor diet and alcohol misuse, are more prevalent in veterans than in civilian populations, and veterans are also more likely to be obese than civilian populations [16].
Veterans also tend to have poorer social support [17], which has been linked to physical inactivity and poor chronic-disease management [18] as well as negative physical [19] and mental-health outcomes [20]. These factors may present greater challenges and barriers to lifestyle and behavioural change; hence, the feasibility and effectiveness of implementing dietary interventions in a veteran population may differ from that of other populations with specific health conditions.
Considering that veterans have a high incidence of poor health behaviours, physical and psychological comorbidities and face unique challenges in undertaking behavioural and lifestyle modifications, it is important to understand how dietary interventions have been utilized and whether they are effective in improving health, specifically in veteran populations. Therefore, the purpose of this systematic review is to evaluate the scope of health conditions targeted with dietary interventions and the effectiveness of these interventions for improving health-related outcomes in veterans.
2. Methods
This systematic review was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21] (Supplementary Material 1, Table S1) and was registered on PROSPERO (International Prospective Register of Systematic Reviews, No CRD42021236259).
2.1. Literature Search
We searched the electronic databases MEDLINE (via PubMed), CINAHL (via EBSCO), Embase, Cochrane CENTRAL and PsycINFO (via Ovid) from inception to 23rd February 2021, using combinations of MeSH and free-text words for “veteran” and “diet” (Supplementary Material 2). The search was originally designed in PubMed, and then the selected terms and their synonyms were translated for the respective databases using Polyglot [22].
2.2. Study Eligibility Criteria
The study inclusion criteria were developed using the Participants, Intervention, Comparator and Outcome (PICO) [23] plus Study design approach (Table 1). Participants were veterans of military or defence forces from any nation or discipline. Interventions were dietary interventions conducted at any time post-discharge from the military or defence force. Dietary interventions could include specific dietary patterns, altered specific nutrients or foods through dietary intake and/or energy intake adjustments.

Table 1.
PICOS study eligibility criteria.
Studies were eligible regardless of the mode of intervention delivery or intervention duration and whether diet was part of a multi-factorial intervention with other lifestyle or behavioural components (e.g., diet and exercise or psychological support). A control or comparator group was not required, and hence single-group pre–post intervention studies and randomised or non-randomised controlled trials involving a usual-care, no-care or alternative-intervention group were eligible.
The outcomes of interest were any health-related outcome measures, including anthropometric or body composition, dietary intake or behaviours, cardiometabolic risk markers, quality of life, mental health, physical function or strength, patient-reported outcome measures and chronic disease incidence or endpoints. All study designs that involved a dietary intervention were included. This included cohort studies if they followed up and evaluated participants undertaking a diet intervention that was considered to be usual care. Studies were required to be published in English for inclusion, and no publication date restrictions were imposed.
2.3. Screening and Data Extraction
Identified references were imported into Endnote X9 reference management software [24], and de-duplication was conducted using the Endnote duplication tool. The de-duplicated set was imported into Covidence [25]. Two reviewers (HM, RM or ESD) independently screened titles and abstracts with disagreements resolved by consensus. The full text of articles that were considered potentially eligible were then screened for eligibility independently by two reviewers (HM, RM or ESD) and agreement was reached via group consensus. Data were extracted from each study into pre-defined tables by one reviewer and cross-checked by a second reviewer (RM, ESD or BS performed either step) and included the study citation, study design, participant eligibility and characteristics, intervention details, control details if relevant, outcomes of interest and results.
2.4. Risk of Bias Assessment
Risk of bias in each included study was assessed independently by two reviewers (RM, ESD or BS) using the Academy of Nutrition and Dietetics Quality Criteria Checklist: Primary Research [26]. Studies were assessed on 10 key criteria assessing the internal and external validity. These criteria related to the clarity of the research question, selection bias, similarity of study groups, methods of handling withdrawals, blinding procedures, intervention details; measurement, validity and reliability of outcomes, statistical analyses methods, appropriateness of conclusions and bias related to funding or sponsorship. Each study was awarded an overall positive, neutral, or negative method quality rating based on the scoring tool instructions. Papers were not excluded based on the quality criteria.
2.5. Data Synthesis
Due to the fact that the characteristics of the studies (the different study designs, the large range of different outcomes measured, the use of different types of dietary interventions and the different health conditions targeted) were too diverse to yield a meaningful summary estimate of effects, meta-analyses were not deemed suitable. Instead, we provide a narrative synthesis of the findings, structured around the types of intervention (i.e., specific dietary regime, dietary education and behavioural change), target population characteristics (e.g., health condition) and type of outcome (e.g., weight change, quality of life and metabolic indices).
3. Results
3.1. Study Selection
The literature search identified 2669 references after the removal of duplicates, and 2548 were removed after title and abstract screening. Of 121 references retrieved for full text screening, 86 were excluded. The remaining 35 studies were included in this review (Figure 1). Eighteen of these studies were related to a U.S. national program, MOVE! (Managing Overweight/Obesity for Veterans Everywhere), which is an ongoing clinical weight management program for veterans and these are discussed separately.
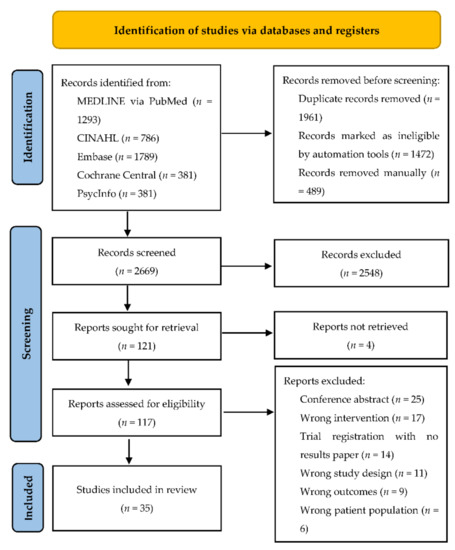
Figure 1.
PRISMA flow diagram for our systematic search.
3.2. Risk of Bias
The results of the quality assessment criteria are presented in detail in Table 2. In summary, 12 studies received a neutral rating and were of moderate methodological quality, while the remaining 23 studies were of high methodological quality, receiving a positive rating. No studies received a negative rating. The main source of bias was a lack of blinding of participants and investigators to the treatment group, as well as of data collectors to the outcome measures.

