Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Human Milk Oligosaccharide Standards
2.3. HMO Isolation and Analysis by HPLC
2.4. Analysis of HMOs by Enzymatic Digest
2.5. Determination of Oligosaccharides by LC-MS
2.6. Statistics
3. Results
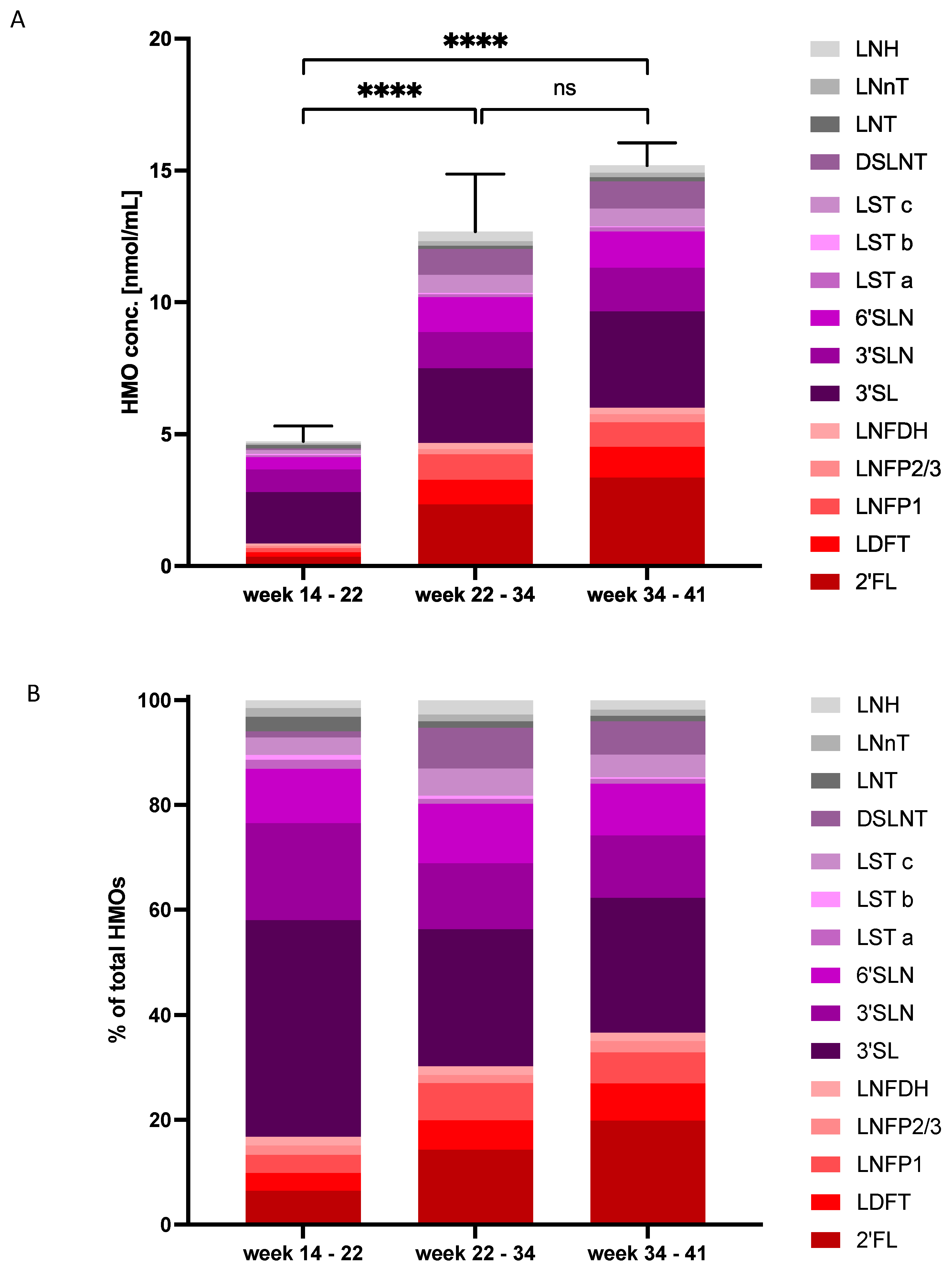
3.1. HMO Profiles in Amniotic Fluid in Early, Mid and Late Pregnancy
3.2. HMO Concentrations and Composition Change with Gestational Age
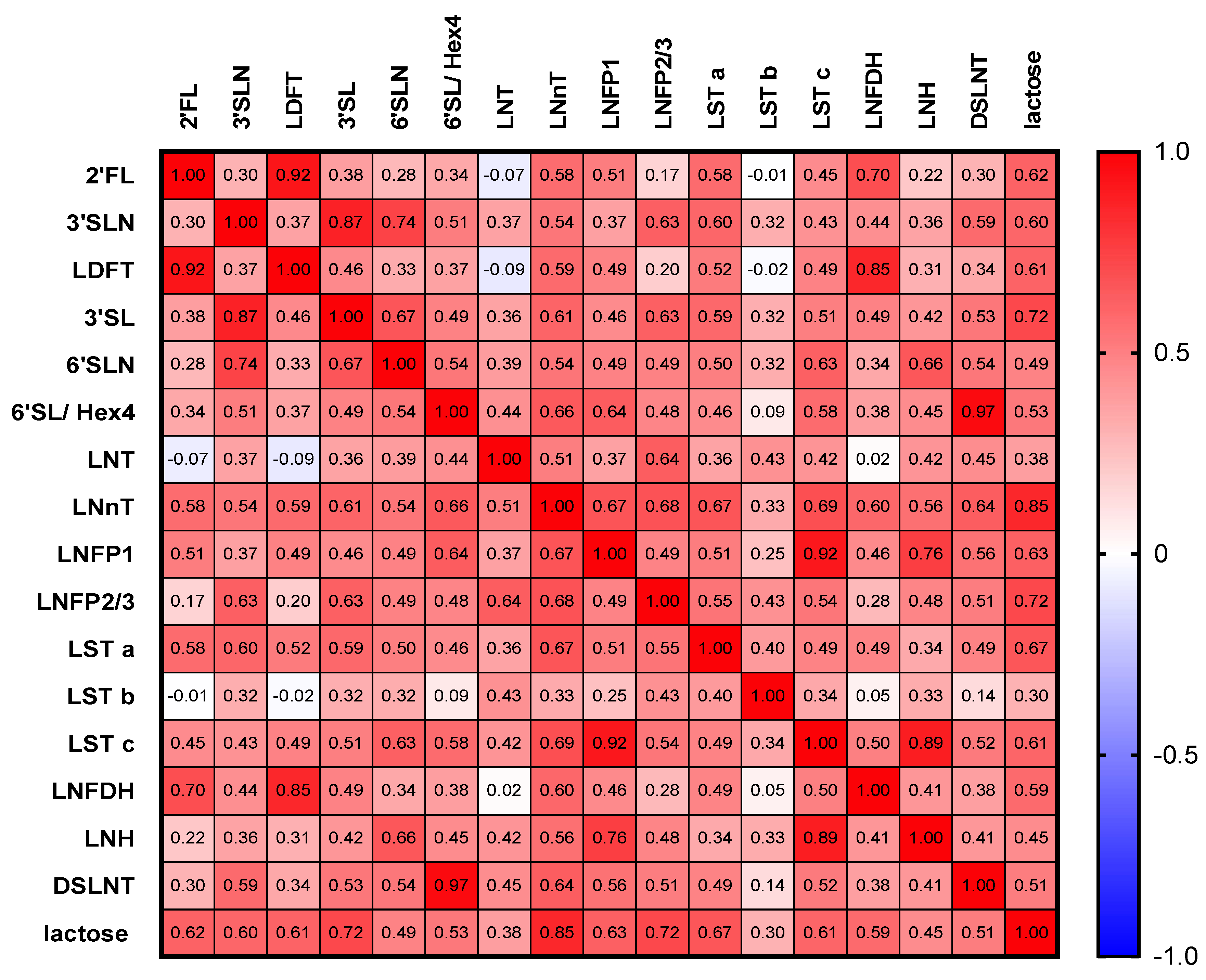
3.3. HMOs Are Highly Correlated with Each Other
3.4. HMOs in Amniotic Fluid Are Associated with Maternal Factors
4. Discussion
4.1. Occurrence of Uterine HMOs and Lactose
4.2. HMO Concentrations in AF over Gestational Periods
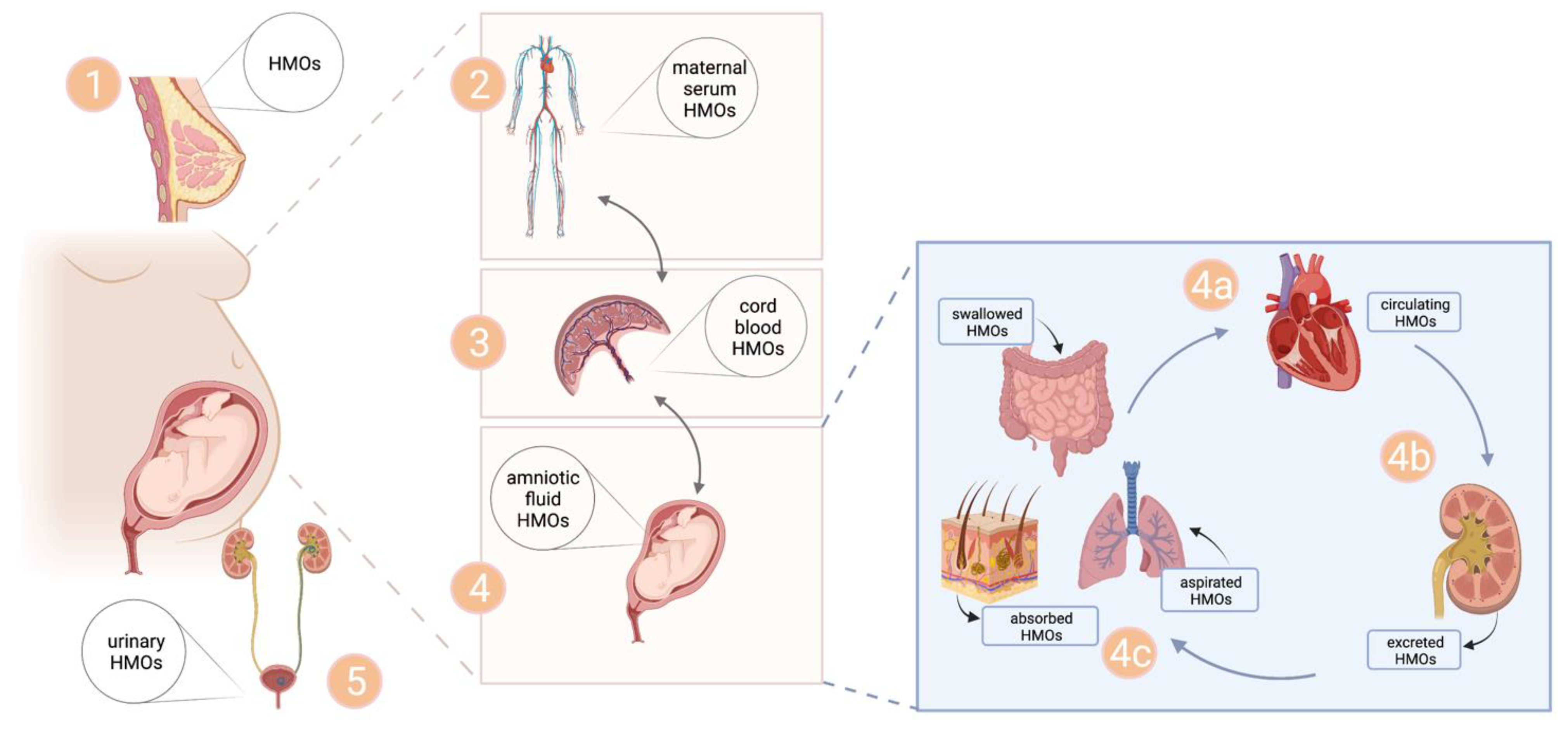
4.3. Origin of Uterine HMOs
4.4. Exposure of Fetal Tissues to HMOs—Implicatitons of HMOs for Fetal Development?
4.5. Potential Clinical Implications for AF-Based Predictive, Preventive or Therapeutic Strategies?
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar] [PubMed]
- Leo, F.; Asakuma, S.; Fukuda, K.; Senda, A.; Urashima, T. Determination of sialyl and neutral oligosaccharide levels in transition and mature milks of Samoan women, using anthranilic derivatization followed by reverse phase high performance liquid chromatography. Biosci. Biotechnol. Biochem. 2010, 74, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Muller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martinez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Stahl, B.; Thurl, S.; Henker, J.; Siegel, M.; Finke, B.; Sawatzki, G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv. Exp. Med. Biol. 2001, 501, 299–306. [Google Scholar]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; Garcia-Mantrana, I.; Bertua-Rios, B.; Martinez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human Milk Oligosaccharides and Associations With