Resting-State Functional Connectivity Differences in College Students with and without Food Insecurity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment of Subjects
2.2. Survey Measures
2.3. fMRI Data Acquisition
2.4. Survey Analyses
2.5. fMRI Preprocessing and Analysis
3. Results
3.1. Demographics and Self-Reported Measures
3.2. Resting-State Functional Connectivity Group Differences and Correlations with BRIEF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman-Jensen, A.; Rabbitt, M.P.; Gregory, C.A.; Singh, A. Statistical Supplement to Household Food Security in the United States in 2020; Economic Researh Service of the US: Kansas City, MO, USA; USDA: Washington, DC, USA, 2021.
- Leung, C.W.; Epel, E.S.; Ritchie, L.D.; Crawford, P.B.; Laraia, B.A. Food insecurity is inversely associated with diet quality of lower-income adults. J. Acad. Nutr. Diet. 2014, 114, 1943–1953.e2. [Google Scholar] [CrossRef]
- Hazzard, V.M.; Loth, K.A.; Hooper, L.; Becker, C.B. Food insecurity and eating disorders: A review of emerging evidence. Curr. Psychiatry Rep. 2020, 22, 74. [Google Scholar] [CrossRef]
- Weaver, L.J.; Fasel, C.B. A systematic review of the literature on the relationships between chronic diseases and food insecurity. Food Nutr. Sci. 2018, 9, 519. [Google Scholar] [CrossRef] [Green Version]
- Pourmotabbed, A.; Moradi, S.; Babaei, A.; Ghavami, A.; Mohammadi, H.; Jalili, C.; Symonds, M.E.; Miraghajani, M. Food insecurity and mental health: A systematic review and meta-analysis. Public Health Nutr. 2020, 23, 1778–1790. [Google Scholar] [CrossRef]
- Arenas, D.J.; Thomas, A.; Wang, J.; DeLisser, H.M. A systematic review and meta-analysis of depression, anxiety, and sleep disorders in US adults with food insecurity. J. Gen. Intern. Med. 2019, 34, 2874–2882. [Google Scholar] [CrossRef]
- Bruening, M.; Argo, K.; Payne-Sturges, D.; Laska, M.N. The struggle is real: A systematic review of food insecurity on postsecondary education campuses. J. Acad. Nutr. Diet. 2017, 117, 1767–1791. [Google Scholar] [CrossRef]
- Nikolaus, C.J.; An, R.; Ellison, B.; Nickols-Richardson, S.M. Food insecurity among college students in the United States: A scoping review. Adv. Nutr. 2020, 11, 327–348. [Google Scholar] [CrossRef]
- Maroto, M.E.; Snelling, A.; Linck, H. Food insecurity among community college students: Prevalence and association with grade point average. Community Coll. J. Res. Pract. 2015, 39, 515–526. [Google Scholar] [CrossRef]
- Phillips, E.; McDaniel, A.; Croft, A. Food insecurity and academic disruption among college students. J. Stud. Aff. Res. Pract. 2018, 55, 353–372. [Google Scholar] [CrossRef]
- Royer, M.F.; Guerithault, N.; Braden, B.B.; Laska, M.N.; Bruening, M. Food Insecurity Is Associated with Cognitive Function: A Systematic Review of Findings across the Life Course. Int. J. Transl. Med. 2021, 1, 205–222. [Google Scholar] [CrossRef]
- Na, M.; Dou, N.; Ji, N.; Xie, D.; Huang, J.; Tucker, K.L.; Gao, X. Food insecurity and cognitive function in middle to older adulthood: A systematic review. Adv. Nutr. 2020, 11, 667–676. [Google Scholar] [CrossRef]
