Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Hematology Analyses
2.3. Hematoxylin and Eosin Staining
2.4. Immunofluorescence Staining
2.5. TUNEL Staining
2.6. Testicular Redox Status
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
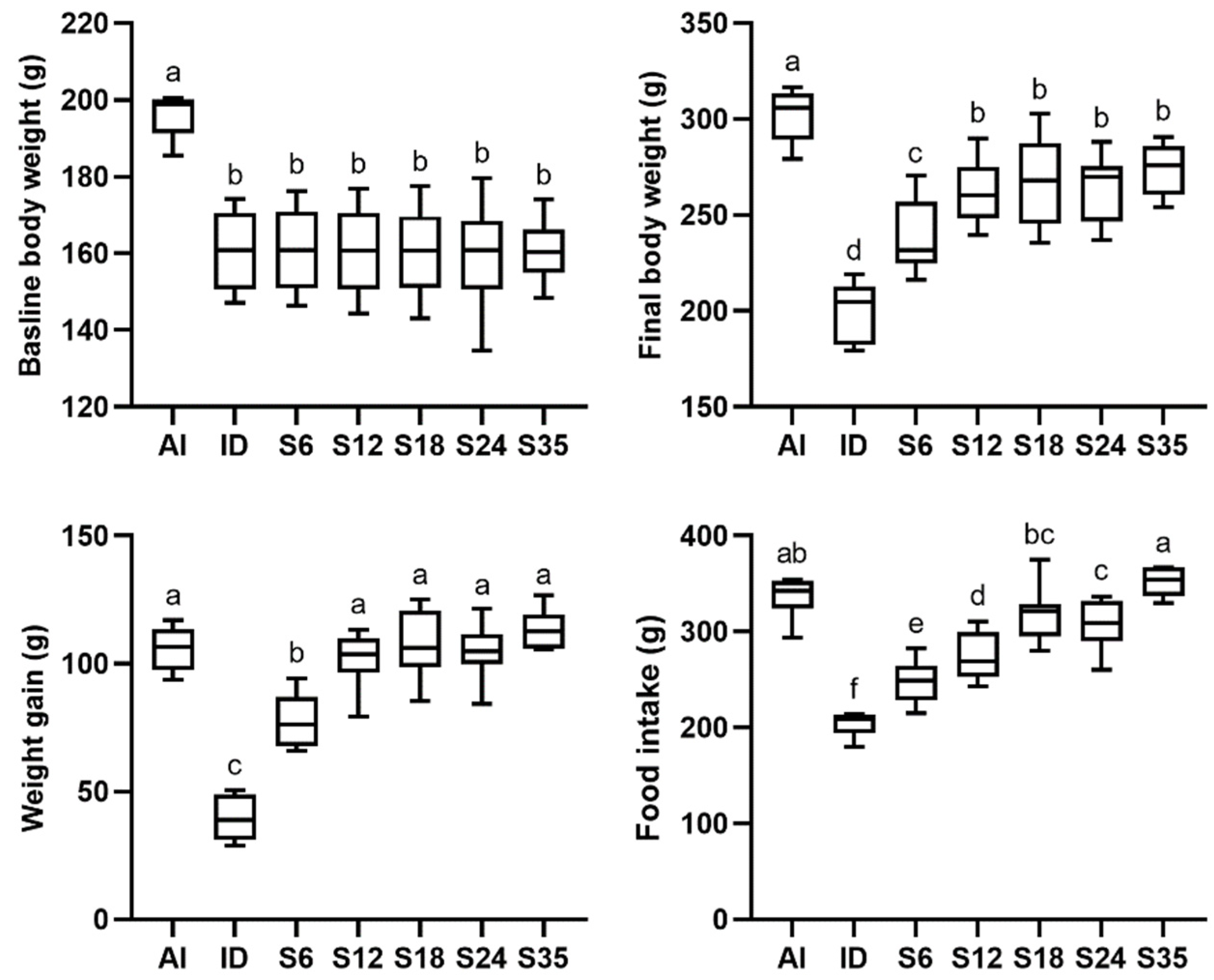
3.1. Body Weight and Food Intake
3.2. Biochemical Values
3.3. Protein Expressions of Testosterone Biosynthesis Enzymes
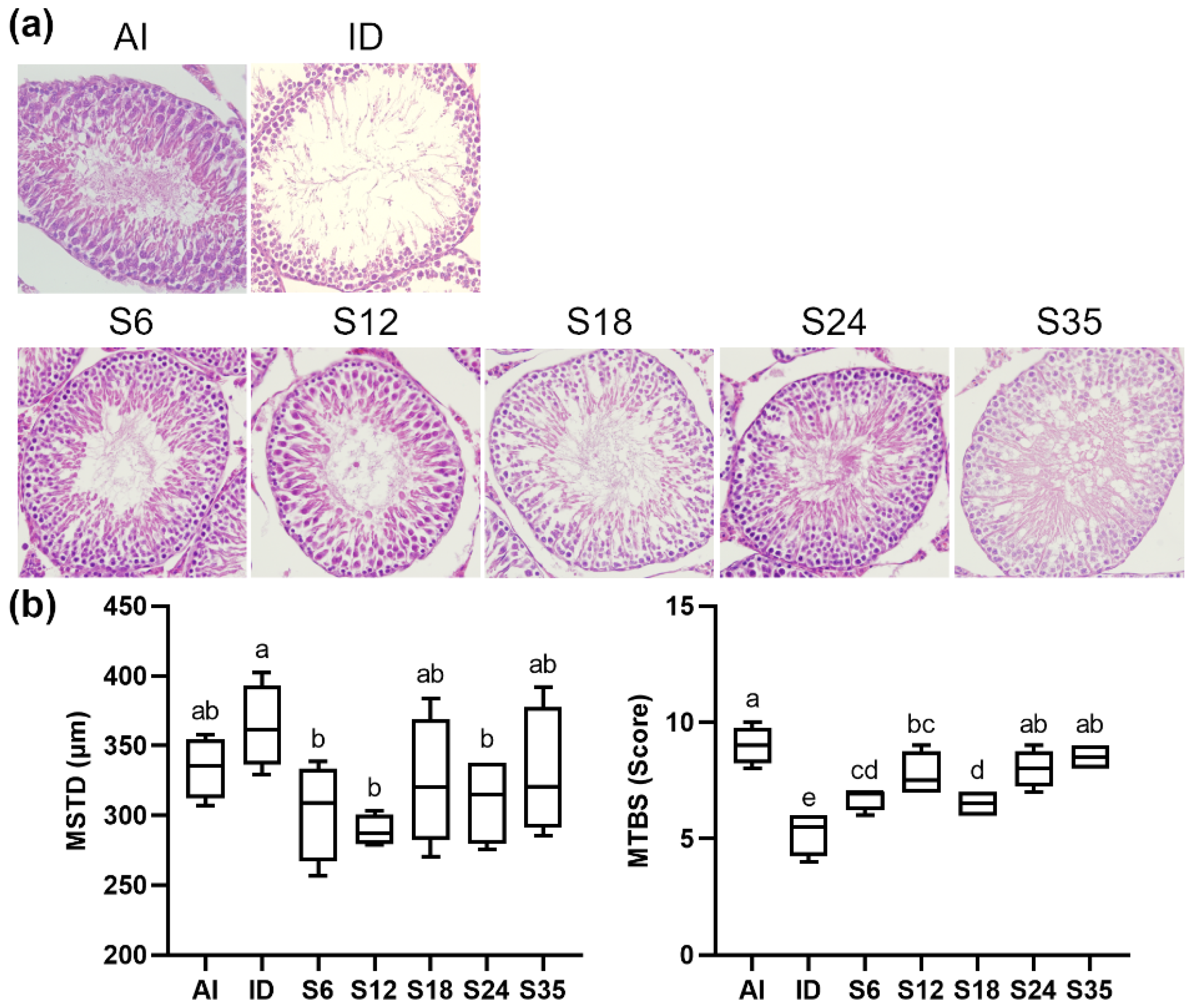
3.4. Testicular Histology
3.5. Testicular Spermatogenesis
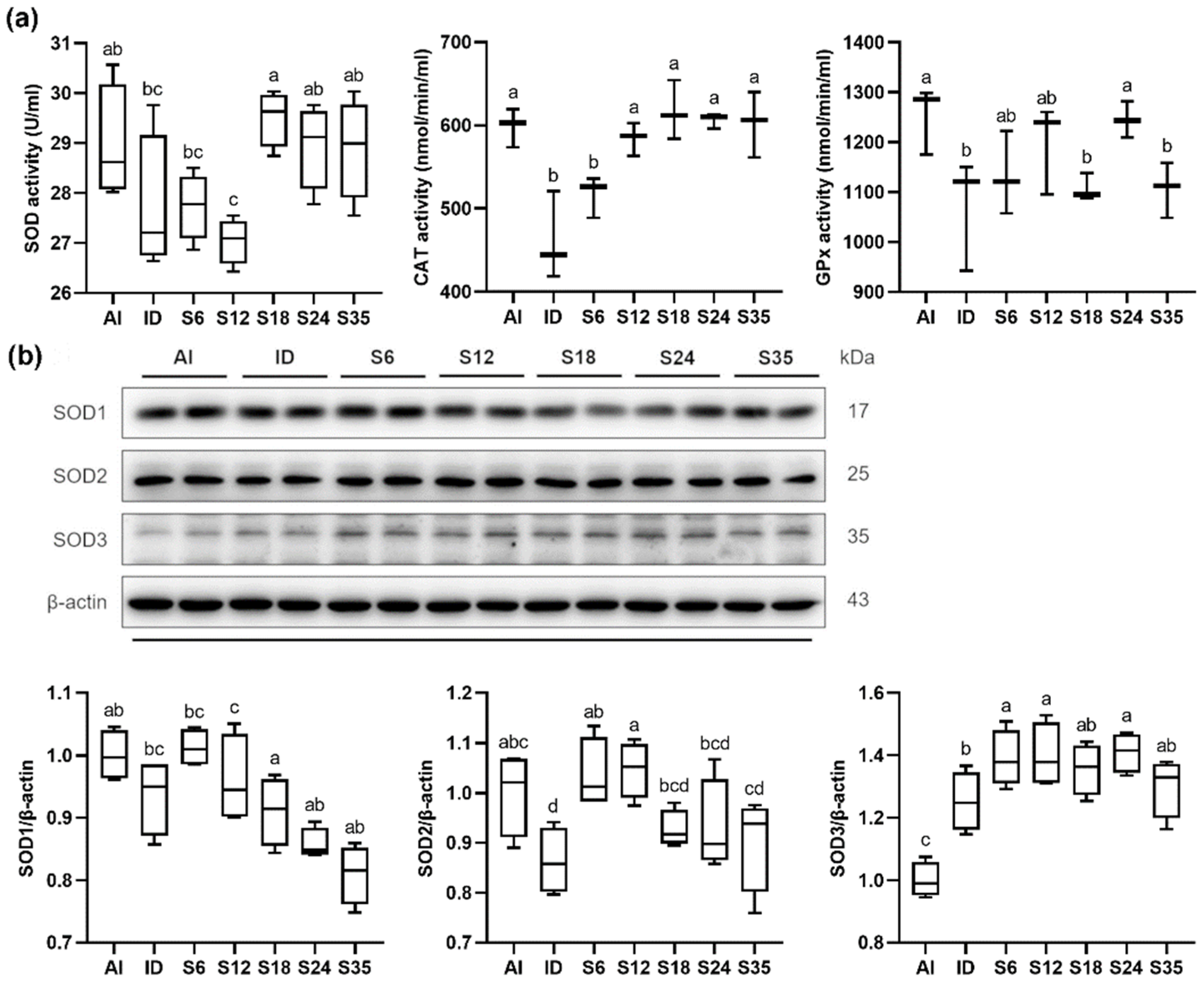
3.6. Testicular Redox Status and Protein Expression of SOD
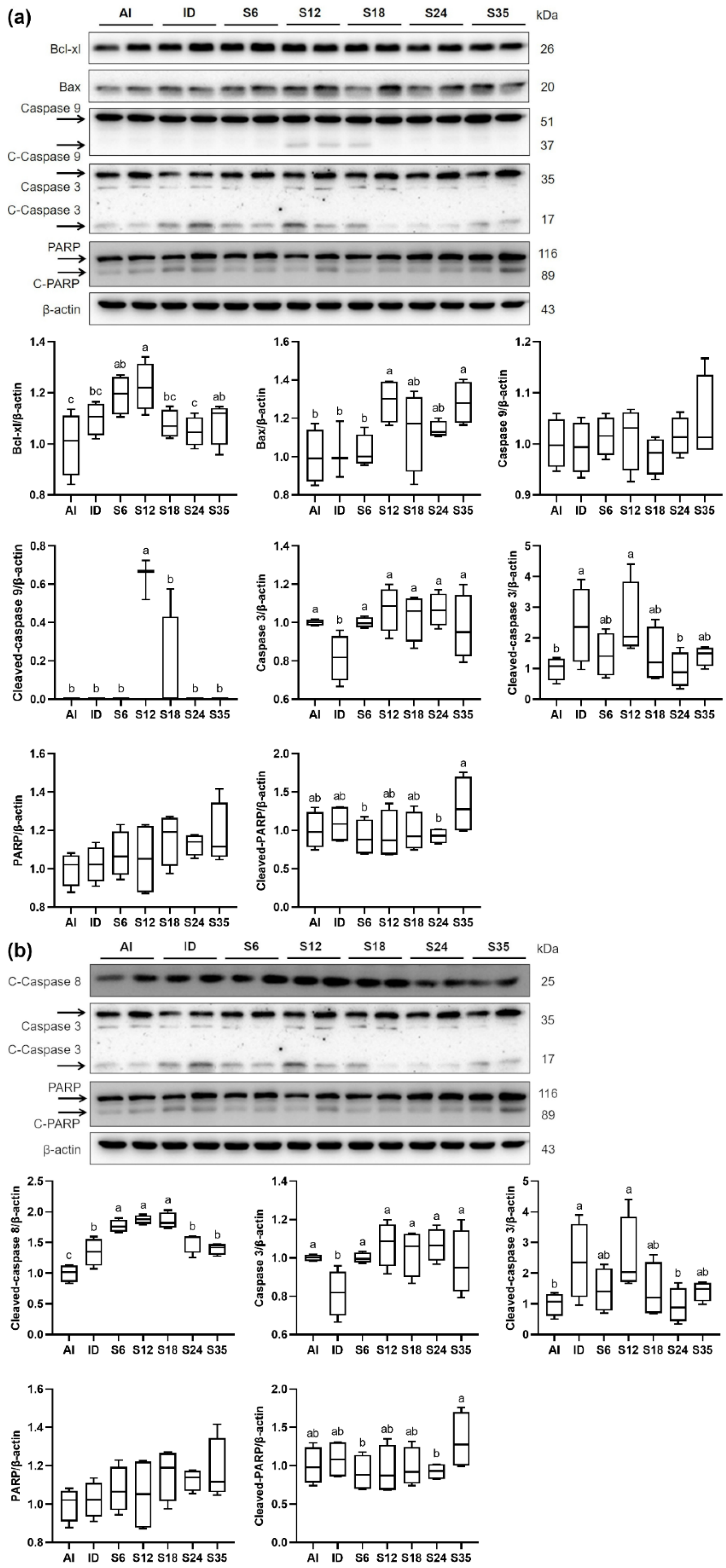
3.7. Testicular Apoptosis and Protein Expressions of Apoptosis Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Short, M.W.; Domagalski, J.E. Iron deficiency anemia: Evaluation and management. Am. Fam. Physician 2013, 87, 98–104. [Google Scholar] [PubMed]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the diagnosis and treatment of iron deficiency across indications: A systematic review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef]
- Zeller, T.; Waldeyer, C.; Ojeda, F.; Schnabel, R.B.; Schäfer, S.; Altay, A.; Lackner, K.J.; Anker, S.D.; Westermann, D.; Blankenberg, S.; et al. Adverse Outcome Prediction of Iron Deficiency in Patients with Acute Coronary Syndrome. Biomolecules 2018, 8, 60. [Google Scholar] [CrossRef]
- Grantham-Mcgregor, S.; Baker-Henningham, H. Iron Deficiency in Childhood: Causes and Consequences for Child Development. Ann. Nestlé 2010, 68, 105–119. [Google Scholar] [CrossRef]