Immune-Mediated Disease and Infection in Childhood: A Systematic Review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Autran, C.A.; Szyszka, A.; Escajadillo, T.; Huang, M.; Godula, K.; Prudden, A.R.; Boons, G.J.; Lewis, A.L.; Doran, K.S.; et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J. Biol. Chem. 2017, 292, 11243–11249. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.; Hansman, G.; Hanisch, F.G.; Schroten, H.; Kunz, C. Human Milk Oligosaccharides as Promising Antivirals. Mol. Nutr. Food Res. 2018, 62, e1700679. [Google Scholar] [CrossRef]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Nino, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2′-fucosyllactose and 6′-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Hauser, J.; Pisa, E.; Arias Vasquez, A.; Tomasi, F.; Traversa, A.; Chiodi, V.; Martin, F.P.; Sprenger, N.; Lukjancenko, O.; Zollinger, A.; et al. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol. Psychiatry 2021, 26, 2854–2871. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macri, S. Exposure to 3′Sialyllactose-Poor Milk during Lactation Impairs Cognitive Capabilities in Adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Botts, S.R.; Johnson-Henry, K.C.; Landberg, E.; Abrahamsson, T.R.; Sherman, P.M. Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study. Nutrients 2022, 14, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; Overbeek, S.A.; Kostadinova, A.I.; Stahl, B.; Garssen, J.; Van’t Land, B.; Willemsen, L.E.M. Exposure of Intestinal Epithelial Cells to 2′-Fucosyllactose and CpG Enhances Galectin Release and Instructs Dendritic Cells to Drive Th1 and Regulatory-Type Immune Development. Biomolecules 2020, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal. Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Kofeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Treichler, C.; Brandl, W.; Schonbacher, L.; Kofeler, H.; van Poppel, M.N.M. The association of human milk oligosaccharides with glucose metabolism in overweight and obese pregnant women. Am. J. Clin. Nutr. 2019, 110, 1335–1343. [Google Scholar] [CrossRef]