Table 2.
Risk of bias outcomes.
3.3. Study Characteristics
The characteristics of the 17 studies not associated with the MOVE! program are described in Table 3. Most studies were conducted in the USA (n = 15 of 17), and there was one each from Australia and Taiwan. Of these, ten were randomised controlled trials (RCTs) [27,28,29,30,31,32,33,34,35], three were dietary interventions in usual care that were evaluated using routinely collected data accessed retrospectively [36,37,38], three were single arm interventions [39,40,41], and one was a cohort study [42]. The trial intervention duration ranged from 1 to 24 months. The mean age of the study participants ranged from 39.0 ± 6.7 to 69.7 ± 0.7 years. Most studies had a predominance of male participants, with the percentages of males ranging from 42% to 100%. The mean body mass index (BMI) of participants ranged from 26.4 ± 2.6 to 42.9 ± 7.7 kg/m2.

Table 3.
Characteristics of the included studies, grouped and listed in order of health condition discussed in text.
3.4. Veteran Populations and Dietary Interventions
In studies of overweight or obese veterans (n = 8), commonly the primary aim was to achieve weight loss. Three studies investigated a low-carbohydrate diet compared to either a low-fat/low-calorie diet [33,43] or a low-fat diet combined with Orlistat therapy [35]. One study compared an intermittent energy restriction diet plan (the 5:2 diet) with a standard energy restricted diet [29]. One trialled individualized wellness coaching, which addressed healthy eating habits, shopping and cooking advice and used the stage-of-change model to alter eating behaviours [32].
One examined the feasibility and efficacy of weekly educational mailings and telephone consultations that addressed weight management issues in reducing weight and improving dietary habits [28]. Two studies included overweight veterans with schizophrenia: one was an inpatient regime consisting of a calorie-restricted diet and physical exercise [34], whilst the other evaluated a psychosocial weight management program that focused on tailored nutritional and behavioural change education [39].
Three studies were conducted in veterans with type II diabetes. One study compared a low-carbohydrate diet to a low-fat diet [31]. Another study incorporated dietitian-led sessions in a group education programme with a focus on carbohydrate intake, dietary fats and general healthy eating, including for weight loss [37]. The last study evaluated The Healthy Teaching Kitchen programme, which comprised cooking and nutrition education classes with topics on carbohydrate counting and meal planning [36].
All other study populations varied. One study in veterans with uncontrolled hypertension compared a telephone-delivered intervention of monthly counselling for exercise, diet and medications based on the current stage of change with sessions of non-tailored information and usual care [30]. As part of a preliminary phase of a larger trial, patients with primary hypercholesterolaemia received two to four dietitian intervention sessions over 6–8 weeks focused on reducing the intake of total and saturated fat, cholesterol and energy [38]. A ketogenic diet was implemented in a population of veterans with advanced malignant cancers [41] to evaluate safety and tolerability.
In a population of veterans with Gulf War Illness (GWI), a low-glutamate diet was implemented to examine its effectiveness on symptomatology [42]. A study in participants with amnestic mild cognitive impairment compared a low-saturated-fat/low-glycaemic-index diet to a high-saturated-fat/high-glycaemic-index diet [27]. Finally, a study in nursing home residents reported the feasibility of implementing a dietary intervention that addressed age-related nutrition topics, such as hydration, meal planning, the Dietary Approaches to Stop Hypertension (DASH) diet and protein intake, as part of an exercise- and health-promotion programme [40].
3.5. Attendance and Attrition
A number of interventions utilized group sessions and reported the number of sessions attended (Table 3). Shorter or less-intense group interventions appeared to have better attendance rates [36,37]. However, in the program for nursing home residents where attendance was optional, a mean of only 2.9 of 7 available classes were attended [40], and mean attendance at 16 offered weekly sessions in the psychosocial weight management program for obese veterans with schizophrenia was poor at 3.8 [39]. Longer duration studies that reassessed participants at 48 weeks reported reasonable percentages of participants that attended for final follow-up measures, ranging from 79% [35] to 88% [44]. In a study offering group sessions over 24 months, only 47.22% completed the 24 month assessment [31].
Other studies utilized individual sessions to deliver the intervention. Number of attended sessions was only reported in one study, in which participants attended a mean of 2.8 of the required two to four dietitian consultations over 6 to 8 weeks [38]. Two 6-month RCTs reported that 65% [32] to 75% [29] of participants in the intervention groups completed the studies. An RCT that provided weekly counselling sessions for four weeks, then monthly sessions for 11 months [33] reported that 60% attended the 6-month follow up appointment, and 67% attended the 12-month follow up appointment.
Some studies delivered intervention content via phone calls. One study reported that 87% of the intervention group, which received weekly mailings and phone calls, and 55% of the comparison group, which received “usual care” from a hospital clinic, attended 8-week follow-up [28]. Another study using dietary training via Skype reported that 87% completed the trial, and 74% completed the 3-month follow up [42]. Of these, 88% were still following the diet.
In one study in which the intervention was strongly controlled, with food delivered to the homes of participants twice weekly, all participants completed the trial [27], whilst in another study in which inpatient participants were provided with a diet overseen by the hospital dietitian for six months [34], all participants who remained in hospital completed the trial. In a trial to evaluate the safety and tolerability of a ketogenic diet in patients with advanced malignant cancer, 64.7% completed 4–16 weeks of dieting, and at 16 weeks, only 36% (four participants) still maintained the diet [41].
3.6. Health-Related Outcome Measures
Data and statistical outcomes for all study results are reported in detail in Table 4, with the main results for health-related outcomes, as described by the individual studies, summarised in the following text.

Table 4.
Health-related outcome measures of the included studies, grouped and listed in order of health condition discussed in the text.
3.7. Weight and BMI
We found that 11 of 17 studies were interested in weight change as their primary outcome, reporting either total weight loss, weight loss percentage, or percentage of participants that lost ≥5% of baseline body weight. Of these, seven studies reported a statistically significant reduction in weight post-intervention (range of mean change: −11.4 kg to −0.2 kg) [29,32,34,35,36,37,44]. Four studies also included BMI as a primary outcome, and three reported a statistically significant reduction in BMI with diet intervention (range of mean change: −1.5 to −0.35 kg/m2) [32,34,36]. Only two of these studies reported a significant between-group difference in weight and/or BMI change at the final follow-up [34,37].