- Fretham, S.J.; Carlson, E.S.; Georgieff, M.K. The role of iron in learning and memory. Adv. Nutr. 2011, 2, 112–121. [Google Scholar] [CrossRef]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the Hypothalamic Pituitary Gonadal Axis in the Male. Urol. Clin. N. Am. 2016, 43, 151–162. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Maeng, H.Y.; Sun, Y.K.; Kim, Y.A.; Park, D.W.; Park, T.S.; Lee, S.T.; Choi, J.R. Oxidative status in iron-deficiency anemia. J. Clin. Lab. Anal. 2009, 23, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update 2003, 9, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Gulyani, S.; Earley, C.J.; Cutler, R.G.; Mattson, M.P.; Rifkind, J.M. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic. Res. 2008, 42, 824–829. [Google Scholar] [CrossRef]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular Biopsy Score Count—A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Nakata, H.; Wakayama, T.; Takai, Y.; Iseki, S. Quantitative Analysis of the Cellular Composition in Seminiferous Tubules in Normal and Genetically Modified Infertile Mice. J. Histochem. Cytochem. 2014, 63, 99–113. [Google Scholar] [CrossRef]
- Shaw, N.-S.; Yeh, W.-T.; Pan, W.-H. Prevalence of Iron Deficiency in the General Population in Taiwan. Nutr. Sci. J. 1999, 24, 119–138. [Google Scholar] [CrossRef]
- Moghaddam Tabrizi, F.; Barjasteh, S. Maternal Hemoglobin Levels during Pregnancy and their Association with Birth Weight of Neonates. Iran. J. Pediatr. Hematol. Oncol. 2015, 5, 211–217. [Google Scholar]