- Knouse, L.E.; Feldman, G.; Blevins, E.J. Executive functioning difficulties as predictors of academic performance: Examining the role of grade goals. Learn. Individ. Differ. 2014, 36, 19–26. [Google Scholar] [CrossRef]
- Baars, M.A.; Nije Bijvank, M.; Tonnaer, G.H.; Jolles, J. Self-report measures of executive functioning are a determinant of academic performance in first-year students at a university of applied sciences. Front. Psychol. 2015, 6, 1131. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.S.; Buckworth, J.; Kirby, T.E.; Sherman, W.M. Characteristics of exercise behavior among college students: Application of social cognitive theory to predicting stage of change. Prev. Med. 2000, 31, 494–505. [Google Scholar] [CrossRef]
- Xu, X.; Pu, Y.; Sharma, M.; Rao, Y.; Cai, Y.; Zhao, Y. Predicting physical activity and healthy nutrition behaviors using social cognitive theory: Cross-sectional survey among undergraduate students in Chongqing, China. Int. J. Environ. Res. Public Health 2017, 14, 1346. [Google Scholar] [CrossRef] [Green Version]
- Grineski, S.E.; Morales, D.X.; Collins, T.W.; Rubio, R. Transitional dynamics of household food insecurity impact children’s developmental outcomes. J. Dev. Behav. Pediatrics 2018, 39, 715. [Google Scholar] [CrossRef]
- Portela-Parra, E.T.; Leung, C.W. Food insecurity is associated with lower cognitive functioning in a national sample of older adults. J. Nutr. 2019, 149, 1812–1817. [Google Scholar] [CrossRef]
- Wong, J.C.; Scott, T.; Wilde, P.; Li, Y.-G.; Tucker, K.L.; Gao, X. Food insecurity is associated with subsequent cognitive decline in the Boston Puerto Rican Health Study. J. Nutr. 2016, 146, 1740–1745. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.Y.; Sheira, L.A.; Frongillo, E.A.; Adimora, A.A.; Tien, P.C.; Konkle-Parker, D.; Golub, E.T.; Merenstein, D.; Levin, S.; Cohen, M. Food insecurity and neurocognitive function among women living with or at risk for HIV in the United States. Am. J. Clin. Nutr. 2020, 112, 1280–1286. [Google Scholar] [CrossRef]
- Van Den Heuvel, M.P.; Pol, H.E.H. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [Green Version]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Zanto, T.P.; Gazzaley, A. Fronto-parietal network: Flexible hub of cognitive control. Trends Cogn. Sci. 2013, 17, 602–603. [Google Scholar] [CrossRef] [Green Version]
- Mond, J.M.; Hay, P.J.; Rodgers, B.; Owen, C.; Beumont, P. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behav. Res. Ther. 2004, 42, 551–567. [Google Scholar] [CrossRef]
- Friederich, H.C.; Wu, M.; Simon, J.J.; Herzog, W. Neurocircuit function in eating disorders. Int. J. Eat. Disord. 2013, 46, 425–432. [Google Scholar] [CrossRef]
- Bickel, G.; Nord, M.; Price, C.; Hamilton, W.; Cook, J. Guide to Measuring Household Food Security; U.S. Department of Agriculture, Food and Nutrition Service: Alexandria, VA, USA, 2000.