3.8. Blood Pressure
There were 2 of 17 studies reported change in blood pressure (BP) as a primary outcome [30,37]. Of these, only one study, comparing a diet intervention tailored to stage of change and a non-tailored health education intervention with usual care, reported a significant reduction in BP in both intervention groups at six months, as well as a significantly greater number of participants with controlled BP in the tailored group compared with other groups [30].
3.9. Blood Composition or Metabolic Parameters
Haemoglobin A1c (HbA1c), glucose, insulin and serum lipids were often included as primary outcome measures. A basic diabetes education intervention compared with standard diabetes management significantly reduced HbA1c levels in veterans with type II diabetes [37], whilst a Healthy Teaching Kitchen program reported no significant change [36]. A calorie-restricted diet for hospitalized obese veterans with schizophrenia produced a significant reduction in insulin levels over the six month intervention, although there were no significant differences in glucose or insulin levels compared with the control group [34].
Three studies assessed serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglyceride levels. There was a significant reduction in triglyceride levels after therapy focused on reduction in saturated fat, cholesterol and energy intake [38] in veterans with primary hypercholesterolaemia and an inpatient calorie-restricted diet and exercise regime in obese veterans with schizophrenia [34]. However, only Sikand and colleagues reported a significant decrease in TC, LDL and HDL [38]. There were no significant changes in serum lipids post-intervention in the Healthy Teaching Kitchen intervention [36].
3.10. Other Health-Related Outcomes
One study examined fruit and vegetable intake as the primary outcome of interest and found no significant change in daily servings in older veterans after participating in a dietary education component within an exercise and health promotion program [40]. The total symptom score (total number of typical GWI symptoms experienced) was significantly decreased in veterans with GWI compared to a wait-listed control group after following a low glutamate diet [42].
The safety and feasibility of a ketogenic diet for participants with advanced cancers examined measures, such as adverse effects, weight, BMI, BP, haematology, ketones, lipids, glucose/ketone indices, quality of life parameters and effects on the tumour. This trial demonstrated that the ketogenic diet was well tolerated by those who remained on the diet (the reasons for drop-outs were not dietary related), and adverse effects were minimal in those remaining on the diet throughout the observation period [41].
3.11. The MOVE! Weight Management Program
In the US, the Veterans Health Administration (VHA) identified a need for effective weight management interventions for their veterans, which prompted the development of the MOVE! (Managing Overweight/Obesity for Veterans Everywhere) Weight-Management Program. Details about the current MOVE! program are reported elsewhere (www.move.va.gov, accessed on 9 March 2021). The MOVE! program has evolved over time to include TeleMOVE (i.e., delivering the MOVE! program via videoconferencing technology), MOVE! plus adjunctive treatments, as well as variance in the length of program and content advancement.
Numerous studies have been conducted, including evaluative, feasibility and comparative studies, to ensure that the MOVE! programs and other weight loss opportunities continue to be developed and refined. The database search for this review identified 18 studies related to the MOVE! program. The study characteristics, data and outcomes for all MOVE! study results are reported in detail in the Supplementary Material 3 (Tables S2 and S3) with a very brief overview reported in the following text.
3.12. Health-Related Study Outcomes
Weight change was the primary outcome measure in the majority of the MOVE!-related studies. Two trajectory studies reported that veterans had been progressively gaining weight prior to enrolment in the MOVE! program and, once enrolled, began to lose weight at a steady rate, ranging from an average of −1.6 kg/year [45] to −2.2 kg/year in the first year of participation [46], depending on the level of involvement.
Mental health had an impact on effectiveness of the program, as those with mental health diagnoses were not as successful in losing weight [47], despite modifications and adaptations to the program for those with serious mental illness [48,49]. However, a short pilot study (over 10 weeks) showed that the MOVE! program was able to reduce the severity of depression in obese veterans with severe depression to a similar extent as a 2-week intense residential-based program [50].
The introduction of telemedicine (e.g., TeleMOVE!) was demonstrated to be a successful method of delivery, as all related studies reported significant weight loss for the intervention group (despite different study comparators and study durations) ranging from −3.9 to −5.3 kg [51,52,53]. The addition of personal digital assistants to self-monitor diet and physical activity and biweekly coaching calls was also successful in optimising weight loss [54].
However, the concurrent treatment of apathy with pharmacotherapy was no more effective than the standard MOVE! program in achieving weight loss [55]. The introduction of a nutrigenetic-guided diet into the MOVE! program was no better than a standard balanced diet in its ability to achieve a loss of ≥5% of body weight in participants [56]. Improved healthy eating habits, in terms of a greater intake of fruit and vegetables, was enhanced by the introduction of tailored newsletters [57].
When compared to two other national weight-loss programs, MOVE! was not inferior over the longer term. When compared to the Aspiring for Lifelong Health (ASPIRE) program, participants in an ASPIRE-Group arm lost more weight than an ASPIRE-phone group and MOVE! participants over 12 months [58]. However, by 24 months, none of the three programs proved superior in weight-loss success [59]. In addition, in obese veterans with type II diabetes, those participating in the Veterans Affairs Diabetes Prevention Program (VA-DPP) had lost more weight than the MOVE! group by six months; however, by 12 months, there was no significant difference in weight loss between the groups [60].
Promisingly, it has been found that MOVE! participation was associated with a reduced incidence of total cardiovascular disease, coronary artery disease, cerebrovascular disease, peripheral vascular disease and heart failure over a follow-up period of almost five years [61].
4. Discussion
This systematic review aimed to explore the scope of use and effectiveness of dietary interventions for improving health-related outcomes in veterans. The majority of health conditions that were addressed by dietary interventions were chronic diseases or illnesses, such as obesity, type II diabetes, hypercholesterolaemia, uncontrolled hypertension and advanced malignant cancers. Psychological conditions were also targeted, such as amnestic mild cognitive impairment, schizophrenia and the symptoms of GWI.
This range of conditions targeted with dietary interventions is unsurprising. In the United States, 35% of the Veterans’ Health Administration (VHA) primary care enrollees (which represents 90% of all of VHA patients) are estimated to be obese [62], which puts them at a higher risk for chronic diseases, such as hypertension, dyslipidaemia, stroke, diabetes, coronary heart disease and osteoarthritis as well as various forms of cancer [63].
