Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gastro-AD®
2.2. Animals and Treatments
2.3. Measurement of Gastric Juice Secretion and pH
2.4. Measurement of the Gastric Ulcerative Lesions and Ulceration Index
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Serum Histamine and PGE2
2.7. Primary Gastric Parietal Cell Culture
2.8. Determination of the cAMP Level in Primary Gastric Parietal Cellsii
2.9. Real-Time Polymerase Chain Reaction (RT-PCR)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
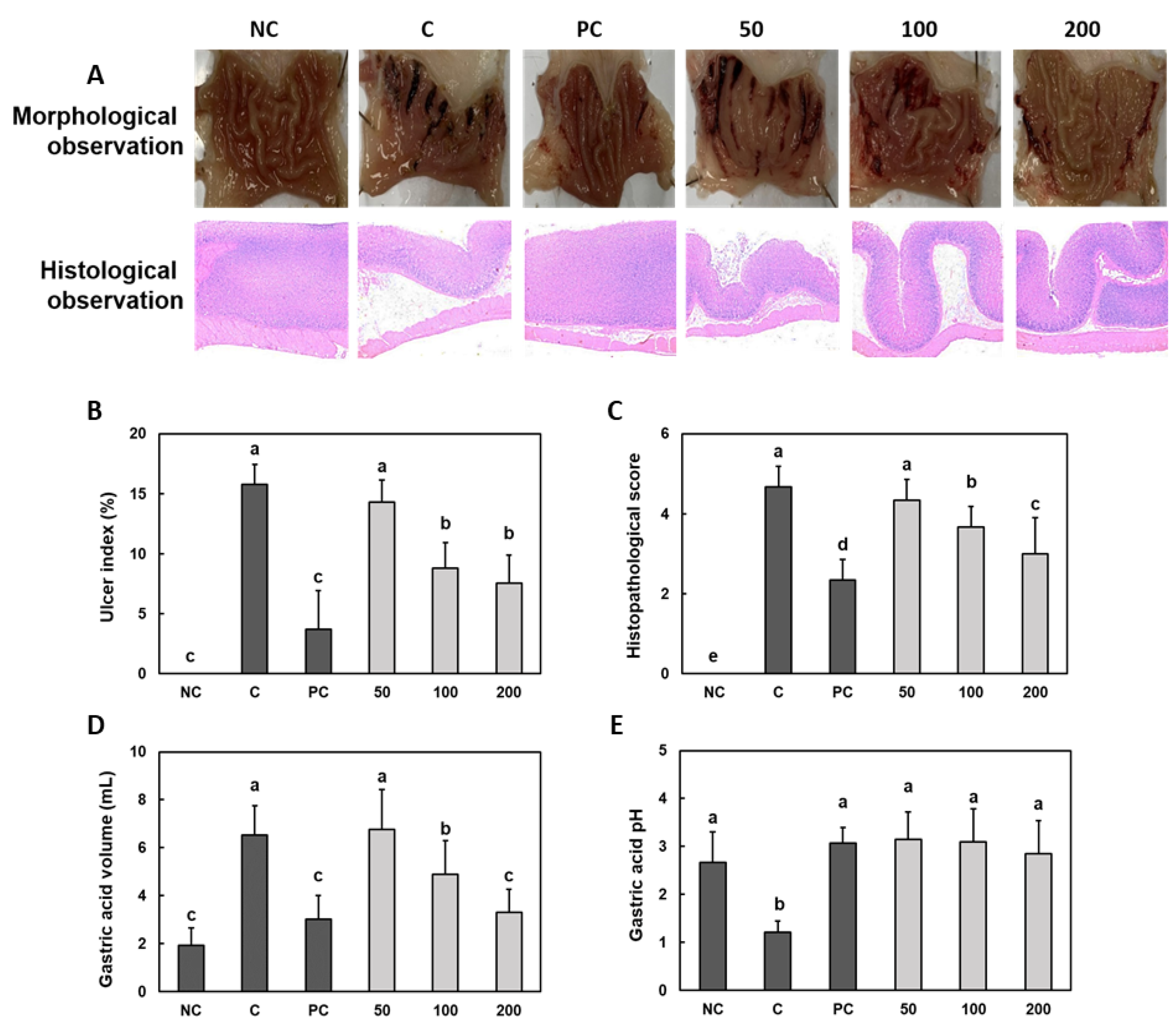
3.1. Effect of Gastro-AD® on the Gastric Mucosal Damage and Gastric Acid Secretion Induced by Ethanol/HCl Treatment in SD Rats
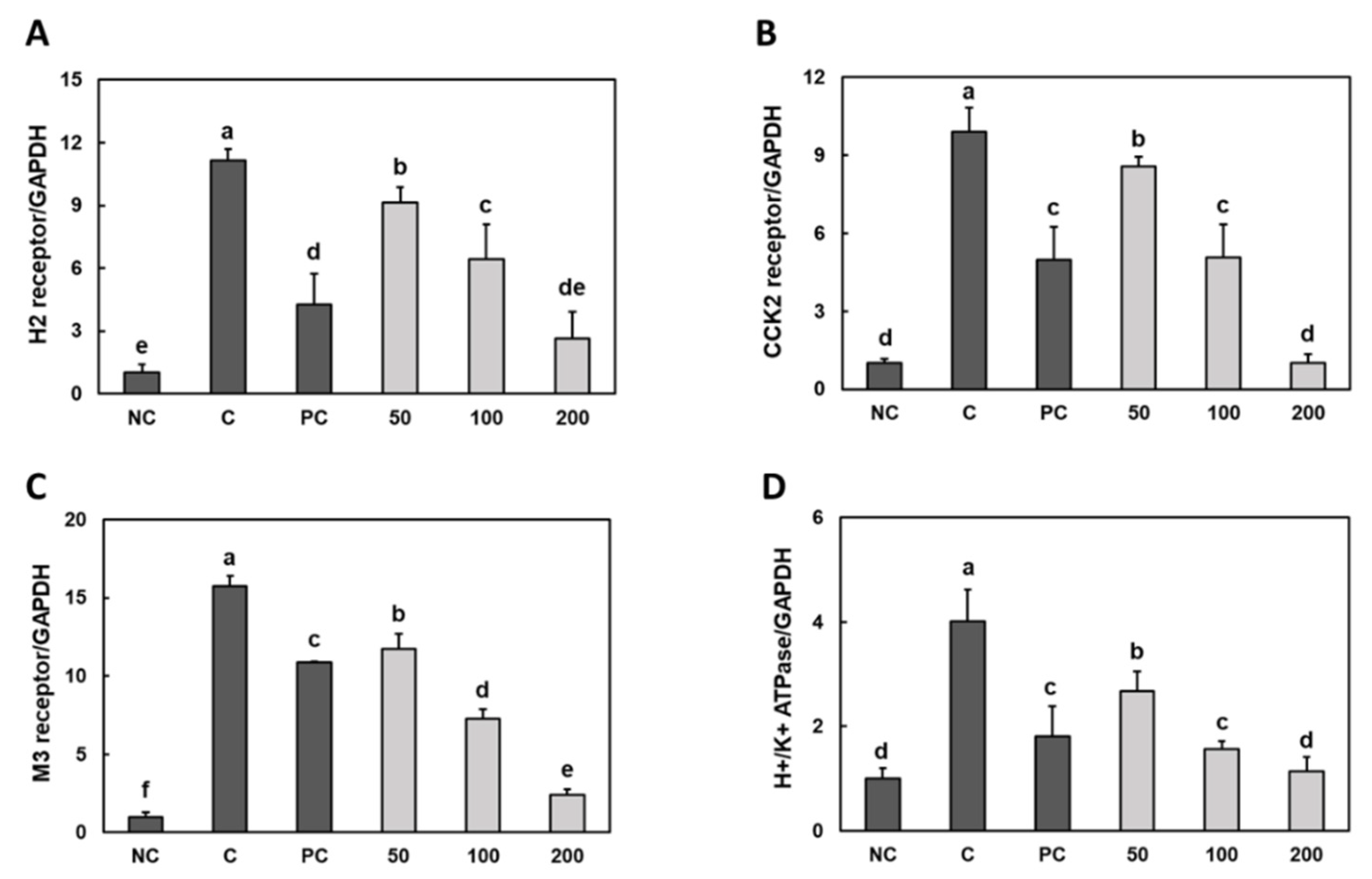
3.2. Effect of Gastro-AD® on the Expression of Gastric Acid Secretion-Related Receptors in Gastric Damage Induced by Ethanol/HCl Treatment in SD Rats
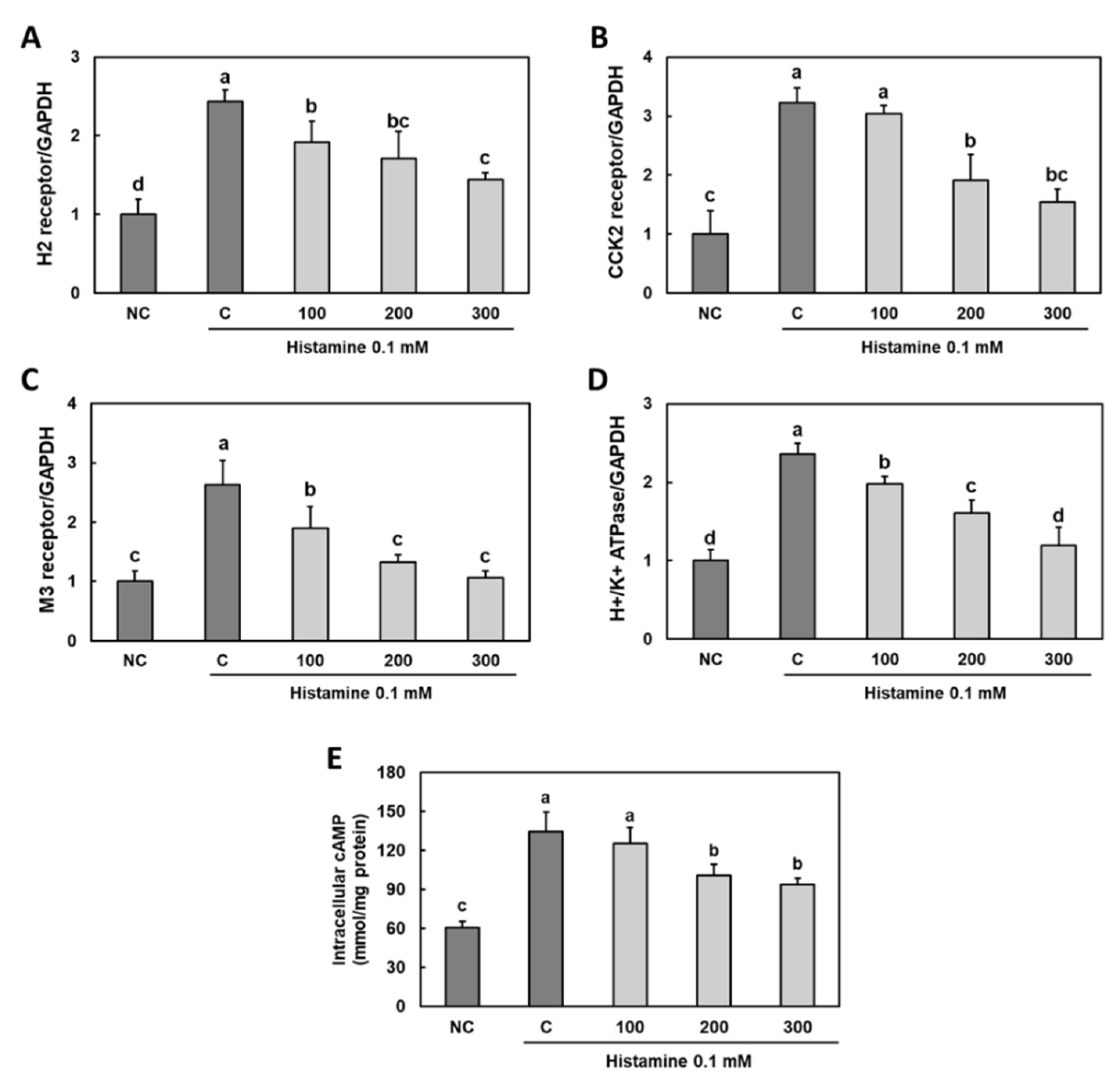
3.3. Effect of Gastro-AD® on the Expression of Gastric Acid Secretion-Related Receptors in Histamine-Treated Primary Gastric Parietal Cells
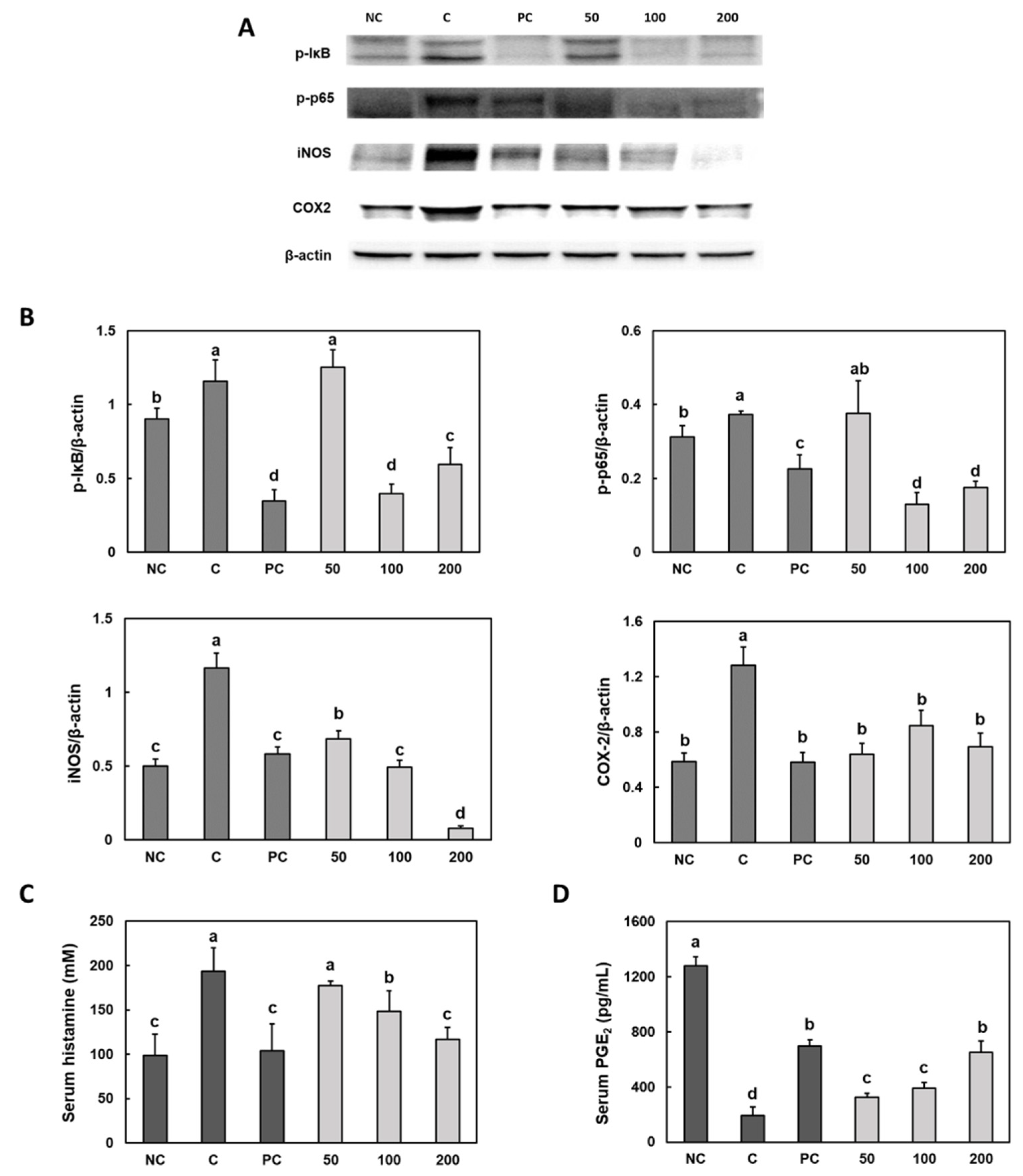