- Pausan, M.R.; Kolovetsiou-Kreiner, V.; Richter, G.L.; Madl, T.; Giselbrecht, E.; Obermayer-Pietsch, B.; Weiss, E.C.; Jantscher-Krenn, E.; Moissl-Eichinger, C. Human Milk Oligosaccharides Modulate the Risk for Preterm Birth in a Microbiome-Dependent and -Independent Manner. mSystems 2020, 5, e00334-20. [Google Scholar] [CrossRef]
- Zopf, D.; Ginsburg, V.; Hallgren, P.; Jonsson, A.; Lindberg, B.; Lundblad, A. Determination of Leb-active oligosaccharides in urine of pregnant and lactating women by radioimmunoassay. Eur. J. Biochem. FEBS 1979, 93, 431–435. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; van Poppel, M.; Kofeler, H.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of Human Milk Oligosaccharides in Cord Blood and Maternal-to-Fetal Transport across the Placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef]
- Wise, A.; Robertson, B.; Choudhury, B.; Rautava, S.; Isolauri, E.; Salminen, S.; Bode, L. Infants Are Exposed to Human Milk Oligosaccharides Already in utero. Front. Pediatr. 2018, 6, 270. [Google Scholar] [CrossRef]
- Mandelbaum, B.; Evans, T.N. Life in the amniotic fluid. Am. J. Obstet. Gynecol. 1969, 104, 365–377. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.A. Fetal swallowing and amniotic fluid volume. Obstet. Gynecol. 1966, 28, 606–610. [Google Scholar]
- Good, M.; Siggers, R.H.; Sodhi, C.P.; Afrazi, A.; Alkhudari, F.; Egan, C.E.; Neal, M.D.; Yazji, I.; Jia, H.; Lin, J.; et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl. Acad. Sci. USA 2012, 109, 11330–11335. [Google Scholar] [CrossRef] [PubMed]