Additionally, the prevalence of mental-health disorders and alcohol-use disorders is higher in veterans than in the civilian population [64], and post-traumatic stress disorder (PTSD) is associated with greater BMI through depression–that is, higher symptoms of depression are associated with poor lifestyle behaviours, such as less physical activity, poorer diet and a greater likelihood of smoking in veterans [65]. All of these conditions can potentially benefit from dietary interventions and lifestyle changes.
Dietary interventions were either delivered as a specific form of diet or incorporated as part of a holistic lifestyle management approach. The range of specific diet regimes evaluated included low-carbohydrate diets, low-fat diets, ketogenic diets, continuous energy restricted diets and the 5:2 diet. One study even examined the feasibility of implementing a nutrigenetic-guided diet into individuals’ management in order to improve weight-loss outcomes [56].
Notably, most of the dietary components of interventions were focused on nutrients or restriction-based recommendations rather than healthy dietary patterns or overall diet quality. Dietary guidelines for prevention and management of chronic disease have evolved such that overall eating patterns that focus on whole foods and their combinations, rather than isolated nutrients, such as Mediterranean and DASH dietary patterns, are recommended [12,66,67,68,69]. The current review suggests there is a gap in the literature investigating the effect of dietary patterns on health outcomes in veteran populations.
Holistic weight management programs address many lifestyle factors (diet, exercise, psychological factors and interaction/socialization), and the dietary components of these types of programs place emphasis on education and strategies to facilitate behaviour modification. Obesity is considered to be a chronic disease with multifactorial aetiology, and thus addressing modifiable lifestyle factors (such as proper nutrition, regular physical activity and attention to eating behaviours) is key to success. Even moderate weight loss can lower the risk of other obesity-related comorbidities [70].
A number of studies implemented a stage-of-change model, which recognises that people move through a series of stages when adopting a new behaviour and that different treatment approaches and health communication strategies may be necessary for individuals in the different stages of change [71]. Research across numerous health conditions has shown improvements in recruitment, retention and progress using stage-matched interventions, with promising outcomes also found with computer-based individualised and interactive interventions [72].
This strategy appeared to be successful in reducing and controlling BP in veterans with hypertension and uncontrolled BP [30], as well as improving dietary intake and reducing weight in a population of obese veterans [32]. However, both of these studies were performed over six months, and it remains to be seen if these behavioural and dietary changes are sustained over the longer term.
Adults with serious mental illness (SMI) are more likely to be overweight or obese, which contributes to a greater risk of comorbid medical conditions, such as type II diabetes and cardiovascular disease [73]. Lifestyle interventions in people with SMI show moderate promise but are not always successful [74]. People with SMI may also have cognitive deficits, limited literacy and challenging social situations, which can affect the success of interventions to address their health conditions. For example, one study found that participants with PTSD and other mental health conditions who were enrolled in the MOVE! program lost less weight than those with no mental health conditions [47].
However, attempts to improve outcomes by adapting the methods of delivery of the MOVE! program appear disappointing. Strategies, such as concurrent treatment for apathy [55], made no additional difference to weight loss. A modified, tailored MOVE! program for veterans with schizophrenia failed to improve uptake—only 56% of participants completed the six month assessment, only seven participants lost 5% of their body weight [48], and there were no differences in weight loss, metabolic, dietary, physical activity, attitudinal or functional measures from a control group who received only basic lifestyle information.
Delivery of MOVE! to obese veterans with SMI via internet browser-based educational modules, self-tracking activity and weight, individualized homework and weekly telephone calls from peer coaches (WebMOVE!) also failed to improve outcomes, as these participants only completed 49% of the modules, and an in-person MOVE! group only completed 41% of the modules. A sub-analysis of only the obese participants reported that merely 26% lost 5% of their body weight at six months [49]. These studies emphasize the need for continued efforts to customise and adapt lifestyle and health interventions to improve uptake and adherence in veterans with mental-health conditions.
Often isolation, distance from healthcare facilities, long travel times, inconvenience and expense are substantial barriers to attending face-to-face weight management programs or accessing healthcare professionals. Of the studies that examined whether videoconferencing, telemedicine, phone counselling/coaching and mail-outs were effective methods of delivering weight management programs to veterans who face these difficulties, all reported successful weight loss with the intervention, and of the five studies with a comparator group/s, two reported significantly more weight loss with the intervention. This is an encouraging finding and a positive step towards using telemedicine and phone-based methods to expand and facilitate delivery of these services to veterans where accessibility and remoteness may otherwise preclude them from valuable healthcare.
Apart from two trajectory studies conducted over four and eight years [45,46] the duration of the remaining studies ranged from one month to 24 months. As the majority of studies were focused on weight loss, limitations to the interpretation of results, including inadequate study duration, large proportions of subjects lost to follow-up, or a lack of appropriate usual care or control group, should be recognised. Many of the studies on specific diet regimes were of shorter duration, which may not allow for significant weight loss, or reflect the continuation of healthy eating habits and sustained weight management. Generally, nutrition education interventions that last for longer than five months report a higher level of success, particularly as behavioural changes take time and practice [75]. It has been reported that dietary and lifestyle therapy generally provides <5 kg of weight loss after 2–4 years [76].
Adherence, engagement and compliance with intervention protocols is vital to improving health-related outcomes. Not all studies reported attendance rates at group or face-to-face interventions or adherence to dietary regimes. A number of studies reported the proportions of how many participants attended final follow-up sessions but not whether participants actually completed or adhered to the intervention. Adequate adherence is one of the difficulties encountered in weight loss and dietary studies and is an aspect that must be considered when interpreting the results from these studies. In general, attendance was better for shorter-term group sessions.
However, in longer-term group session programs, adherence was not particularly high. The importance of engagement on successful outcomes was emphasized by Yancy and colleagues, where participants were given their preferred choice of diet. Those in the intervention group attended an average of 13.5 of 19 attendances (71% of those possible). However, in a sub-analysis, it was shown that participants who attended at least 15 group sessions had a greater mean weight loss than those who attended fewer than 15 sessions [44]. Some identified barriers to maintaining participant engagement in dietary intervention studies include disinterest in the study, difficulty attending sessions or frustration with a lack of weight loss [31].
