Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies
Abstract
:1. Introduction
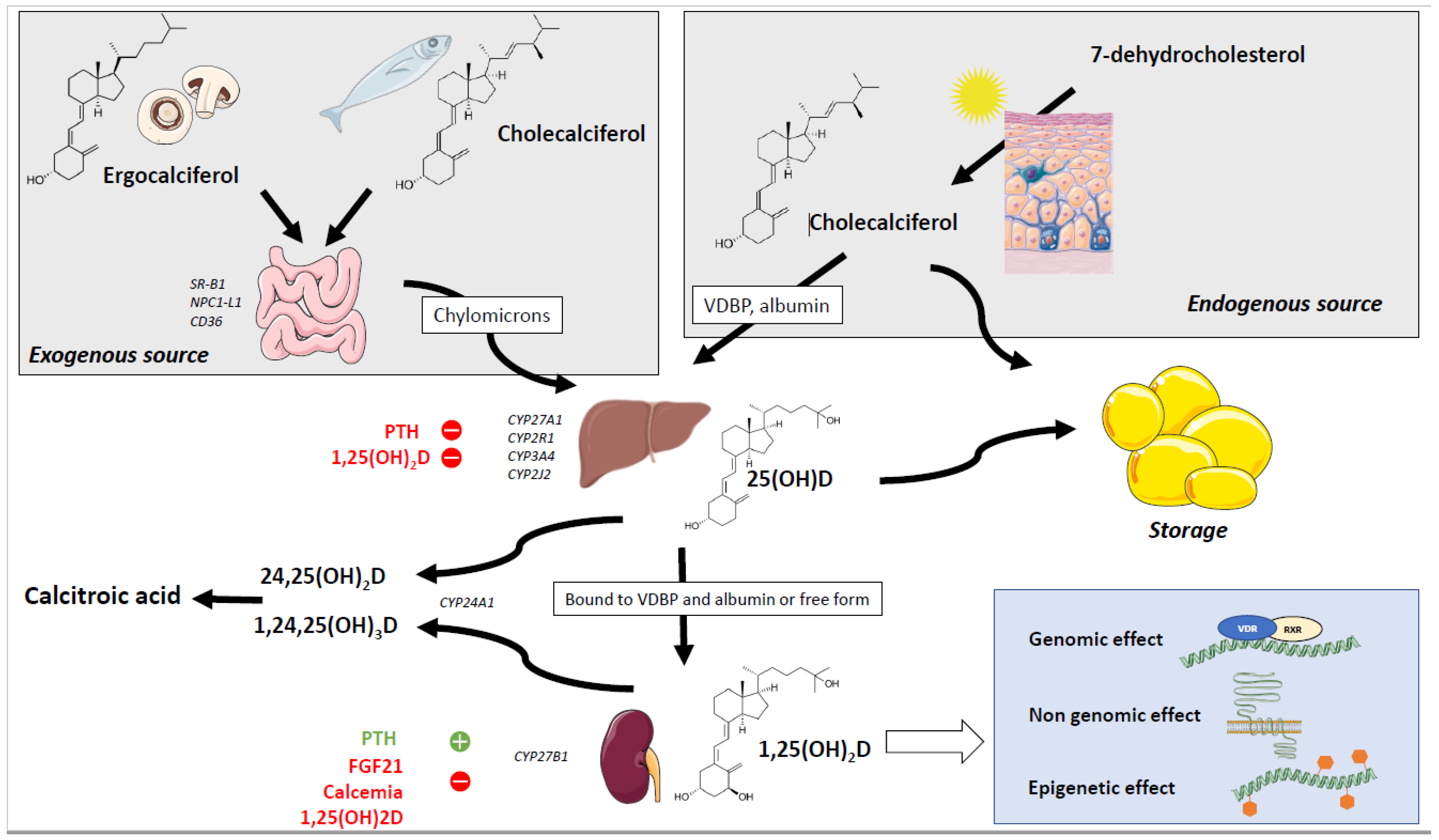
2. Sources and Absorption of Vitamin D
3. Metabolism of the Vitamin D
3.1. Hydroxylation of Vitamin D and Metabolites
3.2. Mechanisms of Action of Vitamin D
4. Relationship between Obesity and Vitamin D in Rodents
5. Relationship between VD and Obesity in Human Observational Studies
6. Relationship between VD and Obesity in Human Interventional Studies
| In Humans | References | |
|---|---|---|
| 25(OH)D plasma levels | Reduced in obesity, inversely correlated to markers of obesity and adiposity | [94,95,96,97,98,99] |
| Free 25(OH)D plasma levels | Reduced in obesity | [85] |
| 1,25(OH)2D plasma levels | Reduced in obesity | [100,101] |
| Impact of obesity on VD supplementation | Obesity reduced the efficacity of VD supplementation | [106] |
| Low 25(OH)D predictor for obesity onset (Prospective studies) | Yes | [107,108,109,110,111,112] |
| Effect of polymorphisms in genes coding for proteins involved in VD metabolism (Genetic studies) | Relationship between obesity and SNP in VDR, VDBP, and Cyp27b1 in small cohorts No link between polymorphisms and obesity in larger cohorts | [113,114,115,116,117] [118,119] |
| Causal effect of low 25(OH)D on obesity (Mendelian randomization) | No | [120] |
| Causal effect of obesity on low 25(OH)D (Mendelian randomization) | Yes | [120] |
| Weight lost increase 25(OH)D plasma levels | Yes | [121] |
| Impact of VD supplementation on obesity (RCT) | Lack of clear-cut results (2 meta-analysis showing no effect, 1 meta-analysis showing improvement of obesity parameters following VD supplementation | [123,124,125] |
7. Maternal Vitamin D Deficiency and Links to Obesity and Adipose Tissue Biology
7.1. Lines of Epidemiological and Clinical Evidence for a Link between VD and Obesity in Offspring
7.2. Lines of Preclinical Evidence for a Link between VD and Obesity in Offspring
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency in 2010: Health benefits of vitamin D and sunlight: A D-bate. Nat. Rev. Endocrinol. 2011, 7, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Sliwinska, A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef]
- Nimitphong, H.; Park, E.; Lee, M.-J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pract. 2020, 14, 553–567. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A d-lightful solution for health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef]
- Heaney, R.P.; Armas, L.A.G.; French, C. All-Source Basal Vitamin D Inputs Are Greater Than Previously Thought and Cutaneous Inputs Are Smaller. J. Nutr. 2013, 143, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Savastano, S.; Di Somma, C.; Savanelli, M.C.; Nappi, F.; Albanese, L.; Orio, F.; Colao, A. Low serum vitamin D-status, air pollution and obesity: A dangerous liaison. Rev. Endocr. Metab. Disord. 2016, 18, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2013, 55, 1193–1205. [Google Scholar] [CrossRef]
