The Cow’s Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. CoMiSS in Presumed Healthy Infants
3.2. Factors Potentially Affecting CoMiSS in Presumed Healthy Infants
- Country
- Gender
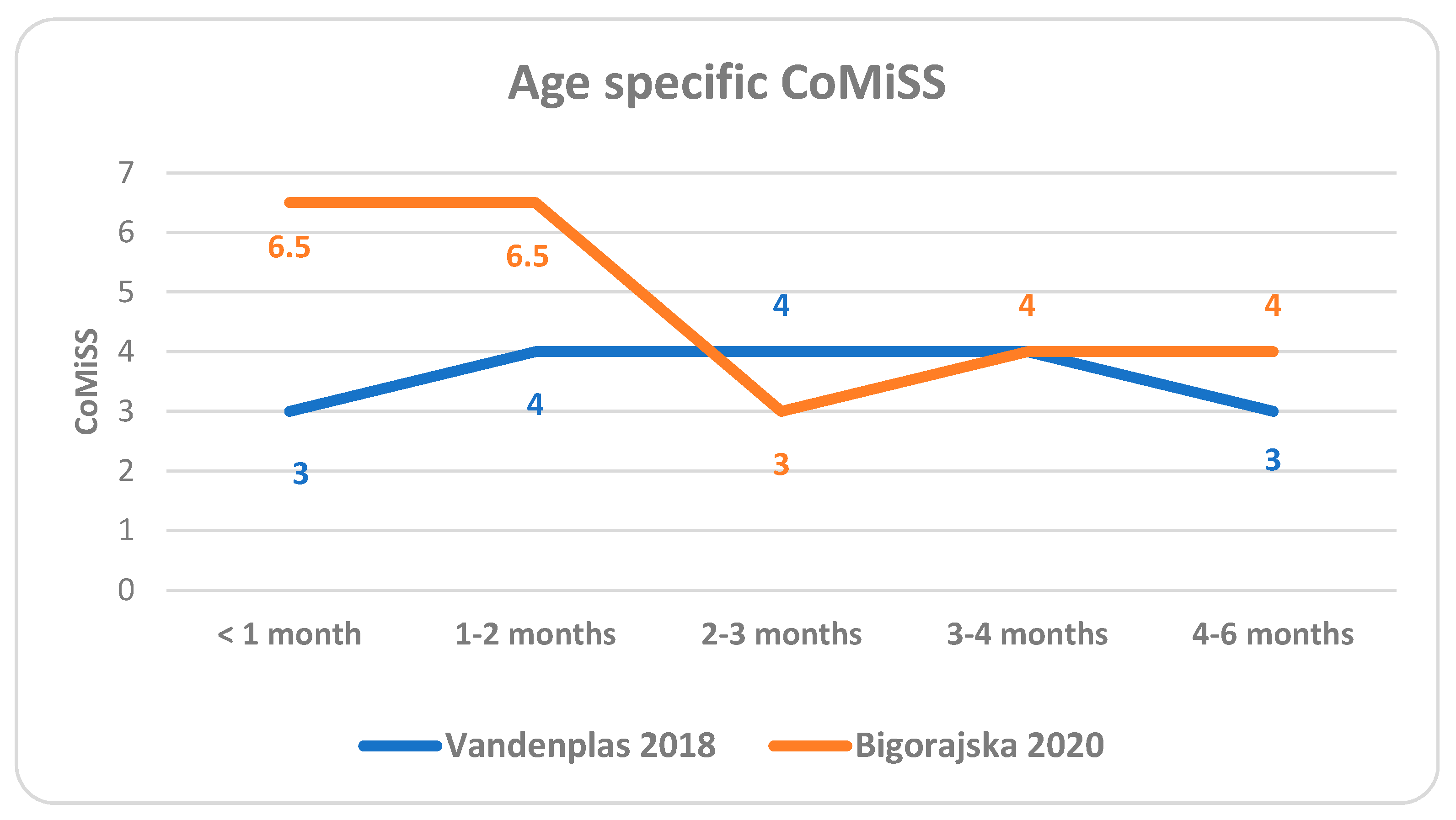
- Age (Table 4)
- Breast and formula feeding
- Inter-rater and day-to-day variability (Table 5)
3.3. CoMiSS in Symptomatic and Allergic Infants
- Response to cow’s milk elimination
- Sensitivity and specificity
3.4. CoMiSS in Conditions Other Than CM Allergy
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s milk allergy or gastroesophageal reflux disease—Can we solve the dilemma in infants? Nutrients 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Perkin, M.R.; Palmer, D.J.; Allen, K.J.; Boyle, R.J. Assessment of evidence about common infant symptoms and cow’s milk allergy. JAMA Pediatr. 2020, 174, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Høst, A. Frequency of cow’s milk allergy in childhood. Ann. Allergy Asthma Immunol. 2002, 89, 33–37. [Google Scholar] [CrossRef]
- Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--Europrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Muraro, A.; Agache, I.; Clark, A.; Sheikh, A.; Roberts, G.; Akdis, C.A.; Borrego, L.M.; Higgs, J.; Hourihane, J.O.; Jorgensen, P.; et al. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy 2014, 69, 1046–1057. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Berin, M.C.; Mehr, S. Food protein-induced enterocolitis syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 24–35. [Google Scholar] [CrossRef]
- Pensabene, L.; Salvatore, S.; D’Auria, E.; Parisi, F.; Concolino, D.; Borrelli, O.; Thapar, N.; Staiano, A.; Vandenplas, Y.; Saps, M. Cow’s milk protein allergy in infancy: A risk factor for functional gastrointestinal disorders in children? Nutrients 2018, 10, 1716. [Google Scholar] [CrossRef]
- Diaferio, L.; Caimmi, D.; Verga, M.C.; Palladino, V.; Trovè, L.; Giordano, P.; Verduci, E.; Miniello, V.L. May failure to thrive in infants be a clinical marker for the early diagnosis of cow’s milk allergy? Nutrients 2020, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-mediated gastrointestinal food allergies in children: An update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Høst, A.; Kuitunen, M.; Ribes-Koninck, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the cow’s milk-related symptom score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D.; Althera Study Group. Treating cow’s milk protein allergy: A double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Belohlavkova, S.; Enninger, A.; Frühauf, P.; Makwana, N.; Järvi, A. How are infants suspected to have cow’s milk allergy managed? A real world study report. Nutrients 2021, 13, 3027. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Althera Study Group; Steenhout, P.; Grathwohl, D. A pilot study on the application of a symptom-based score for the diagnosis of cow’s milk protein allergy. SAGE Open Med. 2014, 2, 2050312114523423. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Hauser, B.; Paradice Study Group. Safety and tolerance of a new extensively hydrolyzed rice protein-based formula in the management of infants with cow’s milk protein allergy. Eur. J. Pediatr. 2014, 173, 1209–1216. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E. Extensive protein hydrolysate formula effectively reduces regurgitation in infants with positive and negative challenge tests for cow’s milk allergy. Acta Paediatr. 2014, 103, e243–e250. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Xinias, I.; Vrani, O.; Mavroudi, A.; Hammoud, M.; Al Refai, F.; Khalife, M.C.; Sayad, A.; Noun, P.; et al. Safety of a thickened extensive casein hydrolysate formula. Nutrition 2016, 32, 206–212. [Google Scholar] [CrossRef]
- Dupont, C.; Bradatan, E.; Soulaines, P.; Nocerino, R.; Berni-Canani, R. Tolerance and growth in children with cow’s milk allergy fed a thickened extensively hydrolyzed casein-based formula. BMC Pediatr. 2016, 16, 96. [Google Scholar] [CrossRef]