The majority of studies were performed in overweight or obese veterans, or those with type II diabetes, and most of these reported improvements in body weight, BMI, waist circumference, as well as physiological biomarkers. This is promising in terms of improved health-related outcomes, as it has been shown that, in overweight individuals with impaired glucose tolerance, for every kilogram of weight lost, there is a 16% reduction in the risk for progression to type II diabetes [77]. In type II diabetes, improvement in fasting glucose, HbA1c, triglycerides and systolic BP may begin at only 2–5 kg of weight loss, with greater weight loss producing greater benefits, while improvements in diastolic BP and HDL cholesterol may be seen at 5–10 kg of weight loss [78].
Although most studies reported significant reductions in the baseline weight or BMI post intervention, fewer studies reported significant differences between the intervention group and comparator or control groups post intervention, as both groups lost significant amounts of weight. In the studies that compared different dietary regimes, none were able to demonstrate superiority in weight loss potential between the diets, and only two of these studies noted improvements in blood pressure, triglycerides or HbA1c in obese participants following low calorie diets, many of whom also had type II diabetes.
Although the majority of papers focused on weight loss as their primary outcome measure, dietary interventions may also have a positive impact on other health outcomes, such as mental health and health-related quality of life, particularly in populations with multiple comorbidities. Consuming a wide variety of healthy foods, including fruits, vegetables, whole grains, legumes and fish containing omega-3 fatty acids, can have positive effects on mood [79] and levels of depression [80], and the quality of life can be enriched by improved physical functioning, tolerance to pain, general health perceptions and vitality and can lead to fewer role limitations due to physical health problems [81,82].
Strengths and Limitations
The strengths of this study include adherence to a systematic methodology and use of the PRISMA guidelines. However, a number of limitations must be acknowledged in this review. It is widely recognized that randomized controlled trials are the gold standard in terms of study design and provide the highest quality scientific evidence. Non-randomized studies are inherently at risk of bias. However, it is also acknowledged that, in order to accommodate the evaluation of various research questions (such as the effectiveness or efficacy and outcomes, such as survival or severe adverse events), the inclusion of more than one study design appears to be necessary in systematic reviews of healthcare interventions [83].
In order to address the aim of our research question, which was to evaluate the scope of health conditions targeted with dietary interventions and the effectiveness of these interventions for improving health-related outcomes in veterans, it was necessary to design our search strategy to identify papers with numerous study designs involving interventions. This resulted in heterogeneous populations, different outcome measures and often small populations. Subsequently, meta-analysis of the results was not suitable.
It is acknowledged that the inclusion of dietary interventions that were part of multifactorial lifestyle or behavioural interventions in our search criteria does not allow for discrimination of the effects of diet from effects due to the other lifestyle components in the program (e.g., exercise or psychological approaches). However, this is commonly how numerous medical and psychological conditions are addressed, and it aligns with clinical guidelines. The inclusion of diet-only and diet as part of combined interventions allowed exploration of the full scope of dietary interventions that have been investigated across specific veteran populations and to include pragmatic studies.
This search strategy identified 18 studies related to the MOVE! program. Although available to all eligible US veterans, participation and attendance at sessions is voluntary. Therefore, a major selection bias is evident in these studies as well as being limited by a high drop-out rate. Most of the MOVE! studies were focused on the evaluation of program success or the effectiveness of adaptations for sub-populations (e.g., mental health conditions) and commonly used retrospective chart reviews in order to achieve this. This often resulted in a degree of missing data. In acknowledgement of these differences, we reported the MOVE! results separately from those of the other identified papers.
Although there were no overall negative ratings for the quality of the studies, a number of biases can be identified. Although lack of blinding was almost inherent, methods of handling withdrawals was a common source of bias, largely due to inadequate descriptions of the number and characteristics of the withdrawals or whether all enrolled subjects were accounted for.
A number of studies with small subject numbers were included, impacting the power of the results and analyses. The majority of studies included in this systematic review were US-based and predominantly involved male veterans; thus, the findings are not able to be generalised to the wider international veteran population, nor to female veterans. Additionally, few papers reported longer term follow-up assessments to determine whether the behavioural and lifestyle changes achieved in the initial relevant interventions were maintained over time–an important component in lifelong health and wellbeing.
An overview of these studies provide a sense of the difficulties and challenges faced during dietary research and when attempting to implement dietary interventions to veterans with various physical and psychological conditions. Although generally successful to some degree in achieving the primary aims of the studies, such as weight loss or metabolic/physiologic improvement, dietary studies must continue to strive for better methods to improve attendance and adherence rates.
Many of these studies had small subject numbers, and thus larger studies need to be performed to strengthen the validity of the results and the quality of the evidence. Strategies to address psychological issues (such as PTSD, depression, SMI, apathy and disinterest) need to be further developed in conjunction with dietary interventions, particularly for veterans where these issues are prevalent. Additional longer term studies ought to be performed in order to assess the sustainability of the interventions and their effect on lifelong health and wellbeing, as behavioural change takes time.
It could be suggested that more successful outcomes might be achieved by considering use of a stage-of-change model to improve recruitment, retention and progress and by adopting implementation strategies seen to be of benefit, such as computer-based individualized and interactive interventions. Promoting the use of healthy diet patterns in conjunction with lifestyle modifications and addressing social and psychological issues not only aligns with dietary guidelines for the prevention and management of chronic disease but also contributes to improvements in lifelong health, wellbeing and quality of life.
5. Conclusions
This review identified a large range of conditions in military veterans that have been targeted by dietary interventions with only moderate success. Most commonly, obesity and associated comorbid chronic conditions were addressed with either single dietary regimes or holistic lifestyle management programs with diet addressed as a component. Limited studies targeted an overall healthy dietary pattern. Barriers to success included poor attendance and adherence rates, mental health conditions and often large drop-out rates. However, the findings from this review illustrate the need for further refinement and the development of dietary and lifestyle interventions for the management of veterans with chronic health conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14102094/s1. Supplementary Material 1, Table S1: PRISMA 2020 Checklist; Supplementary Material 2: Final executed search strategies for relevant databases; Supplementary Material 3: Table S2: Main characteristics of studies that included the MOVE! weight-loss program as the intervention group or the control/comparison group, ordered and grouped by comorbid health conditions (in addition to meeting the eligibility criteria for the MOVE! program); Supplementary Material 3: Table S3: Health-related-outcome measures in studies that included the MOVE! weight-loss program as the intervention group or the control/comparison group, ordered and grouped by comorbid health condition (in addition to meeting eligibility criteria for MOVE! program).