3.4. Effect of Gastro-AD® on the Inflammatory Factors in Gastric Damage Induced by Ethanol/HCl Treatment in SD Rats
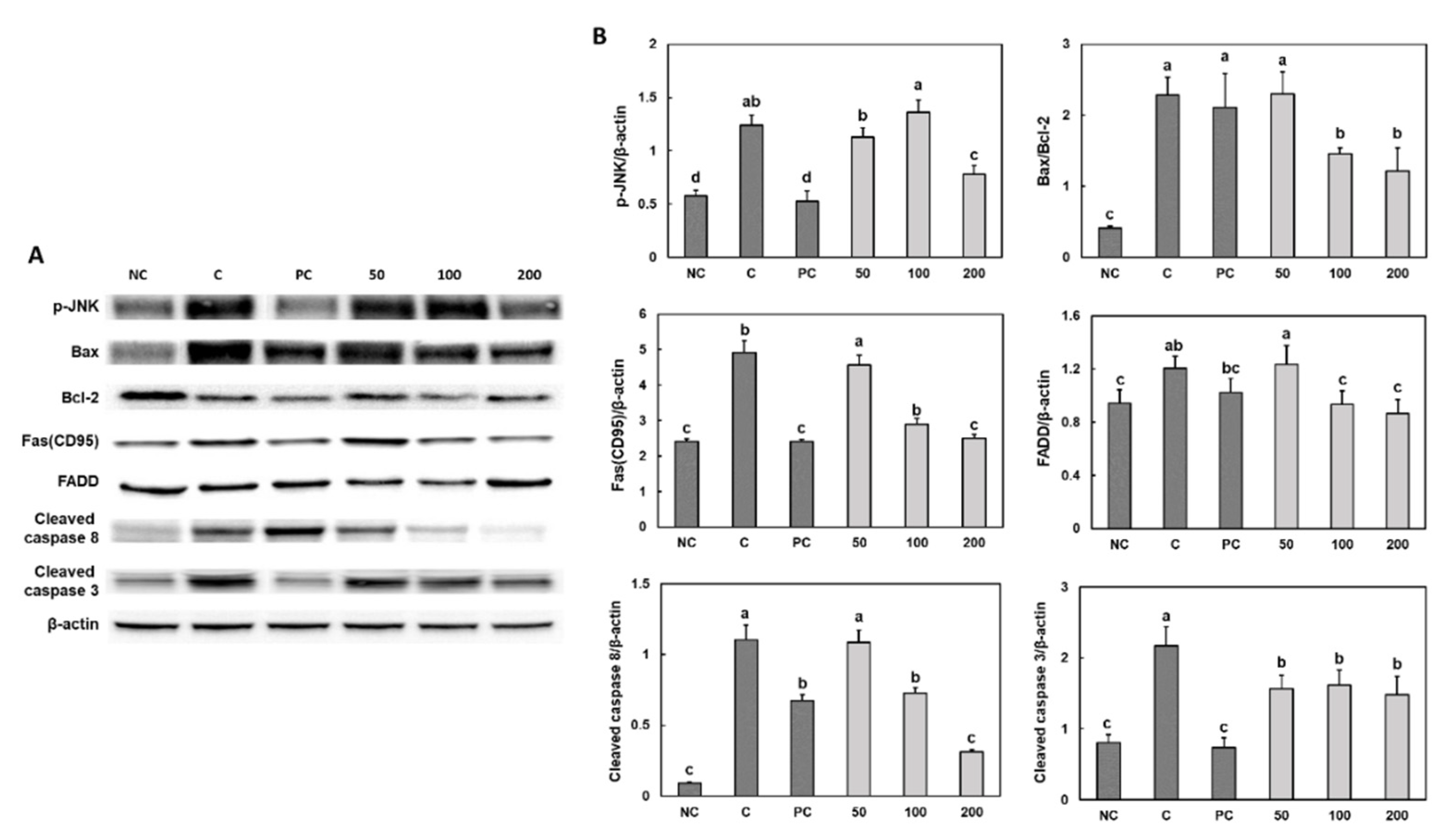
3.5. Effect of Gastro-AD® on the Apoptosis Factors in Gastric Damage Induced by Ethanol/HCl Treatment in SD Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calam, J.; Baron, J.H. ABC of the upper gastrointestinal tract: Pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ 2001, 323, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.N.; Antonioli, D.A. Gastritis. Gastrointest. Endosc. Clin. N. Am. 2001, 11, 717–740. [Google Scholar] [CrossRef]
- Roberts, D.M. Chronic gastritis, alcohol, and non-ulcer dyspepsia. Gut 1972, 13, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.; Teyssen, S.; Singer, M.V. Alcohol and gastric acid secretion in humans. Gut 1993, 34, 843–847. [Google Scholar] [CrossRef] [Green Version]
- Kopic, S.; Geibel, J.P. Update on the mechanisms of gastric acid secretion. Curr. Gastroenterol. Rep. 2010, 12, 458–464. [Google Scholar] [CrossRef]