- Prasad, R.; Venkata, R.S.A.; Ghokale, P.; Chakravarty, P.; Anwar, F. Cow’s milk-related symptom score as a predictive tool for cow’s milk allergy in Indian children aged 0–24 months. Asia Pac. Allergy 2018, 8, e36. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Salvatore, S.; Ribes-Koninckx, C.; Carvajal, E.; Szajewska, H.; Huysentruyt, K. The cow milk symptom score (CoMiSSTM) in presumed healthy infants. PLoS ONE 2018, 13, e0200603. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Bertoni, E.; Bogni, F.; Bonaita, V.; Armano, C.; Moretti, A.; Baù, M.; Luini, C.; D’Auria, E.; Marinoni, M.; et al. Testing the cow’s milk-related symptom score (CoMiSSTM) for the response to a cow’s milk-free diet in infants: A prospective study. Nutrients 2019, 11, 2402. [Google Scholar] [CrossRef]
- Rossetti, D.; Cucchiara, S.; Morace, A.; Leter, B.; Oliva, S. Hypoallergenicity of a thickened hydrolyzed formula in children with cow’s milk allergy. World J. Clin. Cases 2019, 7, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, J.; Dong, G.; Liu, P.; Xiao, F.; Li, W.; Wang, L.; Wu, Q. Assessment of cow’s milk-related symptom scores in early identification of cow’s milk protein allergy in Chinese infants. BMC Pediatr. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Sirin Kose, S.; Atakul, G.; Asilsoy, S.; Uzuner, N.; Anal, O.; Karaman, O. The efficiency of the symptom-based score in infants diagnosed with cow’s milk protein and hen’s egg allergy. Allergol. Immunopathol. 2019, 47, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Adriana, B.; Cristina, M.; Irina, P.; Tatiana, C.; Diana, D.; Adina, U.; Sergiu, C.; Larisia, M. Assessment of IgE-mediated and non-IgE-mediated cow’s milk protein allergy in children. ARS Med. Tomitana 2020, 25, 129–131. [Google Scholar] [CrossRef][Green Version]
- Selbuz, S.K.; Altuntaş, C.; Kansu, A.; Kırsaçlıoğlu, C.T.; Kuloğlu, Z.; İlarslan, N.E.Ç.; Doğulu, N.; Günay, F.; Topçu, S.; Ulukol, B. Assessment of cows milk-related symptom scoring awareness tool in young Turkish children. J. Paediatr. Child Health 2020, 56, 1799–1805. [Google Scholar] [CrossRef]
- Bigorajska, K.; Filipiak, Z.; Winiarska, P.; Adamiec, A.; Trent, B.; Vandenplas, Y.; Ruszczyński, M.; Szajewska, H. Cow’s milk-related symptom score in presumed healthy polish infants aged 0–6 months. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 154–162. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carvajal, E.; Peeters, S.; Balduck, N.; Jaddioui, Y.; Ribes-Koninckx, C.; Huysentruyt, K. The cow’s milk-related symptom score (CoMiSSTM): Health care professional and parent and day-to-day variability. Nutrients 2020, 12, 438. [Google Scholar] [CrossRef]
- Fierro, V.; Valluzzi, R.L.; Banzato, C.; Plaza, M.A.; Bosque, M.; Íbero, M.; Echeverría, L.A.Z.; Mennini, M.; Dahdah, L.; de Castellar, R.; et al. A well-tolerated new amino acid–based formula for cow’s milk allergy. Immun. Inflamm. Dis. 2020, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Petriashvili, M.; Jorjoliani, L. The peculiarities of clinical course of atopic dermatitis and the comorbid conditions in early infancy. Georgian Med. News 2020, 98, 53–57. [Google Scholar]
- Kozłowska-Jalowska, A.; Horvath, A.; Vandenplas, Y.; Szajewska, H. Retrospective and prospective determination of the cow’s milk-related symptom score (CoMiSSTM) values in symptomatic infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- El Desouky, A.I.; Anany, H.G.; Mohammed, I.S.I. Assessment of CoMiSS among children with cow’s milk allergy at Zagazig University Hospital. Egypt. J. Hosp. Med. 2021, 83, 838–843. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Gerlier, L.; Caekelbergh, K.; Nan-Study-Group; Possner, M. An observational real-life study with a new infant formula in infants with functional gastro-intestinal disorders. Nutrients 2021, 13, 3336. [Google Scholar] [CrossRef]