- Reboul, E. Intestinal absorption of vitamin D: From the meal to the enterocyte. Food Funct. 2014, 6, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-soluble vitamin intestinal absorption: Absorption sites in the intestine and interactions for absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef]
- Hollander, D.; Muralidhara, K.S.; Zimmerman, A. Vitamin D-3 intestinal absorption in vivo: Influence of fatty acids, bile salts, and perfusate pH on absorption. Gut 1978, 19, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Margier, M.; Collet, X.; May, C.; Desmarchelier, C.; André, F.; Lebrun, C.; Defoort, C.; Bluteau, A.; Borel, P.; Lespine, A.; et al. ABCB1 (P-glycoprotein) regulates vitamin D absorption and contributes to its transintestinal efflux. FASEB J. 2018, 33, 2084–2094. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; DeLuca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.P.; Hollis, B.W.; Patel, S.B.; Patrick, K.S.; Bell, N.H. CYP3A4 is a Human Microsomal Vitamin D 25-Hydroxylase. J. Bone Miner. Res. 2003, 19, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Aiba, I.; Yamasaki, T.; Shinki, T.; Izumi, S.; Yamamoto, K.; Yamada, S.; Terato, H.; Ide, H.; Ohyama, Y. Characterization of rat and human CYP2J enzymes as Vitamin D 25-hydroxylases. Steroids 2006, 71, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.D.; Strugnell, S.; Back, D.W.; Jones, G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc. Natl. Acad. Sci. USA 1993, 90, 8668–8672. [Google Scholar] [CrossRef] [Green Version]
- Bell, N.H.; Epstein, S.; Greene, A.; Shary, J.; Oexmann, M.J.; Shaw, S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J. Clin. Investig. 1985, 76, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef]
- Park, J.M.; Park, C.Y.; Han, S.N. High fat diet-Induced obesity alters vitamin D metabolizing enzyme expression in mice. BioFactors 2015, 41, 175–182. [Google Scholar] [CrossRef]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Tourniaire, F.; Mounien, L.; Landrier, J.-F. Four days high fat diet modulates vitamin D metabolite levels and enzymes in mice. J. Endocrinol. 2021, 248, 87–93. [Google Scholar] [CrossRef]
- Elkhwanky, M.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues. JBMR Plus 2020, 4. [Google Scholar] [CrossRef]
- Aatsinki, S.-M.; Elkhwanky, M.-S.; Kummu, O.; Karpale, M.; Buler, M.; Viitala, P.; Rinne, V.; Mutikainen, M.; Tavi, P.; Franko, A.; et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes 2019, 68, 918–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.S.; Schoenmakers, I.; Bluck, L.J.C.; Ding, S.; Prentice, A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br. J. Nutr. 2011, 107, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Daiger, S.P.; Schanfield, M.S.; Cavalli-Sforza, L.L. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc. Natl. Acad. Sci. USA 1975, 72, 2076–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E.C. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Haddad, J.G.; Jennings, A.S.; Aw, T.C. Vitamin D Uptake and Metabolism by Perfused Rat Liver: Influences of Carrier Proteins. Endocrinology 1988, 123, 498–504. [Google Scholar] [CrossRef]