Author Contributions
Conceptualization: R.M., H.L.M. and E.S.-D.; Methodology: H.L.M., R.M. and E.S.-D.; Literature Search: H.L.M.; Screening: H.L.M., R.M. and E.S.-D.; Data Extraction, Collation and Quality Assessment: H.L.M., R.M. and E.S.-D.; Writing—Original Draft Preparation, Review and editing: R.M., H.L.M. and E.S.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported and funded by the Gallipoli Medical Research Foundation (GMRF) Research Advisory Committee.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to acknowledge Ben Singh for contributions towards screening, quality assessment and data extraction.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boscarino, J.A. Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosom. Med. 1997, 59, 605–614. [Google Scholar] [CrossRef]
- Yoon, J.; Zulman, D.; Scott, J.Y.; Maciejewski, M.L. Costs associated with multimorbidity among VA patients. Med. Care 2014, 52 (Suppl. S3), S31–S36. [Google Scholar] [CrossRef]
- Kazis, L.E.; Miller, D.R.; Clark, J.; Skinner, K.; Lee, A.; Rogers, W.; Spiro, A., 3rd; Payne, S.; Fincke, G.; Selim, A.; et al. Health-related quality of life in patients served by the Department of Veterans Affairs: Results from the Veterans Health Study. Arch. Intern. Med. 1998, 158, 626–632. [Google Scholar] [CrossRef]
- Hundt, N.E.; Barrera, T.L.; Robinson, A.; Cully, J.A. A systematic review of cognitive behavioral therapy for depression in Veterans. Mil. Med. 2014, 179, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Puetz, T.W.; Youngstedt, S.D.; Herring, M.P. Effects of Pharmacotherapy on Combat-Related PTSD, Anxiety, and Depression: A Systematic Review and Meta-Regression Analysis. PLoS ONE 2015, 10, e0126529. [Google Scholar] [CrossRef] [Green Version]
- Stelmach-Mardas, M.; Walkowiak, J. Dietary Interventions and Changes in Cardio-Metabolic Parameters in Metabolically Healthy Obese Subjects: A Systematic Review with Meta-Analysis. Nutrients 2016, 8, 455. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.R.; Bernardo, A.; Costa, J.; Cardoso, A.; Santos, P.; de Mesquita, M.F.; Vaz Patto, J.; Moreira, P.; Silva, M.L.; Padrao, P. Dietary interventions in fibromyalgia: A systematic review. Ann. Med. 2019, 51, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2021, 79, 410–428. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef]
- Knowles, K.A.; Sripada, R.K.; Defever, M.; Rauch, S.A.M. Comorbid mood and anxiety disorders and severity of posttraumatic stress disorder symptoms in treatment-seeking veterans. Psychol. Trauma 2019, 11, 451–458. [Google Scholar] [CrossRef]
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.H.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, T.J.W.; Cervenka, M.C. Lessons learned from recent clinical trials of ketogenic diet therapies in adults. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Sanchez-Tainta, A.; Estruch, R.; Lamuela-Raventos, R.M.; Schroder, H.; Salas-Salvado, J.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: The PREDIMED study. J. Am. Diet. Assoc. 2008, 108, 1134–1144. [Google Scholar] [CrossRef]
- Hoerster, K.D.; Lehavot, K.; Simpson, T.; McFall, M.; Reiber, G.; Nelson, K.M. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am. J. Prev. Med. 2012, 43, 483–489. [Google Scholar] [CrossRef]
- Campbell, S.B.; Gray, K.E.; Hoerster, K.D.; Fortney, J.C.; Simpson, T.L. Differences in functional and structural social support among female and male veterans and civilians. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 56, 375–386. [Google Scholar] [CrossRef]
- Gallant, M.P. The influence of social support on chronic illness self-management: A review and directions for research. Health Educ. Behav. 2003, 30, 170–195. [Google Scholar] [CrossRef]
- Wang, H.X.; Mittleman, M.A.; Orth-Gomer, K. Influence of social support on progression of coronary artery disease in women. Soc. Sci. Med. 2005, 60, 599–607. [Google Scholar] [CrossRef]
- Leavy, R.L. Social support and psychological disorder: A review. J. Community Psychol. 1983, 11, 3–21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The EndNote Team. EndNote; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia. Available online: www.covidence.org (accessed on 23 February 2021).