- Bagci, S.; Brosens, E.; Tibboel, D.; De Klein, A.; Ijsselstijn, H.; Wijers, C.H.; Roeleveld, N.; de Blaauw, I.; Broens, P.M.; van Rooij, I.A.; et al. More than fetal urine: Enteral uptake of amniotic fluid as a major predictor for fetal growth during late gestation. Eur. J. Pediatr. 2016, 175, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Burjonrappa, S.C.; Crete, E.; Bouchard, S. The role of amniotic fluid in influencing neonatal birth weight. J. Perinatol. 2010, 30, 27–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaart, M.W.; Yamanouchi, T.; van Nispen, D.J.; Raatgeep, R.H.; van Goudoever, J.B.; de Krijger, R.R.; Tibboel, D.; Einerhand, A.W.; Renes, I.B. Does small intestinal atresia affect epithelial protein expression in human newborns? J. Pediatr. Gastroenterol. Nutr. 2006, 43, 576–583. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Rudloff, S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br. J. Nutr. 2009, 101, 1306–1315. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Kunz, C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br. J. Nutr. 2008, 99, 462–471. [Google Scholar] [CrossRef]
- Koos, B.J.; Rajaee, A. Fetal breathing movements and changes at birth. Adv. Exp. Med. Biol. 2014, 814, 89–101. [Google Scholar] [CrossRef]
- Siggers, J.; Ostergaard, M.V.; Siggers, R.H.; Skovgaard, K.; Molbak, L.; Thymann, T.; Schmidt, M.; Moller, H.K.; Purup, S.; Fink, L.N.; et al. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G864–G875. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, M.V.; Bering, S.B.; Jensen, M.L.; Thymann, T.; Purup, S.; Diness, M.; Schmidt, M.; Sangild, P.T. Modulation of intestinal inflammation by minimal enteral nutrition with amniotic fluid in preterm pigs. JPEN J. Parenter Enter. Nutr. 2014, 38, 576–586. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed]