- Ursino, F.G.; Orsi, M.; Mehaudy, R.; Micheletti, M.E.; Parisi, C.; Petriz, N.; Parente, C.; Jauregui, M.B.; Pagoto, V. Cultural adaptation and validation of the Spanish version of the cow’s milk-related symptom score (CoMiSS) for cow’s milk protein allergy. Rev. Gastroenterol. M. 2021; in press. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zhao, Z.-Y.; Mukherjee, R.; Dupont, C.; Eigenmann, P.; Kuitunen, M.; Ribes Koninckx, C.; Szajewska, H.; von Berg, A.; Bajerová, K.; et al. Assessment of the cow’s milk-related symptom score (CoMiSS) as a diagnostic tool for cow’s milk protein allergy: A prospective, multicentre study in China (MOSAIC Study). BMJ Open 2022, 12, e056641. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Żołnowska, M.; Berni Canani, R.; Ludman, S.; Tengelyi, Z.; Moreno-Álvarez, A.; Goh, A.E.N.; Gosoniu, M.L.; Kirwan, B.-A.; Tadi, M.; et al. Effects of an extensively hydrolyzed formula supplemented with two human milk oligosaccharides on growth, tolerability, safety and infection risk in infants with cow’s milk protein allergy: A randomized, multi-center trial. Nutrients 2022, 14, 530. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Järvi, A.; Garreau, A.-S.; Mukherjee, R. Pooled analysis of the cow’s milk-related-symptom-score (CoMiSSTM) as a predictor for cow’s milk related symptoms. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 22–26. [Google Scholar] [CrossRef]
- Thompson, G.; Zhelev, Z.; Peters, J.; Khalid, S.; Briscoe, S.; Shaw, L.; Nunns, M.; Ludman, S.; Hyde, C. Symptom scores in the diagnosis of pediatric cow’s milk protein allergy: A systematic review. Pediatr. Allergy Immunol. 2021, 32, 1497–1507. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non–IgE- or mixed IgE/non–IgE-mediated gastrointestinal food allergies in the first years of life: Old and new tools for diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef]
- Gibbons, T.E.; Patil, S.N.; Frem, J.C.; Smith, C.; Wakwe, J.; Swearingen, C.J. Non-IgE-mediated cow milk allergy is linked to early childhood clusters of commonly seen illnesses: A pilot study. Clin. Pediatr. 2012, 51, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S.; Maynard, F.; Järvi, A.; Rytz, A.; Simons, P.J.; Heine, R.G.; Kuslys, M. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolyzed infant formulas. Allergy 2020, 75, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Sladkevicius, E.; Nagy, E.; Lack, G.; Guest, J.F. Resource implications and budget impact of managing cow milk allergy in the UK. J. Med. Econ. 2010, 13, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Arvola, T.; Ruuska, T.; Keränen, J.; Hyöty, H.; Salminen, S.; Isolauri, E. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Pediatrics 2006, 117, e760–e768. [Google Scholar] [CrossRef]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Di Nardo, G. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ. J. 2020, 13, 100471. [Google Scholar] [CrossRef]
- Schallreuter, K.; Levenig, C.; Berger, J.; Umbert, J.; Winkelmann, R.; Wegener, L.; Correia, O.; Chosidow, O.; Saiag, P.; Bastuji-Garin, S.; et al. Severity scoring of atopic dermatitis: The SCORAD index. Consensus report of the European task force on atopic dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Turner, D.; Griffiths, A.M.; Walters, T.D.; Seah, T.; Markowitz, J.; Pfefferkorn, M.; Keljo, D.; Waxman, J.; Otley, A.; LeLeiko, N.S.; et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm. Bowel Dis. 2012, 18, 55–62. [Google Scholar] [CrossRef]
- Muñoz-Urribarri, A.; Sabrá, A.; Sabrá, S.; Condorhuamán, Y.M. A trial of an anamnesis-based score applied as a diagnostic tool for cow’s milk protein allergy in children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e86–e89. [Google Scholar] [CrossRef]
| Crying | ||||
| ≤1 h/day | 0 | |||
| 1 to 1.5 h/day | 1 | |||
| 1.5 to 2 h/day | 2 | |||
| 2 to 3 h/day | 3 | |||
| 3 to 4 h/day | 4 | |||
| 4 to 5 h/day | 5 | |||
| ≥5 h/day | 6 | |||
| Regurgitation | ||||
| 0 to 2 episodes/day | 0 | |||
| ≥3 to ≤5 of small volume | 1 | |||
| >5 episodes of >1 coffee spoon | 2 | |||
| >5 episodes of ± half of the feed in <half of the feedings | 3 | |||
| Continuous regurgitations of small volume >30 min after each feeding | 4 | |||
| Regurgitation of half to complete volume of a feeding in at least half of the feedings | 5 | |||