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. Assessment of executive functioning using the Behavior Rating Inventory of Executive Function (BRIEF). In Handbook of Executive Functioning; Springer: Berlin/Heidelberg, Germany, 2014; pp. 301–331. [Google Scholar]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Testa, A.; Jackson, D.B. Adverse childhood experiences and food insecurity in adulthood: Evidence from the national longitudinal study of adolescent to adult health. J. Adolesc. Health 2020, 67, 218–224. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Layman, H.M.; Ganson, K.T.; Tabler, J.; Ciciolla, L.; Tsotsoros, C.E.; Nagata, J.M. Adverse childhood events and cognitive function among young adults: Prospective results from the national longitudinal study of adolescent to adult health. Child Abus. Negl. 2021, 115, 105008. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Van Woerden, I.; Hruschka, D.; Bruening, M. Food insecurity negatively impacts academic performance. J. Public Aff. 2019, 19, e1864. [Google Scholar] [CrossRef]
- Martinez, S.M.; Frongillo, E.A.; Leung, C.; Ritchie, L. No food for thought: Food insecurity is related to poor mental health and lower academic performance among students in California’s public university system. J. Health Psychol. 2020, 25, 1930–1939. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, C.; Pike, J.R.; Riggs, N.R.; Warren, C.M.; Pentz, M.A. Executive function and probabilities of engaging in long-term sedentary and high calorie/low nutrition eating behaviors in early adolescence. Soc. Sci. Med. 2019, 237, 112483. [Google Scholar] [CrossRef]