- Walker, J.; Hell, J.; Liszt, K.I.; Dresel, M.; Pignitter, M.; Hofmann, T.; Somoza, V. Identification of beer bitter acids regulating mechanisms of gastric acid secretion. J. Agric. Food Chem. 2012, 60, 1405–1412. [Google Scholar] [CrossRef]
- Aihara, T.; Nakamura, E.; Amagase, K.; Tomita, K.; Fujishita, T.; Furutani, K.; Okabe, S. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: Past, present, and future. Pharmacol. Ther. 2003, 98, 109–127. [Google Scholar] [CrossRef]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Fatani, A.; Vaher, K.; Rivero-Mendoza, D.; Alabasi, K.; Dahl, W.J. Fermented soy supplementation improves indicators of quality of life: A randomized, placebo-controlled, double-blind trial in adults experiencing heartburn. BMC Res. Notes 2020, 13, 364. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xie, B.; Zhang, W.; Chen, Q.; Gan, D. Investigating the Influence of Early Gastric Discomfort Symptoms on Life Quality and the Interventional Effects of a Stomach-Protecting Formula. GMR 2022, 6, 566–569. [Google Scholar]
- Nam, D.E.; Kim, O.K.; Shim, T.J.; Lee, J.K.; Hwang, K.T. Inhibitory Effects of Chios Mastic Gum on Gastric Acid Secretion by Histamine-Related Pathway in a Rat Model and Primary Parietal Cells. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1500–1509. [Google Scholar] [CrossRef]