| Regurgitation of the “complete feeding” after each feeding | 6 | |||
| Stools (Bristol scale) | ||||
| Type 1 and 2 (hard stools) | 4 | |||
| Type 3 and 4 (normal stools) | 0 | |||
| Type 5 (soft stools) | 2 | |||
| Type 6 (liquid stools, if unrelated to infection) | 4 | |||
| Type 7 (watery stools) | 6 | |||
| Skin Symptoms | absent | mild | moderate | severe |
| Atopic eczema | ||||
| Head, neck, and trunk | 0 | 1 | 2 | 3 |
| Arms, hands, legs, and feet | 0 | 1 | 2 | 3 |
| Urticaria | no | yes | ||
| 0 | 6 | |||
| Respiratory symptoms | ||||
| No respiratory symptoms | 0 | |||
| Slight symptoms | 1 | |||
| Mild symptoms | 2 | |||
| Severe symptoms | 3 |
| 1st Author [Ref] | Year | Type of Study * | Sample Size Included (PP) | Title | Conflict of Interest |
|---|---|---|---|---|---|
| Vandenplas [14] | 2013 | observation prospective | 116 (85) | Treating cow’s milk protein allergy: a double-blind randomized trial comparing two extensively hydrolyzed formulas with probiotics. | yes |
| Vandenplas [16] | 2014 | validation prospective | 116 (84) | A pilot study on the application of a symptom-based score for the diagnosis of cow’s milk protein allergy. | yes |
| Vandenplas [17] | 2014 | observation prospective | 40 (36) | Safety and tolerance of a new extensively hydrolyzed rice protein-based formula in the management of infants with cow’s milk protein allergy. | yes |
| Vandenplas [18] | 2014 | observation prospective | 72 (52) | Extensive protein hydrolysate formula effectively reduces regurgitation in infants with positive and negative challenge tests for cow’s milk allergy. | yes |
| Vandenplas [19] | 2016 | observation prospective | 71 (50) | Safety of a thickened extensive casein hydrolysate formula. | yes |
| Dupont [20] | 2016 | observation prospective | 30 | Tolerance and growth in children with cow’s milk allergy fed a thickened extensively hydrolyzed casein-based formula. | yes |
| Prasad [21] | 2018 | validation prospective | 83 | Cow’s milk-related Symptom Score as a predictive tool for cow’s milk allergy in Indian children aged 0–24 months. | no |
| Vandenplas [22] | 2018 | observation prospective | 891 (563) | The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. | yes |
| Salvatore [23] | 2019 | validation prospective | 47 | Testing the cow’s milk-related symptom score (CoMiSS) for the response to a cow’s milk-free diet in infants: a prospective study. | no |
| Rossetti [24] | 2019 | observation prospective | 30 (29) | Hypoallergenicity of a thickened hydrolyzed formula in children with cow’s milk allergy. | no |
| Zeng [25] | 2019 | validation prospective | 38 | Assessment of Cow’s milk-related symptom scores in early identification of cow’s milk protein allergy in Chinese infants. | no |
| Sirin Kose [26] | 2019 | validation prospective | 49 CMA 39 HEA 24 CMA + HEA | The efficiency of the symptom-based score in infants diagnosed with cow’s milk protein and hen’s egg allergy. | no |
| Balasa [27] | 2019 | observation retrospective | 40 | Assessment of IgE-Mediated and Non-IgE-Mediated Cow’s Milk Protein Allergy in Children. | no |
| Selbuz [28] | 2020 | validation prospective | 168 | Assessment of cow’s milk-related symptom scoring awareness tool in young Turkish children. | no |
| Bigorajska [29] | 2020 | observation prospective | 226 | Cow’s Milk-Related Symptom Score in Presumed Healthy Polish Infants Aged 0–6 Months. | no |
| Vandenplas [30] | 2020 | observation prospective | 220 (Spain 148 Belgium 72) | The Cow’s Milk-Related Symptom Score (CoMiSSTM): Health Care Professional and Parent and Day-to-Day Variability. | yes |
| Fierro [31] | 2020 | observation prospective | 41 (30) | A well-tolerated new amino acid–based formula for cow’s milk allergy. | yes |
| Petrashvili [32] | 2020 | validation prospective | 68 | The peculiarities of clinical course of atopic dermatitis and the comorbid conditions in early infancy. | no |
| Kozlowska–Jalowska [33] | 2021 | observation prospective | 110 | Retrospective and prospective determination of the Cow’s Milk-related Symptom Score (CoMiSS™) values in symptomatic infants. | no |