| Maternal Characteristics | Total Group | Early Pregnancy (14.0–21.9 Weeks of Gestation) | Mid Pregnancy (22.0–33.9 Weeks of Gestation) | Late Pregnancy; (34.0–41.0 Weeks of Gestation) | ANOVA p Value |
|---|---|---|---|---|---|
| n = 77 | n = 8 | n = 15 | n = 54 | ||
| Maternal age (years) ± SD | 33.3 ± 5.4 | 32.8 ± 4.1 | 31.7 ± 5.5 | 33.8 ± 5.5 | 0.823 |
| BMI (kg/m2) ± SD | 28.4 ± 5.8 | 27.0 ± 4.0 | 26.1 ± 4.5 | 29.3 ± 6.1 | 0.126 |
| Gestational age (weeks) [min–max] | 34.0 [14.3–40.9] | 18.3 [14.3–21.7] | 28.1 [22.6–32.6] | 37.8 [34.1–40.9] | |
| sample collection | |||||
| Fetal surgery (n) | 9 (12%) | 5 (63%) | 4 (27%) | 0 (0%) | |
| Amniocentesis (n) | 3 (4%) | 3 (38%) | 0 (0%) | 0 (0%) | |
| C-section (n) | 65 (84%) | 0 (0%) | 11 (73%) | 54 (100%) | |
| Twin pregnancies (n) | 17 (22%) | 5 (63%) | 6 (40%) | 6 (11%) | |
| IUGR/placenta insufficiency (n) | 10 (13%) | 1 (13%) | 8 (53%) | 1 (2)% | |
| HDP (n) | 6 (8%) | 0 (0) | 1 (7%) | 5 (9%) | |
| GDM (n) | 7 (9%) | 0 (0%) | 0 (0%) | 7 (13%) | |
| Primary C-section * (n) | 19 (25%) | 0 (0%) | 2 (13%) | 17 (31%) |
| Early Pregnancy (Weeks 14.0–21.9) | Mid Pregnancy (Weeks 22.0–33.9) | Late Pregnancy (Weeks 34.0–41.0) | |||||
|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 15) | (n = 54) | ANOVA | ||||
| Median | IQR | Median | IQR | Median | IQR | p Value | |
| Lactose | 1.561 | 0.995–1.792 | 2.598 | 1.572–5.190 | 4.512 | 3.826–5.603 | <0.0001 |
| 2′FL | 0.272 | 0.103–0.569 | 0.970 | 0.165–3.500 | 2.769 a | 0.754–5.362 | 0.0011 |
| 3′SLN | 0.873 | 0.600–1.067 | 1.282 | 0.795–1.822 | 1.607 a | 1.318–1.891 | 0.0011 |
| LDFT | 0.129 | 0.074–0.311 | 0.422 | 0.108–1.667 | 0.959 a | 0.411–1.699 | <0.0001 |
| 3′SL | 1.801 | 1.282–2.379 | 2.355 | 1.803–3.781 | 3.379 a | 2.896–4.078 | 0.0003 |
| 6′SLN | 0.472 | 0.397–0.565 | 1.225 a | 0.553–1.615 | 1.296 a | 1.024–1.499 | <0.0001 |
| 6′SL/Hex4 | 0.458 | 0.357–0.670 | 6.981 a | 4.355–12.160 | 6.096 a | 4.090–12.680 | <0.0001 |