- Academy of Nutrition and Dietetics (Ed.) Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2012; pp. 90–92. [Google Scholar]
- Bayer-Carter, J.L.; Green, P.S.; Montine, T.J.; VanFossen, B.; Baker, L.D.; Watson, G.S.; Bonner, L.M.; Callaghan, M.; Leverenz, J.B.; Walter, B.K.; et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch. Neurol. 2011, 68, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Boutelle, K.N.; Dubbert, P.; Vander Weg, M. A pilot study evaluating a minimal contact telephone and mail weight management intervention for primary care patients. Eat. Weight Disord. 2005, 10, e1–e5. [Google Scholar] [CrossRef]
- Conley, M.; Le Fevre, L.; Haywood, C.; Proietto, J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. 2018, 75, 65–72. [Google Scholar] [CrossRef]
- Friedberg, J.P.; Rodriguez, M.A.; Watsula, M.E.; Lin, I.; Wylie-Rosett, J.; Allegrante, J.P.; Lipsitz, S.R.; Natarajan, S. Effectiveness of a tailored behavioral intervention to improve hypertension control: Primary outcomes of a randomized controlled trial. Hypertension 2015, 65, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Vetter, M.L.; Moore, R.H.; Chittams, J.L.; Dalton-Bakes, C.V.; Dowd, M.; Williams-Smith, C.; Cardillo, S.; Wadden, T.A. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity 2010, 18, 1733–1738. [Google Scholar] [CrossRef]
- Shahnazari, M.; Ceresa, C.; Foley, S.; Fong, A.; Zidaru, E.; Moody, S. Nutrition-focused wellness coaching promotes a reduction in body weight in overweight US veterans. J. Acad. Nutr. Diet. 2013, 113, 928–935. [Google Scholar] [CrossRef]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Intern. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.K.; Wang, C.K.; Bai, Y.M.; Huang, C.Y.; Lee, S.D. Outcomes of obese, clozapine-treated inpatients with schizophrenia placed on a six-month diet and physical activity program. Psychiatr. Serv. 2007, 58, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S., Jr.; Westman, E.C.; McDuffie, J.R.; Grambow, S.C.; Jeffreys, A.S.; Bolton, J.; Chalecki, A.; Oddone, E.Z. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med. 2010, 170, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexter, A.S.; Pope, J.F.; Erickson, D.; Fontenot, C.; Ollendike, E.; Walker, E. Cooking Education Improves Cooking Confidence and Dietary Habits in Veterans. Diabetes Educ. 2019, 45, 442–449. [Google Scholar] [CrossRef] [PubMed]
- North, S.L.; Palmer, G.A. Outcome analysis of hemoglobin A1c, weight, and blood pressure in a VA diabetes education program. J. Nutr. Educ. Behav. 2015, 47, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sikand, G.; Kashyap, M.L.; Yang, I. Medical nutrition therapy lowers serum cholesterol and saves medication costs in men with hypercholesterolemia. J. Am. Diet. Assoc. 1998, 98, 889–894. [Google Scholar] [CrossRef]
- Niv, N.; Cohen, A.N.; Hamilton, A.; Reist, C.; Young, A.S. Implementation and Effectiveness of a Psychosocial Weight Management Program for Individuals with Schizophrenia. J. Behav. Health Serv. Res. 2014, 41, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Serra, M.C.; Addison, O.; Giffuni, J.; Barton-Ort, K.; Parker, E.; Katzel, L. Changes in Self-Reported Fruit and Vegetable Intake following Nutritional Modification in High Risk Older Veterans. J. Nutr. Gerontol. Geriatr. 2021, 40, 1–8. [Google Scholar] [CrossRef]
- Tan-Shalaby, J.L.; Carrick, J.; Edinger, K.; Genovese, D.; Liman, A.D.; Passero, V.A.; Shah, R.B. Modified Atkins diet in advanced malignancies—Final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr. Metab. 2016, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Holton, K.F.; Kirkland, A.E.; Baron, M.; Ramachandra, S.S.; Langan, M.T.; Brandley, E.T.; Baraniuk, J.N. The Low Glutamate Diet Effectively Improves Pain and Other Symptoms of Gulf War Illness. Nutrients 2020, 12, 2593. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; McVay, M.A.; Voils, C.I. Effect of allowing choice of diet on weight loss—In response. Ann. Intern. Med. 2015, 163, 805–806. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Mayer, S.B.; Coffman, C.J.; Smith, V.A.; Kolotkin, R.L.; Geiselman, P.J.; McVay, M.A.; Oddone, E.Z.; Voils, C.I. Effect of Allowing Choice of Diet on Weight Loss: A Randomized Trial. Ann. Intern. Med. 2015, 162, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahn, J.R.; Fitzpatrick, S.L.; Llabre, M.M.; Apterbach, G.S.; Helms, R.L.; Cugnetto, M.L.; Klaus, J.; Florez, H.; Lawler, T. Weight management for veterans: Examining change in weight before and after MOVE! Obesity 2011, 19, 977–981. [Google Scholar] [CrossRef]
- Romanova, M.; Liang, L.J.; Deng, M.L.; Li, Z.; Heber, D. Effectiveness of the MOVE! Multidisciplinary weight loss program for veterans in Los Angeles. Prev. Chronic. Dis. 2013, 10, E112. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, K.D.; Lai, Z.; Goodrich, D.E.; Damschroder, L.J.; Littman, A.J.; Klingaman, E.A.; Nelson, K.M.; Kilbourne, A.M. Weight loss after participation in a national VA weight management program among veterans with or without PTSD. Psychiatr. Serv. 2014, 65, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.W.; Reeves, G.; Tapscott, S.; Medoff, D.; Dickerson, F.; Goldberg, A.P.; Ryan, A.S.; Fang, L.J.; Dixon, L.B. “MOVE!” Outcomes of a weight loss program modified for veterans with serious mental illness. Psychiatr. Serv. 2013, 64, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.S.; Cohen, A.N.; Goldberg, R.; Hellemann, G.; Kreyenbuhl, J.; Niv, N.; Nowlin-Finch, N.; Oberman, R.; Whelan, F. Improving Weight in People with Serious Mental Illness: The Effectiveness of Computerized Services with Peer Coaches. J. Gen. Intern. Med. 2017, 32 (Suppl. 1), 48–55. [Google Scholar] [CrossRef] [Green Version]
- Shiroma, P.R.; Velasquez, T.; Usset, T.J.; Wilhelm, J.H.; Thuras, P.; Baltutis, E. Antidepressant Effect of the VA Weight Management Program (MOVE) among Veterans with Severe Obesity. Mil. Med. 2020, 185, e586–e591. [Google Scholar] [CrossRef] [Green Version]
- Ahrendt, A.D.; Kattelmann, K.K.; Rector, T.S.; Maddox, D.A. The effectiveness of telemedicine for weight management in the MOVE! Program. J. Rural Health 2014, 30, 113–119. [Google Scholar] [CrossRef]