| El Desouky [34] | 2021 | observation prospective | 120 | Assessment of CoMiSS among children with cow’s milk allergy at Zagazig University Hospital. | no |
| Vandenplas [35] | 2021 | observation prospective | 196 (171) | An observational real-life study with a new infant formula in infants with functional gastro-intestinal disorders. | yes |
| Vandenplas [15] | 2021 | observation prospective | 268 (208) | How are infants suspected to have cow’s milk allergy managed? A real world study report. | yes |
| Ursino [36] | 2021 | observation prospective | 32 | Cultural adaptation and validation of the Spanish version of the Cow’s Milk-related Symptom Score (CoMiSS) for cow’s milk protein allergy. | no |
| Vandenplas [37] | 2022 | validation prospective | 299 (250) | Assessment of the Cow’s Milk-related Symptom Score (CoMiSS) as a diagnostic tool for cow’s milk protein allergy- A prospective, multicenter study in China (MOSAIC study). | yes |
| Vandenplas [38] | 2022 | validation prospective | 194 (137) | Effects of an extensively hydrolyzed formula supplemented with two human milk oligosaccharides on growth, tolerability, safety and infection risk in infants with cow’s milk protein allergy: a randomized, multicenter trial. | yes |
| Vandenplas [39] | 2017 | validation | 170 | Pooled analysis of the Cow’s Milk-related-Symptom-Score (CoMiSS™) as a predictor for cow’s milk related symptoms. | yes |
| Thompson [40] | 2021 | systematic review | 15 studies | Symptom scores in the diagnosis of pediatric cow’s milk protein allergy: A systematic review. | no |
| Calvani [41] | 2020 | review | 13 studies and 10 congress abstracts | Non-IgE- or mixed IgE/non-IgE-mediated gastrointestinal food allergies in the first years of life: old and new tools for diagnosis. | no |
| Publication [Ref] | Country F/M | Type of Milk FF/BF/FF + Bf at Enrollment | Gestational Age | Age at Enrolment Median (IQR) | CoMiSS Median (IQR) | CoMiSS Mean ± SD | P 95 | CoMiSS ≥ 12 N (%) | CoMiSS >9 N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Vandenplas [22] | Belgium Italy Spain Poland | 204/283/76 | on term | 8.7 (1.9) weeks | 3 (1–5) | 3.7 ± 2.9 | 9 | 9 (1.5%) | 28 (5) % |
| Bigorajska [29] | Poland | 34/176/16 | median (IQR) 39 (39–40) weeks | 4 (3–4) months | 4 (2–7) | 4.7 ± 2.9 | 11 | 11 (4.9) % | n.r. |
| Salvatore [23] | Italy | n.r. | n.r. | 3 (n.r) months | 3 (0–11) (min-max) IQR n.r. | n.r. | n.r. | 0 | 1(1.1%) |
| Vandenplas Spanish cohort [30] | Spain | n.r. | n.r. | 2.3 (2.9) months | n.r. | n.r. | n.r. | 7 (4.7%) | 11(7.4%) |
| Vandenplas Belgian cohort [30] | Belgium | n.r. | n.r. | 3 (0.5) months | 3.7 (5.0) | n.r. | n.r. | 1 (1.4%) | 1(1.4%) |
| Petriashvili [32] | Georgia | n.r. | n.r. | up to 2 years | n.r. | 3.6± 1.8 | n.r. | 0% | n.r. |
| Age | No | Min | P05 | P25 | Median | P75 | P95 | Max | |
|---|---|---|---|---|---|---|---|---|---|
| V | <1 mo | 139 | 0 | 0 | 1 | 3 | 5 | 8 | 10 |
| B | 1 mo | 28 | 3 | 3 | 5 | 6.5 | 9 | 13.3 | 15 |
| V | 1–2 mo | 129 | 0 | 0 | 2 | 4 | 6 | 10 | 14 |
| B | 2 mo | 22 | 0 | 1 | 4 | 6.5 | 8.8 | 11 | 12 |
| V | 2–3 mo | 94 | 0 | 0 | 2 | 4 | 6 | 9 | 10 |
| B | 3 mo | 55 | 0 | 0 | 1 | 3 | 5 | 9.3 | 14 |
| V | 3–4 mo | 88 | 0 | 0 | 1 | 4 | 6 | 11 | 15 |
| B | 4 mo | 72 | 0 | 0 | 2 | 4 | 7 | 11.5 | 15 |
| V | 4–6 mo | 113 | 0 | 0 | 1 | 3 | 5 | 8 | 12 |
| B | 5 mo | 34 | 0 | 1.3 | 3 | 4 | 6 | 9 | 10 |
| B | 6 mo | 15 | 0 | 0 | 0.5 | 4 | 4.5 | 9 | 9 |
| Publication [Ref] Clinical Presentation | Age | Inter-rater Variability (IV) | No. of Subjects | Repetition Variability (RV) | Total Scores Compared (IV) | Total Scores Compared (RV) | CoMiSS ≥ 12 N | CoMiSS ≥ 10 | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Vandenplas Spanish cohort [30] PH | median (IQR) 2.3 (2.9) mo | HCP vs. parent | 148 vs. 148 | no | ICC 0.981 95% CI 0.974–0.986 p < 0.001 | n.r. | 7/8 HCP/parent | 11/12 HCP/parent | excellent agreement |