| LNT | 0.114 | 0.088–0.164 | 0.101 | 0.066–0.218 | 0.101 | 0.081–0.167 | 0.9367 |
| LNnT | 0.074 | 0.065–0.082 | 0.152 a | 0.067–0.238 | 0.161 a | 0.113–0.214 | 0.0003 |
| LNFP1 | 0.154 | 0.115–0.233 | 0.808 a | 0.164–1.32 | 0.681 a | 0.433–1.122 | 0.0002 |
| LNFP2/3 | 0.078 | 0.045–0.120 | 0.085 | 0.065–0.282 | 0.266 a,b | 0.175–0.368 | <0.0001 |
| LSTa | 0.073 | 0.057–0.102 | 0.089 | 0.059–0.114 | 0.128 a | 0.084–0.185 | 0.026 |
| LSTb | 0.040 | 0.031–0.053 | 0.051 | 0.037–0.081 | 0.040 | 0.028–0.060 | 0.1337 |
| LSTc | 0.131 | 0.101–0.224 | 0.570 a | 0.168–0.871 | 0.510 a | 0.355–0.800 | 0.0002 |
| LNDFH | 0.075 | 0.049–0.095 | 0.134 | 0.067–0.343 | 0.232 a | 0.111–0.344 | 0.0022 |
| LNH | 0.0623 | 0.044–0.100 | 0.261 a | 0.061–0.372 | 0.189 a | 0.124–0.304 | 0.0011 |
| DSLNT | 0.0437 | 0.040–0.070 | 0.669 a | 0.315–1.608 | 0.693 a | 0.361–1.162 | <0.0001 |
| fucosylated | 0.676 | 0.459–1.322 | 1.998 a | 1.120–6.446 | 5.314 a | 2.703–8.519 | <0.0001 |
| sialylated | 3.521 | 2.534–4.409 | 8.269 a | 4.434–10.660 | 7.980 a | 6.500–9.204 | <0.0001 |
| unmodified | 0.266 | 0.193–0.341 | 0.559 a | 0.220–0.933 | 0.497 a | 0.349–0.803 | 0.0043 |
| total HMOs | 4.323 | 3.435–6.324 | 9.762 a | 6.698–15.390 | 14.740 a | 10.880–17.580 | <0.0001 |
| Gestational Age | Maternal Age | Maternal BMI | ||||
|---|---|---|---|---|---|---|
| Spearman r | p Value | Spearman r | p Value | Spearman r | p Value | |
| 2′FL | 0.35 | 0.0009 | 0.06 | 0.3065 | −0.12 | 0.1585 |
| 3′SLN | 0.56 | <0.0001 | 0.19 | 0.0519 | 0.10 | 0.1963 |
| LDFT | 0.37 | 0.0004 | 0.09 | 0.2168 | −0.05 | 0.3246 |
| 3′SL | 0.48 | <0.0001 | 0.29 | 0.0060 | 0.08 | 0.2550 |
| 6′SLN | 0.35 | 0.001 | 0.25 | 0.0131 | 0.06 | 0.3154 |
| 6′SL/Hex4 | 0.3 | 0.0037 | −0.13 | 0.1254 | −0.07 | 0.2661 |
| LNT | 0 | 0.497 | 0.00 | 0.4928 | −0.08 | 0.2465 |
| LNnT | 0.36 | 0.0007 | 0.11 | 0.1650 | −0.08 | 0.2545 |