- Safadi, F.F.; Thornton, P.; Magiera, H.; Hollis, B.W.; Gentile, M.; Haddad, J.G.; Liebhaber, S.A.; Cooke, N.E. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Investig. 1999, 103, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Cubilin, the Intrinsic Factor-Vitamin B12 Receptor in Development and Disease. Curr. Med. Chem. 2020, 27, 3123–3150. [Google Scholar] [CrossRef]
- Gacad, M.A.; Chen, H.; Arbelle, J.E.; LeBon, T.; Adams, J.S. Functional Characterization and Purification of an Intracellular Vitamin D-binding Protein in Vitamin D-resistant New World Primate Cells. J. Biol. Chem. 1997, 272, 8433–8440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrier, J.F.; Karkeni, E.; Marcotorchino, J.; Bonnet, L.; Tourniaire, F. Vitamin D modulates adipose tissue biology: Possible consequences for obesity? Proc. Nutr. Soc. 2016, 75, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Tuoresmäki, P.; Väisänen, S.; Neme, A.; Heikkinen, S.; Carlberg, C. Patterns of Genome-Wide VDR Locations. PLoS ONE 2014, 9, e96105. [Google Scholar] [CrossRef] [Green Version]
- Kazemian, E.; Amouzegar, A.; Akbari, M.E.; Moradi, N.; Gharibzadeh, S.; Jamshidi-Naeini, Y.; Khademolmele, M.; As’habi, A.; Davoodi, S.H. Vitamin D receptor gene polymorphisms affecting changes in visceral fat, waist circumference and lipid profile in breast cancer survivors supplemented with vitamin D3. Lipids Health Dis. 2019, 18, 161. [Google Scholar] [CrossRef] [Green Version]
- Turano, C.; Gaucci, E.; Grillo, C.; Chichiarelli, S. ERp57/GRP58: A protein with multiple functions. Cell. Mol. Biol. Lett. 2011, 16, 539–563. [Google Scholar] [CrossRef]
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 281–285. [Google Scholar] [CrossRef]
- Chen, J.; Doroudi, M.; Cheung, J.; Grozier, A.L.; Schwartz, Z.; Boyan, B.D. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1α,25(OH)2D3. Cell. Signal. 2013, 25, 2362–2373. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Nemere, I.; Hintze, K. Novel hormone “receptors”. J. Cell Biochem. 2008, 103, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.T.C.; Booth, D.R.; Parnell, G.P. Vitamin D and its Effects on DNA Methylation in Development, Aging, and Disease. Mol. Nutr. Food Res. 2020, 64, e2000437. [Google Scholar] [CrossRef] [PubMed]
- Nur, S.M.; Rath, S.; Ahmad, V.; Ahmad, A.; Ateeq, B.; Khan, M.I. Nutritive vitamins as epidrugs. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.-C.; Tourniaire, F.; Landrier, J.-F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid Biochem. Mol. Biol. 2018, 185, 39–46. [Google Scholar] [CrossRef]