| Vandenplas Belgian cohort [30] PH | median (IQR) 3 (0.5) mo | HCP vs. parent *° | 72 vs. 72 ° 72 vs. 72 vs. 72 °° | °° parent 3 times on 3 consecutive days ** | ICC 0.53 95% CI 0.34–0.68 p < 0.001 | ICC 0.93 95% CI 0.90–0.96 p < 0.001 | n.r. | n.r. | * moderate ICC estimate ** excellent ICC estimate |
| Kozlowska-Jalowska [33] Symptomatic | mean (±SD) 18.2 (±11.7) weeks | no | 110 vs. 110 | 1 HCP retrospective vs. prospective | n.r. | MD−1.5 95% CI −2.0 to −1.0 p < 0.001 | 17/11 (p = 0.109) retrospective vs. prospective | 32/19 (p = 0.004) retrospective vs. prospective | scores determined retrospectively and prospectively differed |
| Ursino [36] Symptomatic | median (IQR) 3 (2) mo | HCP 1 vs. HCP 2 | 32 vs. 32 | no | ICC 0.80 95% CI 0.63–0.9 p < 0.001. | n.r. | n.r. | n.r. | substantial ICC estimate |
| Publication [Ref] | No. of Subjects (PP) | Age at Enrolment | Baseline CoMiSS Mean ± SD (min-max) | Elimination Period | Mean ± SD (min-max) After Elimination CMA+ | Mean ± SD (min-max) After Elimination CMA− | Cut-Off | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prasad [21] ° | 83 | 12.5 ± 6.4 w mean ±SD | 16.2 ± 6.8 (2–32) | 15 days | n.r. | n.r. | ≥12 | 0.68 | 77% | 66% | 93% | 33% |
| Zeng [25] ° | 38 | 1–6 mo: 33 7–12 mo: 5 (2–12 mo) (min-max) | 7.4 ± 2.3 (CMPA +) | 4 weeks | n.r. | n.r. | 5.5 | 0.89 | 87.5% | 78.6% ** | n.r. | n.r. |
| Salvatore [23] ° | 47 | median 3 (10 d–8 mo) (min-max) | 8 (2–16) median (min-max) | 2–4 weeks | 2 (n.r.) median (IQR) | n.r. | ≥9 {≥12} | 0.91 {n.r.} | 84% {37%} | 85% {92%} | 80% {77%} | 88% {68%} |
| Selbuz [28] ° CoMiSS ≥12 and decrease after elimination ≥3 | 168 | 87 (16–330) days (limit n.r.) | 13.6 ± 1.9 (12–22) | 4 weeks | 5.8 | 5.9 | 12.5 | 0.57 | 64.8% | 54.4% | n.r. | n.r. |
| Vandenplas [37] ° | 299 (250) | 16.1 (9.9–20.8) median (IQR) | 8 (5–10) median (IQR); 0–24 (min-max) | 2 weeks | 5 (3–7) median (IQR) | 3.5 (2–7) median (IQR) | ≥6 {≥12} | 0.67 {n.r.} | 78.8% {20.3%} | 51.5% {87.9%} | 91.4% {91.7%} | 27.0% {14.4%} |
| El Desouky [34] ° | 120 | 6.60 ± 4.82 mo mean ± SD | 11.2 ± 2.8 (n.r.-n.r.) | n.r. | n.r. | n.r. | >12 | n.r. | 86.4% | 93.4% | 88.3% | 92.2% |
| Sirin Kose [26] ° | 49 | 4.7 ± 1.9 mo mean ± SD *** | 13 (5) median (IQR) | 4 weeks | 4 (4) median (IQR) | n.r. | ≥10 {≥12} | n.r. n.r. | 87,8% {69,4%} | n.r. | n.r. | n.r. |
| Vandenplas [16] ° CoMiSS ≥12 | 116 (84) | 72 (53–122) days median (IQR) *** | 13.65 ± 1.75 (12–21) | 4 weeks | 5.12 ± 3.39 (0–18) | 6.81 ± 3.01 (1–13) | ≥12 | n.r. | n.r. | n.r. | n.r. | n.r. |
| Balasa [27] * | 40 | n.r. | n.r. | n.r. | n.r. | n.r. | ≥12 | n.r. | n.r. | n.r. | n.r. | n.r. |
| Vandenplas [15] ° | 268 (208) | 18,4 (1.4–80.6) w median (min-max) | 11.1± n.r. median 11.0 | 3 weeks | 4.2 after elimination | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | |
| Vandenplas [39] ° | 170 | 86 (60–122) median (Q1–Q3) | 13 (12–15) median (Q1–Q3) | 1 month | 5(3–7) median (Q1–Q3) | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | |
| Publication [Ref] | Inclusion Criteria | Age at Inclusion | CoMiSS Before Treatment Initiation | Elimination Period | Follow Up Period with Treatment Formula | CoMiSS During Treatment |
|---|---|---|---|---|---|---|
| Vandenplas [14] | Suspicion of CMA | median (IQR) days eWHF 80 (57–136) eCHF 64 (48–114) | mean ± SD (range) eWHF 13.58 ± 2.20 (5–21) eCHF 13.79 ± 1.47 (12–17) | 1 mo | 10 months | mean ± SD 1 mo: 5.16 ± 3.16 2 mo: 3.98 ± 2.92 4 mo: 2.79 ± 2.63 6 mo: 2.11 ± 2.17 8 mo: 1.33 ± 1.79 10 mo: 1.04 ± 1.02 |
| Vandenplas [17] | CMA proven before inclusion- positive challenge with CMP | mean ± SD months 3.4 ± 1.5 median (range) 3 (0–6) months | mean ± SD 13.50 ± 5.2 | 1 mo | 6 months | mean ± SD 1 mo: 3.5 ± 2.3 3 mo: 2.4 ± 1.9 6 mo: 1.5 ± 2.0 |
| Vandenplas [18] | Suspicion of CMA | mean ± SD days total 87.5 ± 46.20 TeCHF 80.00 ± 44.00 NTeCHF 94.7 ± 47.7 | mean ± SD total 14.1 ± 3.5 TeCHF 14 ± 3.6 NTeCHF 14.1 ± 3.4 CMA+ 14.3 ± 3.4 CMA- 13.9 ± 3.8 | 1 mo | 6 months | decrease after 1 mo mean ± SD total: −7.4 ± 5.5 TeCHF: −7.7 ± 5.2 NTeCHF: −7.2 ± 5.7 CMA+: −8.6 ± 5.3 CMA-: −5.9 ± 3.2 |