- Susanti, T.; Dirgahayu, P.; Indarto, D. Development of Rat Model with Iron Deficiency Anemia by Modification of Its Standard Food. Adv. Health Sci. Res. 2018, 9, 63–66. [Google Scholar] [CrossRef][Green Version]
- Thakur, M.; Kulkarni, S.; Mohanty, N.; Kadam, N.; Swain, N. Standardization & Development of Rat Model with Iron Deficiency Anaemia Utilising Commercial Available Iron Deficient Food. Biosci. Biotechnol. Res. Asia 2019, 16, 71–77. [Google Scholar] [CrossRef]
- He, H.; Huang, Q.; Liu, C.; Jia, S.; Wang, Y.; An, F.; Song, H. Effectiveness of AOS–iron on iron deficiency anemia in rats. RSC Adv. 2019, 9, 5053–5063. [Google Scholar] [CrossRef] [PubMed]
- Ghada, Z.A.; Mahfouz, M.H.; Emara, I.A. Effect of Different Types of Oral Iron Therapy Used for the Treatment of Iron Deficiency Anemia and Their Effects on Some Hormones and Minerals in Anemic Rats. J. Am. Sci. 2010, 6, 109–118. [Google Scholar]
- Lawen, A.; Lane, D.J. Mammalian iron homeostasis in health and disease: Uptake, storage, transport, and molecular mechanisms of action. Antioxid. Redox Signal. 2013, 18, 2473–2507. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kusuda, M.; Abe, K.; Nagasaka, M. Effects of lron Deficiency Anemia on Growth Rate of Rats. Keitai Kinou (Struct. Funct.) 2009, 7, 67–75. [Google Scholar] [CrossRef]
- Soliman, A.; Yassin, M.; De Sanctis, V. Intravenous iron replacement therapy in eugonadal males with iron-deficiency anemia: Effects on pituitary gonadal axis and sperm parameters; A pilot study. Indian J. Endocrinol. Metab. 2014, 18, 310–316. [Google Scholar] [CrossRef]