- O’Neill, J.; Kamper-DeMarco, K.; Chen, X.; Orom, H. Too stressed to self-regulate? Associations between stress, self-reported executive function, disinhibited eating, and BMI in women. Eat. Behav. 2020, 39, 101417. [Google Scholar] [CrossRef]
- Alves, P.N.; Foulon, C.; Karolis, V.; Bzdok, D.; Margulies, D.S.; Volle, E.; de Schotten, M.T. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol. 2019, 2, 370. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.B.; Hedden, T.; Thompson, T.W.; Anteraper, S.A.; Gabrieli, J.D.; Whitfield-Gabrieli, S. Resting-state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex 2015, 64, 271–280. [Google Scholar] [CrossRef] [Green Version]
- DeSerisy, M.; Ramphal, B.; Pagliaccio, D.; Raffanello, E.; Tau, G.; Marsh, R.; Posner, J.; Margolis, A.E. Frontoparietal and default mode network connectivity varies with age and intelligence. Dev. Cogn. Neurosci. 2021, 48, 100928. [Google Scholar] [CrossRef]
- Christoff, K.; Irving, Z.C.; Fox, K.C.; Spreng, R.N.; Andrews-Hanna, J.R. Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Utevsky, A.V.; Smith, D.V.; Huettel, S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014, 34, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ptak, R.; Schnider, A.; Fellrath, J. The dorsal frontoparietal network: A core system for emulated action. Trends Cogn. Sci. 2017, 21, 589–599. [Google Scholar] [CrossRef] [PubMed]