| Vandenplas [19] | Suspicion of CMA | mean ± SD days total 90.51 ± 49.02 TeCHF 80.77 ± 43.17 NTeCHF 99.97 ± 43.17 | mean ± SD total 14.1 ± 3.5 TeCHF 14 ± 3.6 NTeCHF 14.1 ± 3.5 CMA+ 14.3 ± 3.4 CMA? 13.9 ± 3.8 | 1 mo | 6 months | decrease after 1 mo mean ± SD Total: −7.5 ± 5.2 TeCHF: −7.6 ± 5.2 NTeCHF: −7.4 ± 5.3 CMA+: −8.4 ± 5.2 CMA?: −6.5 ± 4.5 |
| Dupont [20] | CMA proven before inclusion | mean ± SD months 4.8 ± 3.0 | n.r. | n.r. | 120 days | mean ± SD Day 0: 7.4 ± 4.4 Day 14: 3.2 ± 2.3 |
| Rossetti [24] | CMA proven before inclusion | mean ± SD months 8.03 ± 7.43 median (range) 6 (1–31) months | n.r. | n.r. | 3 months | mean ± SD Day 0: 1.4 ± 2.0 Day 7: 0.7 ± 1.2 |
| Fierro [31] | CMA proven before inclusion | mean ± SD months 2.1 ± 2.52 | n.r. | n.r. | 1 week | mean ± SD Visit 1: 1.37 ± 1.59 Visit 4:0.75 ± 0.55 |
| Vandenplas [35] | FGID | mean ± SD months 1.5 ± 1.0 median (Q1–Q3) 1.1 (07–2.1) months | mean ± SD (range) 6.46 ± 3.09 (0–15) median (Q1–Q3) 6 (4–8) | 7 d | 2 weeks | mean ± SD day 3: 5.21 ± 2.90 day 7: 4,98 ± 2.93 day 14: 4.92 ± 3.06 |
| Petriashvili [32] | AD | up to 2 years | SCORAD mean (SD) <20 7.7 (3.0) 20–40 7.3 (3.9) >40 11.3 (5.0) | n.r. | n.r. | n.r. |
| Vandenplas [38] | CMA | mean ± SD months test formula 3.2 ±1.7 control formula 3.2 ± 1.7 | test formula 12.08 (95%CI, 10.75–12.63) control formula 11.65 (95% CI, 10.75–12.63) | 1 mo | 6 months (follow up n.r.) | 1 mo mean test formula 3.38 (95% CI, 1.91–2.69) control formula 2.73 (95%CI, 1.42–291) |
| Publication [Ref] | Group | Crying Score | Crying Score | p | Regurgitation Score | Regurgitation Score | p | Stool Score | Stool Score | p |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After CM Elimination | Baseline | After CM Elimination | Baseline | After CM Elimination | |||||
| Sirin Kose [26] | CMA+ | median (Q1–Q3) 1 (0–4.5) | n.r. | n.r. | median (Q1–Q3) 1 (0–4) | n.r. | n.r. | median (Q1–Q3) 4 (4–6) | n.r. | n.r. |
| Salvatore [23] | CM-free diet responders | 2.7 (1.9) * | n.r. | n.r. | 2.3 (1.9) ** | n.r. | n.r. | 2.6 (1.9) *** | n.r. | n.r. |
| Salvatore [23] | CM-free diet non -responders | 2.1 (1.9) * | n.r. | * NS | 1.8 (1.9) ** | n.r. | ** NS | 0.78 (1.8) *** | n.r. | *** NS |
| Vandenplas [14] | eWHF | 4.2 (2.0) | 1.1 (1.4) | n.r. | 2.6 (1.7) | 1.1 (1.2) | n.r. | 3.5 (1.5) | 1.8 (1.8) | n.r. |
| Vandenplas [14] | eCHF | 4.6 (1.8) | 1.5 (1.7) | n.r. | 3.0 (1.6) | 1.8 (1.2) | n.r. | 3.5 (1.3) | 1.4 (1.6) | n.r. |
| Vandenplas [17] | CMA+ | 3.8 (2.0) | 0.5 (0.8) | <0.001 | 2.4 (2.2) | 0.6 (0.9) | < 0.001 | normal/ abnormal 5.3%/94.7% | normal/ abnormal 52.6%/47.4% | <0.0001 |
| Vandenplas [18] | CMA+ | 3.7 (2.3) | 1.1 (1.6) | <0.001 | 3.2 (1.3) | 0.9 (0.9) | <0.001 | normal 14.7% | normal 44.1%% | 0.0124 |
| Vandenplas [18] | CMA- | 3.0 (2.1) | 0.9 (1.1) | <0.001 | 2.8 (1.2) | 0.9 (0.9) | <0.001 | normal 11.4% | normal 17.1% | 0.527 |
| Vandenplas [19] | CMA+ T-eCHF | n.r. | decrease 2.8 (2.4) | <0.001 | n.r. | decrease 2.3 (1.3) | <0.001 | normal 9.5% | normal 42.9% | 0.020 |
| Vandenplas [19] | CMA+ NT-eCHF | n.r. | decrease 1.9 (2.0) | <0.001 | n.r. | decrease 2.2 (1.8) | <0.001 | normal 12.5% | normal 37.5% | 0.157 |
| Dupont [20] ° | CMA+ | 1.7 (1.1) | 0.8 (0.6) | n.r. | 1.6 (1.6) | 0.9 (1.0) | n.r. | normal 53.3% | normal 66.7% | n.r. |
| Vandenplas [35] | FGIDs | 2.24 | 1.23 | n.r. | 1.31 | 0.72 | n.r. | hard/norm/soft/fluid/watery 19%/21%/36%/21%/4% | hard/norm/soft/fluid/watery 2%/17%/36%/37%/3% | n.r. |
| Vandenplas [37] | CMA+ | median (IQR) 0 (0–2) range 0–6 | median (IQR) 0 (0–1) range 0–5 | <0.001 | median (IQR) 0 (0–1) range 0–6 | median (IQR) 0 (0–0) range 0–3 | <0.001 | median (IQR) 4 (2–4) range 0–6 | median (IQR) 2 (0–4) range 0–6 | <0.0001 |
| Vandenplas [37] | CMA- | median (IQR) 0 (0–2) range 0–6 | median (IQR) 0 (0–1) range 0–3 | 0.089 | median (IQR) 0 (0–1) range 0–4 | median (IQR) 0 (0–0.25) range 0–5 | 0.24 | median (IQR) 2 (0–4) range 0–6 | median (IQR) 0 (0–2) range 0–4 | 0.03 |
| Publication [Ref] | Group | Eczema Score | Eczema Score | p | Urticaria Score | Urticaria Score | p | Respiratory Score | Respiratory Score | p |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Baseline | After CM Elimination | Baseline | After CM Elimination | Baseline | After CM Elimination | |||||