| LNFP1 | 0.24 | 0.017 | −0.02 | 0.4429 | −0.27 | 0.0081 |
| LNFP2/3 | 0.54 | <0.0001 | 0.14 | 0.1089 | 0.00 | 0.4937 |
| LST a | 0.45 | <0.0001 | 0.23 | 0.0206 | −0.10 | 0.1870 |
| LST b | −0.05 | 0.3416 | 0.14 | 0.1137 | −0.11 | 0.1724 |
| LST c | 0.27 | 0.0093 | 0.05 | 0.3327 | −0.18 | 0.0602 |
| LNFDH | 0.43 | <0.0001 | 0.14 | 0.1140 | −0.01 | 0.4533 |
| LNH | 0.18 | 0.0626 | 0.10 | 0.1944 | −0.06 | 0.2913 |
| DSLNT | 0.3 | 0.0037 | −0.09 | 0.2108 | −0.02 | 0.4482 |
| total HMOs * | 0.46 | <0.0001 | 0.15 | 0.0979 | −0.08 | 0.2318 |
| fucosylated | 0.4 | 0.0002 | 0.07 | 0.2784 | −0.15 | 0.1020 |
| sialylated | 0.44 | <0.0001 | 0.18 | 0.0593 | 0.02 | 0.4241 |
| unmodified | 0.21 | 0.0305 | 0.09 | 0.2208 | −0.08 | 0.2515 |
| lactose | 0.56 | <0.0001 | 0.16 | 0.0869 | −0.11 | 0.1737 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantscher-Krenn, E.; von Schirnding, L.; Trötzmüller, M.; Köfeler, H.; Kurtovic, U.; Fluhr, H.; Müller, A.; Bagci, S. Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age. Nutrients 2022, 14, 2065. https://doi.org/10.3390/nu14102065
Jantscher-Krenn E, von Schirnding L, Trötzmüller M, Köfeler H, Kurtovic U, Fluhr H, Müller A, Bagci S. Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age. Nutrients. 2022; 14(10):2065. https://doi.org/10.3390/nu14102065
Chicago/Turabian StyleJantscher-Krenn, Evelyn, Lara von Schirnding, Martin Trötzmüller, Harald Köfeler, Una Kurtovic, Herbert Fluhr, Andreas Müller, and Soyhan Bagci. 2022. "Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age" Nutrients 14, no. 10: 2065. https://doi.org/10.3390/nu14102065
APA StyleJantscher-Krenn, E., von Schirnding, L., Trötzmüller, M., Köfeler, H., Kurtovic, U., Fluhr, H., Müller, A., & Bagci, S. (2022). Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age. Nutrients, 14(10), 2065. https://doi.org/10.3390/nu14102065