| Food Insecure (n = 20) | Food Secure (n = 20) | p-Value | |
|---|---|---|---|
| ACEs (Mean ± SE) | 3.7 ± 0.6 | 1.6 ± 0.3 | 0.006 |
| Pell grant status % (n) | 33.3 (10) | 66.7 (5) | 0.102 |
| Race/Ethnicity % (n) | |||
| White | 47.1 (8) | 52.9 (9) | 0.749 |
| Other | 57.5 (12) | 47.8 (11) |
| Dependent Variable | Predictor | β | Cohen’s D | 95% CI |
|---|---|---|---|---|
| BRIEF Global Executive | FI | 8.36 * | 0.43 | 2.32, 14.40 |
| ACEs | 1.69 * | 0.42 | 0.46, 2.93 | |
| BRIEF Inhibit | FI | 8.25 * | 0.45 | 2.58, 13.9 |
| ACEs | 0.63 | 0.19 | −0.23, 1.78 | |
| BRIEF Shift | FI | 5.58 | 0.25 | −1.26, 12.62 |
| ACEs | 1.70 * | 0.37 | 0.28, 3.11 | |
| BRIEF Emotional Control | FI | 3.01 | 0.14 | −3.71, 9.75 |
| ACEs | 1.45 * | 0.33 | 0.07, 2.83 | |
| BRIEF Self-Monitor | FI | 5.37 | 0.27 | −0.69, 11.43 |
| ACEs | 1.91 * | 0.48 | 0.67, 3.15 | |
| BRIEF Initiate | FI | 7.64 * | 0.32 | 0.23, 15.1 |
| ACEs | 1.52 * | 0.32 | 0.06, 3.03 | |
| BRIEF Working Memory | FI | 9.31 * | 0.45 | 2.90, 15.71 |
| ACEs | 2.13 * | 0.5 | 0.82, 3.44 | |
| BRIEF Plan | FI | 8.02 * | 0.34 | 0.64, 15.39 |
| ACEs | 0.83 | 0.17 | −0.67, 2.33 | |
| BRIEF Task | FI | 0.79 | 0.03 | −6.76, 8.34 |
| ACEs | 1.91 * | 0.38 | 0.37, 3.47 | |
| BRIEF Organize | FI | 11.13 * | 0.48 | 3.96, 18.29 |