- Chepelev, N.; Willmore, W. Regulation of iron pathways in response to hypoxia. Free. Radic. Biol. Med. 2011, 50, 645–666. [Google Scholar] [CrossRef]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Vargas, Á.; Bustos-Obregón, E.; Hartley, R. Effects of hypoxia on epididymal sperm parameters and protective role of ibuprofen and melatonin. Biol. Res. 2011, 44, 161–167. [Google Scholar] [CrossRef]
- Bomhard, E.M.; Gelbke, H.P. Hypoxaemia affects male reproduction: A case study of how to differentiate between primary and secondary hypoxic testicular toxicity due to chemical exposure. Arch. Toxicol. 2013, 87, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Zhang, Y.; Chen, H.; Brown, T.R.; Zirkin, B.R.; Burnett, A.L. Mechanism of testosterone deficiency in the transgenic sickle cell mouse. PLoS ONE 2015, 10, e0128694. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.A.; Shen, M.; Ge, R.-S.; Sottas, C.M.; Hardy, M.P.; Morris, D.J. Role of 11β-OH-C19 and C21 steroids in the coupling of 11β-HSD1 and 17β-HSD3 in regulation of testosterone biosynthesis in rat Leydig cells. Steroids 2011, 76, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. NADPH-generating dehydrogenases: Their role in the mechanism of protection against nitro-oxidative stress induced by adverse environmental conditions. Front. Environ. Sci. 2014, 2, 55. [Google Scholar] [CrossRef]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Leichtmann-Bardoogo, Y.; Cohen, L.A.; Weiss, A.; Marohn, B.; Schubert, S.; Meinhardt, A.; Meyron-Holtz, E.G. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1519–E1530. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Griswold, M.D. The testicular iron shuttle: A “nurse” function of the Sertoli cells. J. Androl. 1994, 15, 381–385. [Google Scholar]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef]
- Griffin, K.P.; Ward, D.T.; Liu, W.; Stewart, G.; Morris, I.D.; Smith, C.P. Differential expression of divalent metal transporter DMT1 (Slc11a2) in the spermatogenic epithelium of the developing and adult rat testis. Am. J. Physiol. Cell Physiol. 2005, 288, C176–C184. [Google Scholar] [CrossRef]
- Elseweidy, M.; Asker, M.; Ali, S.; Atteia, H. Effect of prolonged intake of iron enriched diet on testicular functions of experimental rats. Nat. Sci. 2010, 2, 551–556. [Google Scholar] [CrossRef]
- Kurtoglu, E.; Ugur, A.; Baltaci, A.K.; Undar, L. Effect of iron supplementation on oxidative stress and antioxidant status in iron-deficiency anemia. Biol. Trace Elem. Res. 2003, 96, 117–123. [Google Scholar] [CrossRef]
- Akarsu, S.; Demir, H.; Selek, Ş.; Oguzoncul, A. Iron Deficiency Anemia and Levels of Oxidative Stress Induced by Treatment Modalities. Pediatrics Int. 2013, 55, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.; Gupta, S.; Harlev, A.; Ahmad, G.; Du Plessis, S.S.; Esteves, S.C.; Wang, S.M.; Durairajanayagam, D. Oxidative Stress in Human Reproduction: Shedding Light on a Complicated Phenomenon; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Yan, L.; Liu, J.; Wu, S.; Zhang, S.; Ji, G.; Gu, A. Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J. Assist. Reprod. Genet. 2014, 31, 549–554. [Google Scholar] [CrossRef]