| Sirin Kose [26] | CMA+ | median (Q1–Q3) 2 (0–3.5) | n.r. | n.r. | median (Q-Q3) 0 (0–6) | n.r. | n.r. | Median (Q1–Q3) 0 (0–5) | n.r. | n.r. |
| Salvatore [23] | CM-free diet responders | 2.4 (2.2) **** | n.r. | n.r. | n.r. | n.r. | n.r. | 0.6 (0.7) ***** | n.r. | n.r. |
| Salvatore [23] | CM-free diet non-responders | 0.6 (2.2) **** | n.r. | **** NS | n.r. | n.r. | n.r. | 0.6 (0,8) ***** | n.r. | ***** NS |
| Vandenplas [14] | eWHF | 2.1 (2.0) | 0.8 (1.1) | n.r. | 0.4 (1.6) | 0 (0) | n.r. | 0.8 (1.0) | 0.4 (0.8) | n.r. |
| Vandenplas [14] | eCHF | 1.8 (1.9) | 1.0 (1.5) | n.r. | 0.1 (0.5) | 0 (0) | n.r. | 0.8 (0.9) | 0.4 (0.6) | n.r. |
| Vandenplas [17] | CMA+ | n.r. | n.r. | present 15.8% | present 0% | <0.02 | n.r. | n.r. | n.r. | |
| Vandenplas [18] | CMA+ | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. |
| Vandenplas [18] | CMA- | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. |
| Vandenplas [19] | CMA+ T-eCHF | n.r. | decrease 0.8 (1.3) | <0.01 | n.r. | n.r. | n.r. | n.r. | decrease 0.6 (0.7) | 0.002 |
| Vandenplas [19] | CMA+ NT-eCHF | n.r. | decrease 1.9 (1.6) | <0.01 | n.r. | n.r. | n.r. | n.r. | decrease 0.6 (0.7) | 0.002 |
| Dupont [20] ° | CMA+ | absent 70% | absent 80% | n.r. | absent 76.7% | absent 100% | n.r. | absent 83.3% | absent 93.3% | n.r. |
| Vandenplas [35] | FGIDs | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. |
| Vandenplas [37] | CMA+ | median (IQR) 2 (1–4) range 0–6 | median (IQR) 1 (1–2) range 0–6 | <0.0001 | median (IQR) 0 (0–0) range 0–6 | median (IQR) 0 (0–0) range 0–6 | 0.06 | median (IQR) 1 (0–1) range 0–3 | median (IQR) 0 (0–1) range 0–2 | <0.0001 |
| Vandenplas [37] | CMA- | median (IQR) 1 (1–2) range 0–6 | median (IQR) 1 (0–1) range 0–5 | 0.017 | median (IQR) 0 (0–0) range 0–6 | median (IQR) 0 (0–0) range 0–6 | 0.57 | median (IQR) 1 (0–1) range 0–2 | median (IQR) 1 (0–1) range 0–1 | 0.0056 |
| A. Baseline CoMiSS | ||||
| CoMiSS Cut-Off | Sensitivity | Specificity | PPV | NPV |
| ≥5 | 88.5% | 33.3% | 89.7% | 30.6% |
| ≥6 | 78.8% | 51.5% | 91.4% | 27.0% |
| ≥7 | 68.2% | 57.6% | 91.4% | 21.6% |
| ≥8 | 56.2% | 60.6% | 90.4% | 17.4% |
| ≥9 | 43.8% | 69.7% | 90.5% | 15.9% |
| ≥10 | 35.9% | 69.7% | 88.6% | 14.2% |
| ≥11 | 25.8% | 81.8% | 90.3% | 14.4% |
| ≥12 | 20.3% | 87.9% | 91.7% | 14.4% |
| B. Baseline CoMiSS plus ≥50% reduction from baseline to Visit 2 | ||||
| CoMiSS Cut-Off | Sensitivity | Specificity | PPV | NPV |
| ≥5 | 38.1% | 62.5% | 87.2% | 13.1% |
| ≥6 | 35.3% | 68.8% | 88.4% | 13.7% |
| ≥7 | 34.4% | 71.9% | 89.2% | 14.0% |
| ≥8 | 30.2% | 71.9% | 87.8% | 13.3% |
| ≥9 | 25.6% | 78.1% | 88.7% | 13.5% |
| ≥10 | 20.5% | 78.1% | 86.3% | 12.8% |
| ≥11 | 16.3% | 84.4% | 87.5% | 13.0% |
| ≥12 | 14.0% | 87.5% | 88.2% | 13.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajerova, K.; Salvatore, S.; Dupont, C.; Eigenmann, P.; Kuitunen, M.; Meyer, R.; Ribes-Koninckx, C.; Shamir, R.; Szajewska, H.; Vandenplas, Y. The Cow’s Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool. Nutrients 2022, 14, 2059. https://doi.org/10.3390/nu14102059
Bajerova K, Salvatore S, Dupont C, Eigenmann P, Kuitunen M, Meyer R, Ribes-Koninckx C, Shamir R, Szajewska H, Vandenplas Y. The Cow’s Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool. Nutrients. 2022; 14(10):2059. https://doi.org/10.3390/nu14102059
Chicago/Turabian StyleBajerova, Katerina, Silvia Salvatore, Christophe Dupont, Philippe Eigenmann, Mikael Kuitunen, Rosan Meyer, Carmen Ribes-Koninckx, Raanan Shamir, Hania Szajewska, and Yvan Vandenplas. 2022. "The Cow’s Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool" Nutrients 14, no. 10: 2059. https://doi.org/10.3390/nu14102059
APA StyleBajerova, K., Salvatore, S., Dupont, C., Eigenmann, P., Kuitunen, M., Meyer, R., Ribes-Koninckx, C., Shamir, R., Szajewska, H., & Vandenplas, Y. (2022). The Cow’s Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool. Nutrients, 14(10), 2059. https://doi.org/10.3390/nu14102059