| ACEs | 0.51 | 0.11 | −0.94, 1.97 |
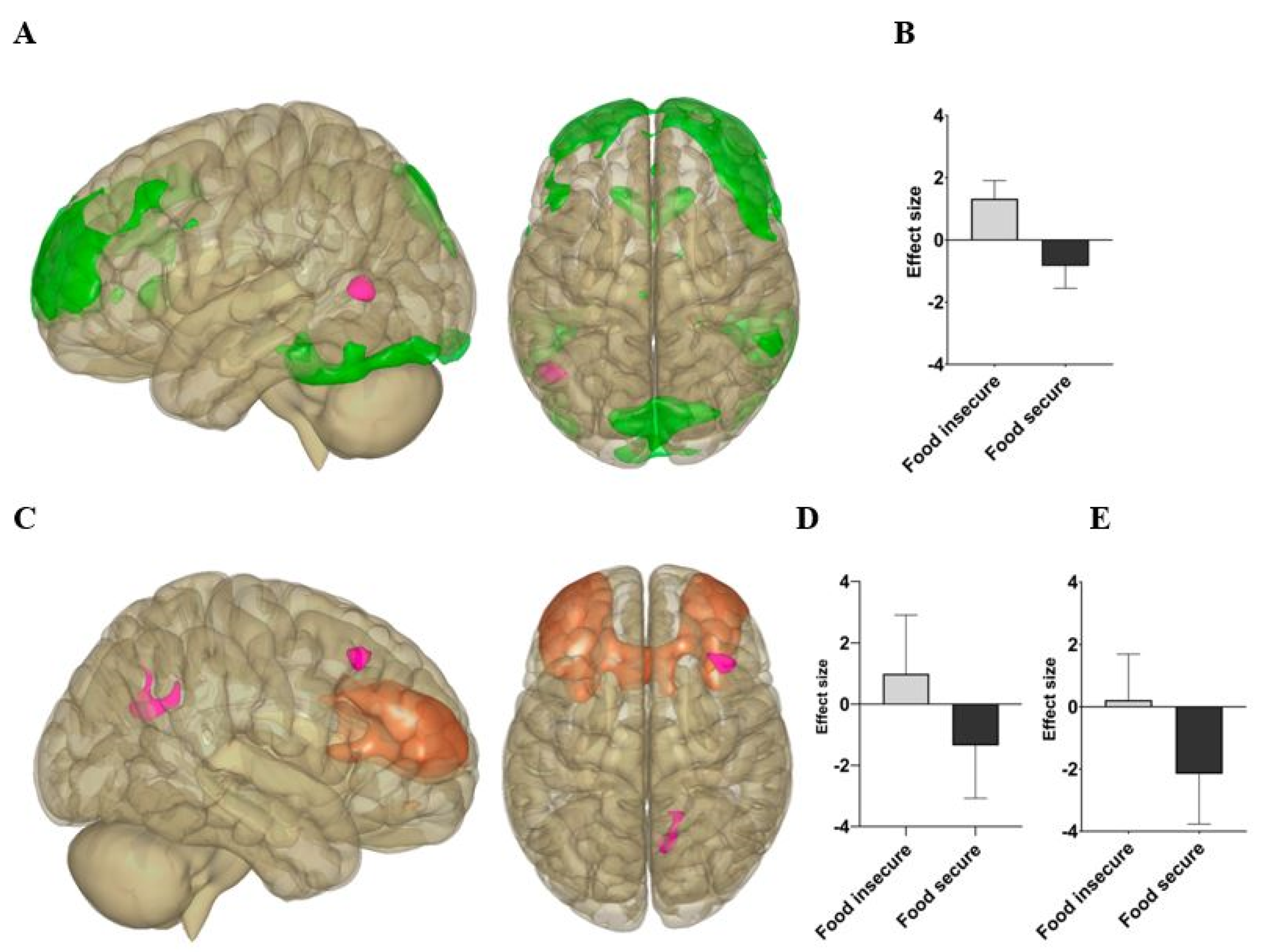
| Component | Cluster (x, y, z) | Region | Size | Cluster p-FWE |
|---|---|---|---|---|
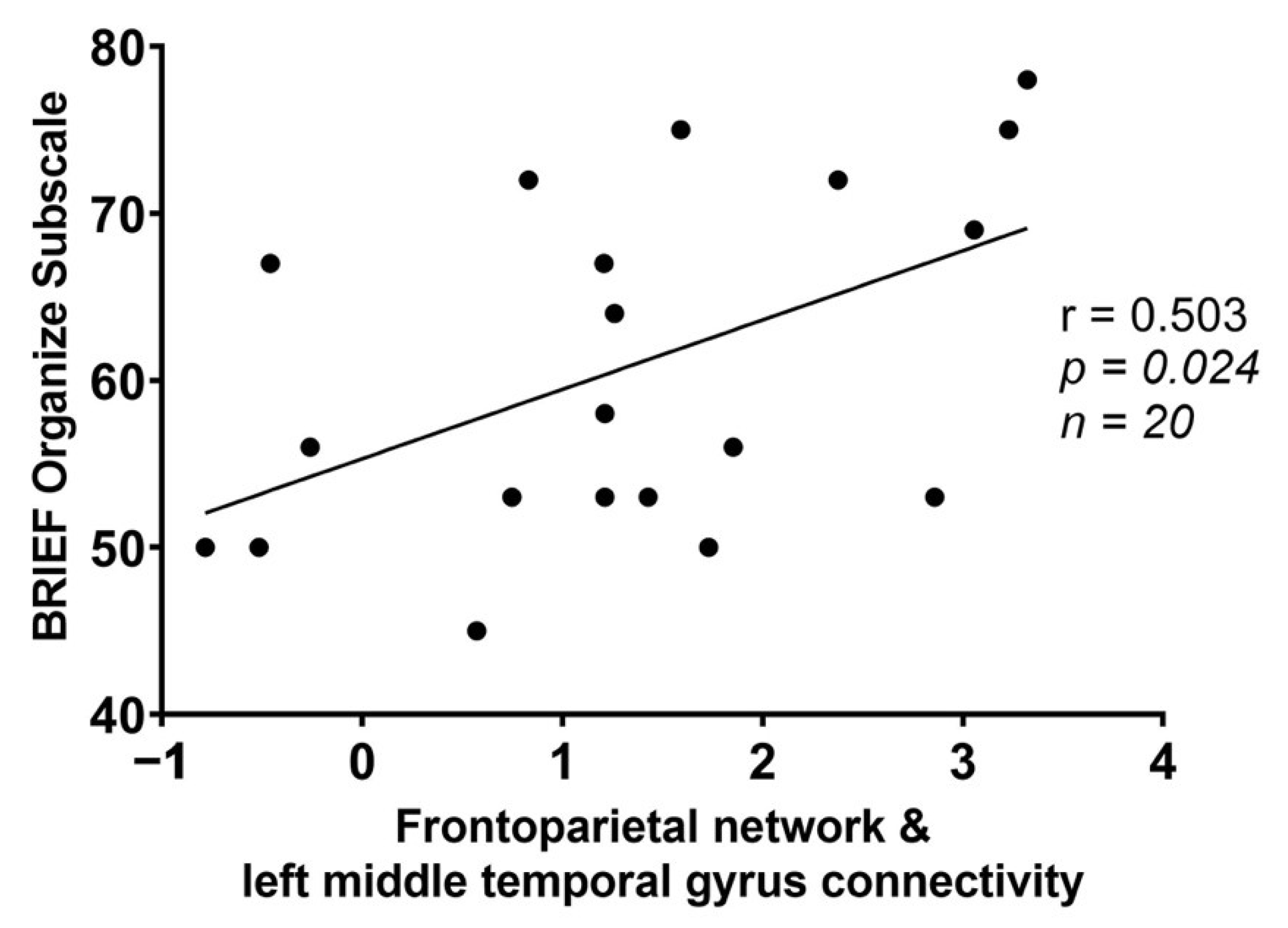
| FPN | −50, −62, +04 | Left middle temporal gyrus | 193 | 0.029429 |
| SN | +16, −50, +30 | Precuneus | 280 | 0.000866 |
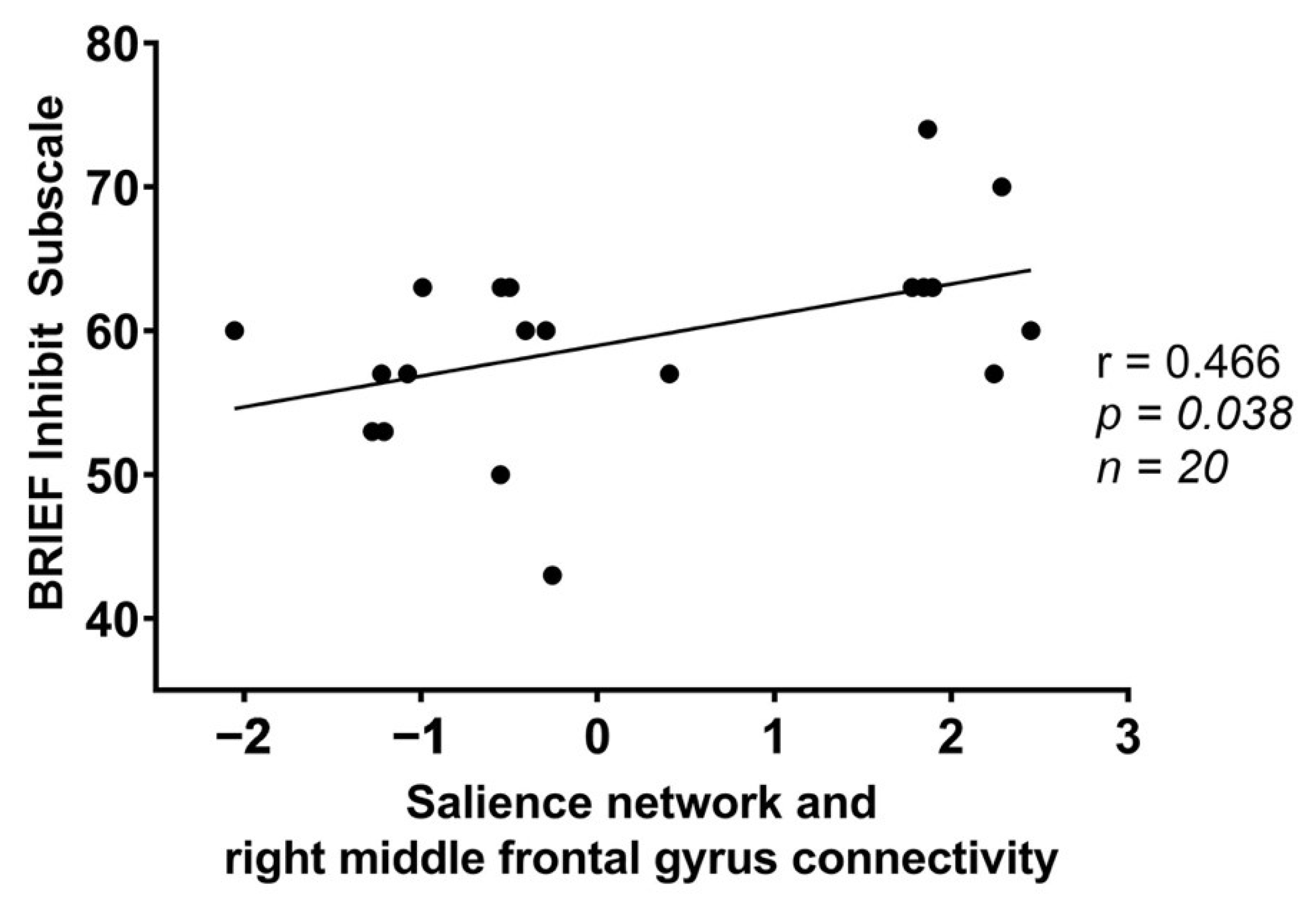
| SN | +38, +24, +46 | Right middle frontal gyrus | 169 | 0.015856 |
| BRIEF Subscale (T-Scores) | Frontoparietal Network (FPN)-Left Middle Temporal Gyrus | Salience Network (SN)-Precuneus | Salience Network (SN)-Right Middle Fontal Gyrus | |||
|---|---|---|---|---|---|---|
| Food Insecure | Food Secure | Food Insecure | Food Secure | Food Insecure | Food Secure | |
| Total (Global Executive) | r = 0.142; | r = −0.315; | r = 0.177; | r = −0.277; | r = −0.090; | r = 0.179; |
| p = 0.550 | p = 0.176 | p = 0.456 | p = 0.237 | p = 0.707 | p = 0.449 | |
| Inhibit | r = 0.307; | r = −0.258; | r = 0.402; | r = −0.345; | r = 0.466; | r = 0.066; |
| p = 0.188 | p = 0.273 | p = 0.079 | p = 0.137 | p = 0.038 * | p = 0.783 | |
| Initiate | r = −0.067; | r = −0.282; | r = 0.148; | r = −0.414; | r = −0.147; | r = 0.044; |
| p = 0.780 | p = 0.228 | p = 0.535 | p = 0.070 | p = 0.535 | p = 0.854 | |
| Working Memory | r = 0.027; | r = −0.280; | r = −0.029; | r = −0.417; | r = −0.081; | r = 0.113; |
| p = 0.910 | p = 0.232 | p = 0.904 | p = 0.067 | p = 0.734 | p = 0.636 | |