- Kaunitz, J.D. Barrier function of gastric mucus. Keio J. Med. 1999, 48, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.D.; Bradley, S.; Buckley, N.D.; Green-Johnson, J.M. Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production. J. Food Prot. 2003, 66, 466–472. [Google Scholar] [CrossRef]
- Osefo, N.; Ito, T.; Jensen, R.T. Gastric acid hypersecretory states: Recent insights and advances. Curr. Gastroenterol. Rep. 2009, 11, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.L. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr. Opin. Gastroenterol. 2017, 33, 430–438. [Google Scholar] [CrossRef]
- Jung, A.R.; Ahn, S.H.; Park, I.S.; Park, S.Y.; Jeong, S.I.; Cheon, J.H.; Kim, K. Douchi (fermented Glycine max Merr.) alleviates atopic dermatitis-like skin lesions in NC/Nga mice by regulation of PKC and IL-4. BMC Complement Altern. Med. 2016, 16, 416. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Kim, J.H.; Palaniyandi, S.A.; Lee, C.C.; You, J.W.; Yang, H.; Yoon Park, J.H.; Yang, S.H.; Lee, K.W. Yak-Kong Soybean (Glycine max) Fermented by a Novel Pediococcus pentosaceus Inhibits the Oxidative Stress-Induced Monocyte-Endothelial Cell Adhesion. Nutrients 2019, 11, 1380. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Kang, S.C. Apoptotic Activity of Lactobacillus plantarum DGK-17-Fermented Soybean Seed Extract in Human Colon Cancer Cells via ROS-JNK Signaling Pathway. J. Food Sci. 2017, 82, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Yoo, H.J.; Song, H.J.; Kim, K.K.; Chun, Y.J.; Matsui, T.; Kim, H.B. Inflammation-related signaling pathways implicating TGFβ are revealed in the expression profiling of MCF7 cell treated with fermented soybean, chungkookjang. Nutr. Cancer 2011, 63, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, M.; Lee, J.S.; Inoue, M.; Sasazuki, S.; Tsugane, S. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: A meta-analysis of observational studies. Cancer Sci. 2011, 102, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Toller, I.M.; Hitzler, I.; Sayi, A.; Mueller, A. Prostaglandin E2 prevents Helicobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology 2010, 138, 1455–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, T.; Tsutsumi, S.; Tomisato, W.; Hwang, H.J.; Tsuchiya, T.; Mizushima, T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J. Biol. Chem. 2003, 278, 12752–12758. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Kim, D.; Kim, H.; Jo, S.; Kim, O.-K.; Lee, J. Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury. Nutrients 2022, 14, 2079. https://doi.org/10.3390/nu14102079
Lee M, Kim D, Kim H, Jo S, Kim O-K, Lee J. Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury. Nutrients. 2022; 14(10):2079. https://doi.org/10.3390/nu14102079
Chicago/Turabian StyleLee, Minhee, Dakyung Kim, Hyunji Kim, Sukyung Jo, Ok-Kyung Kim, and Jeongmin Lee. 2022. "Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury" Nutrients 14, no. 10: 2079. https://doi.org/10.3390/nu14102079
APA StyleLee, M., Kim, D., Kim, H., Jo, S., Kim, O.-K., & Lee, J. (2022). Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury. Nutrients, 14(10), 2079. https://doi.org/10.3390/nu14102079