- Rutledge, T.; Skoyen, J.A.; Wiese, J.A.; Ober, K.M.; Woods, G.N. A comparison of MOVE! versus TeleMOVE programs for weight loss in Veterans with obesity. Obes. Res. Clin. Pract. 2017, 11, 344–351. [Google Scholar] [CrossRef]
- Skoyen, J.A.; Rutledge, T.; Wiese, J.A.; Woods, G.N. Evaluation of TeleMOVE: A Telehealth Weight Reduction Intervention for Veterans with Obesity. Ann. Behav. Med. 2015, 49, 628–633. [Google Scholar] [CrossRef]
- Spring, B.; Duncan, J.M.; Janke, E.A.; Kozak, A.T.; McFadden, H.G.; DeMott, A.; Pictor, A.; Epstein, L.H.; Siddique, J.; Pellegrini, C.A.; et al. Integrating technology into standard weight loss treatment: A randomized controlled trial. JAMA Intern. Med. 2013, 173, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desouza, C.V.; Padala, P.R.; Haynatzki, G.; Anzures, P.; Demasi, C.; Shivaswamy, V. Role of apathy in the effectiveness of weight management programmes. Diabetes Obes. Metab. 2012, 14, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Frankwich, K.A.; Egnatios, J.; Kenyon, M.L.; Rutledge, T.R.; Liao, P.S.; Gupta, S.; Herbst, K.L.; Zarrinpar, A. Differences in Weight Loss between Persons on Standard Balanced vs Nutrigenetic Diets in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1625–1632.e1. [Google Scholar] [CrossRef] [Green Version]
- Allicock, M.; Ko, L.; van der Sterren, E.; Valle, C.G.; Campbell, M.K.; Carr, C. Pilot weight control intervention among US veterans to promote diets high in fruits and vegetables. Prev. Med. 2010, 51, 279–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damschroder, L.J.; Lutes, L.D.; Kirsh, S.; Kim, H.M.; Gillon, L.; Holleman, R.G.; Goodrich, D.E.; Lowery, J.C.; Richardson, C.R. Small-changes obesity treatment among veterans: 12-month outcomes. Am. J. Prev. Med. 2014, 47, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutes, L.D.; Damschroder, L.J.; Masheb, R.; Kim, H.M.; Gillon, L.; Holleman, R.G.; Goodrich, D.E.; Lowery, J.C.; Janney, C.; Kirsh, S.; et al. Behavioral Treatment for Veterans with Obesity: 24-Month Weight Outcomes from the ASPIRE-VA Small Changes Randomized Trial. J. Gen. Intern. Med. 2017, 32 (Suppl. 1), 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moin, T.; Damschroder, L.J.; AuYoung, M.; Maciejewski, M.L.; Datta, S.K.; Weinreb, J.E.; Steinle, N.I.; Billington, C.; Hughes, M.; Makki, F.; et al. Diabetes Prevention Program Translation in the Veterans Health Administration. Am. J. Prev. Med. 2017, 53, 70–77. [Google Scholar] [CrossRef]
- Jackson, S.L.; Safo, S.; Staimez, L.R.; Long, Q.; Rhee, M.K.; Cunningham, S.A.; Olson, D.E.; Tomolo, A.M.; Ramakrishnan, U.; Narayan, K.M.V.; et al. Reduced Cardiovascular Disease Incidence with a National Lifestyle Change Program. Am. J. Prev. Med. 2017, 52, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Das, S.R.; Kinsinger, L.S.; Yancy, W.S., Jr.; Wang, A.; Ciesco, E.; Burdick, M.; Yevich, S.J. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am. J. Prev. Med. 2005, 28, 291–294. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, E.R.; Bergman, H.E.; Walton, T.O.; Walker, D.D.; Kaysen, D.L. Co-Occurring Post-Traumatic Stress Disorder and Alcohol Use Disorder in U.S. Military and Veteran Populations. Alcohol. Res. 2018, 39, 161–169. [Google Scholar] [PubMed]
- Hoerster, K.D.; Campbell, S.; Dolan, M.; Stappenbeck, C.A.; Yard, S.; Simpson, T.; Nelson, K.M. PTSD is associated with poor health behavior and greater Body Mass Index through depression, increasing cardiovascular disease and diabetes risk among U.S. veterans. Prev. Med. Rep. 2019, 15, 100930. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- National Heart Foundation of Australia. Dietary Position Statement. Heart Healthy Eating Patterns. Melbourne: National Heart Foundation of Australia. 2019. Available online: https://www.heartfoundation.org.au/health-professional-tools/nutrition-position-statements (accessed on 9 February 2022).
- U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025. Updated December. 9th ed. Available online: DietaryGuidelines.gov (accessed on 9 February 2022).
- Rippe, J.M.; Crossley, S.; Ringer, R. Obesity as a chronic disease: Modern medical and lifestyle management. J. Am. Diet. Assoc. 1998, 98, S9–S15. [Google Scholar] [CrossRef]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390–395. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Allison, D.B.; Newcomer, J.W.; Dunn, A.L.; Blumenthal, J.A.; Fabricatore, A.N.; Daumit, G.L.; Cope, M.B.; Riley, W.T.; Vreeland, B.; Hibbeln, J.R.; et al. Obesity among those with mental disorders: A National Institute of Mental Health meeting report. Am. J. Prev. Med. 2009, 36, 341–350. [Google Scholar] [CrossRef]
- Cabassa, L.J.; Ezell, J.M.; Lewis-Fernandez, R. Lifestyle interventions for adults with serious mental illness: A systematic literature review. Psychiatr. Serv. 2010, 61, 774–782. [Google Scholar] [CrossRef]
- Murimi, M.W.; Kanyi, M.; Mupfudze, T.; Amin, M.R.; Mbogori, T.; Aldubayan, K. Factors Influencing Efficacy of Nutrition Education Interventions: A Systematic Review. J. Nutr. Educ. Behav. 2017, 49, 142–165.e1. [Google Scholar] [CrossRef]
- Douketis, J.D.; Macie, C.; Thabane, L.; Williamson, D.F. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int. J. Obes. 2005, 29, 1153–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanza-Martinez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Henriquez Sanchez, P.; Ruano, C.; de Irala, J.; Ruiz-Canela, M.; Martinez-Gonzalez, M.A.; Sanchez-Villegas, A. Adherence to the Mediterranean diet and quality of life in the SUN Project. Eur. J. Clin. Nutr. 2012, 66, 360–368. [Google Scholar] [CrossRef]
- Tessier, J.M.; Erickson, Z.D.; Meyer, H.B.; Baker, M.R.; Gelberg, H.A.; Arnold, I.Y.; Kwan, C.; Chamberlin, V.; Rosen, J.A.; Shah, C.; et al. Therapeutic Lifestyle Changes: Impact on Weight, Quality of Life, and Psychiatric Symptoms in Veterans with Mental Illness. Mil. Med. 2017, 182, e1738–e1744. [Google Scholar] [CrossRef] [Green Version]
- Peinemann, F.; Tushabe, D.A.; Kleijnen, J. Using multiple types of studies in systematic reviews of health care interventions—A systematic review. PLoS ONE 2013, 8, e85035. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