| Plan | r = 0.108; | r = −0.424; | r = 0.387; | r = −0.249; | r = 0.052; | r = 0.009; |
| p = 0.650 | p = 0.063 | p = 0.092 | p = 0.290 | p = 0.827 | p = 0.969 | |
| Organize | r = 0.503; | r = 0.017; | r = 0.331; | r = −0.146; | r = −0.161; | r = 0.119; |
| p = 0.024 * | p = 0.944 | p = 0.154 | p = 0.539 | p = 0.498 | p = 0.619 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerithault, N.; McClure, S.M.; Ojinnaka, C.O.; Braden, B.B.; Bruening, M. Resting-State Functional Connectivity Differences in College Students with and without Food Insecurity. Nutrients 2022, 14, 2064. https://doi.org/10.3390/nu14102064
Guerithault N, McClure SM, Ojinnaka CO, Braden BB, Bruening M. Resting-State Functional Connectivity Differences in College Students with and without Food Insecurity. Nutrients. 2022; 14(10):2064. https://doi.org/10.3390/nu14102064
Chicago/Turabian StyleGuerithault, Nicolas, Samuel M. McClure, Chinedum O. Ojinnaka, B. Blair Braden, and Meg Bruening. 2022. "Resting-State Functional Connectivity Differences in College Students with and without Food Insecurity" Nutrients 14, no. 10: 2064. https://doi.org/10.3390/nu14102064
APA StyleGuerithault, N., McClure, S. M., Ojinnaka, C. O., Braden, B. B., & Bruening, M. (2022). Resting-State Functional Connectivity Differences in College Students with and without Food Insecurity. Nutrients, 14(10), 2064. https://doi.org/10.3390/nu14102064