- Perumal, P. Effect of Superoxide Dismutase on Semen Parameters and Antioxidant Enzyme Activities of Liquid Stored (5 °C) Mithun (Bos frontalis) Semen. J. Anim. 2014, 2014, 821954. [Google Scholar] [CrossRef]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Lucesoli, F.; Caligiuri, M.; Roberti, M.F.; Perazzo, J.C.; Fraga, C.G. Dose-dependent increase of oxidative damage in the testes of rats subjected to acute iron overload. Arch. Biochem. Biophys. 1999, 372, 37–43. [Google Scholar] [CrossRef]
- Santambrogio, P.; Biasiotto, G.; Sanvito, F.; Olivieri, S.; Arosio, P.; Levi, S. Mitochondrial ferritin expression in adult mouse tissues. J. Histochem. Cytochem. 2007, 55, 1129–1137. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, C.-W.; Liao, Y.-R.; Chang, T.-C.; Liew, Y.-F.; Liu, C.-Y. Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients 2022, 14, 2063. https://doi.org/10.3390/nu14102063
Tsao C-W, Liao Y-R, Chang T-C, Liew Y-F, Liu C-Y. Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients. 2022; 14(10):2063. https://doi.org/10.3390/nu14102063
Chicago/Turabian StyleTsao, Chih-Wei, Yuan-Ru Liao, Ting-Chia Chang, Yih-Fong Liew, and Chin-Yu Liu. 2022. "Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats" Nutrients 14, no. 10: 2063. https://doi.org/10.3390/nu14102063
APA StyleTsao, C.-W., Liao, Y.-R., Chang, T.-C., Liew, Y.-F., & Liu, C.-Y. (2022). Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients, 14(10), 2063. https://doi.org/10.3390/nu14102063