- Seldeen, K.L.; Pang, M.; Rodriguez-Gonzalez, M.; Hernandez, M.; Sheridan, Z.; Yu, P.; Troen, B.R. A mouse model of vitamin D insufficiency: Is there a relationship between 25(OH) vitamin D levels and obesity? Nutr. Metab. 2017, 14, 26. [Google Scholar] [CrossRef] [Green Version]
- Valle, M.; Mitchell, P.L.; Pilon, G.; St-Pierre, P.; Varin, T.; Richard, D.; Vohl, M.-C.; Jacques, H.; Delvin, E.; Levy, E.; et al. Cholecalciferol Supplementation Does Not Prevent the Development of Metabolic Syndrome or Enhance the Beneficial Effects of Omega-3 Fatty Acids in Obese Mice. J. Nutr. 2021, 151, 1175–1189. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Borges, C.C.; Salles, A.F.; Bringhenti, I.; Souza-Mello, V.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Adverse effects of vitamin D deficiency on the Pi3k/Akt pathway and pancreatic islet morphology in diet-induced obese mice. Mol. Nutr. Food Res. 2015, 60, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean Phenotype and Resistance to Diet-Induced Obesity in Vitamin D Receptor Knockout Mice Correlates with Induction of Uncoupling Protein-1 in White Adipose Tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.; Erben, R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2012, 97, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; DeMay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lou, Y.; Kong, J. VDR regulates energy metabolism by modulating remodeling in adipose tissue. Eur. J. Pharmacol. 2019, 865, 172761. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted Expression of Human Vitamin D Receptor in Adipocytes Decreases Energy Expenditure and Induces Obesity in Mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.G.; D’Angelo, J.; Drelich, J.; Welsh, J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J. Steroid Biochem. Mol. Biol. 2015, 164, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Tao, T.; Kobelski, M.M.; Saini, V.; Demay, M.B. Adipose-specific VDR Deletion Leads to Hepatic Steatosis in Female Mice Fed a Low-Fat Diet. Endocrinology 2021, 163. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Kang, S.; Goyal, A.; Zhang, K.; You, J.Y.; Carey, M.; Jain, S.; Bhansali, S.; Kehlenbrink, S.; Guo, P.; et al. Insulin-sensitizing effects of vitamin D repletion mediated by adipocyte vitamin D receptor: Studies in humans and mice. Mol. Metab. 2020, 42, 101095. [Google Scholar] [CrossRef]
- Jahn, D.; Dorbath, D.; Kircher, S.; Nier, A.; Bergheim, I.; Lenaerts, K.; Hermanns, H.M.; Geier, A. Beneficial Effects of Vitamin D Treatment in an Obese Mouse Model of Non-Alcoholic Steatohepatitis. Nutrients 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Gregorio, B.; Sampaio, F.; Sanchez, R.; Risopatrón, J. Role of Vitamin D in the Development of Obesity. Int. J. Morphol. 2017, 35, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Futawaka, K.; Koyama, R.; Fukuda, Y.; Hayashi, M.; Imamoto, M.; Miyawaki, T.; Kasahara, M.; Tagami, T.; Moriyama, K. Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 2016, 23, 56. [Google Scholar] [CrossRef] [Green Version]
- Aldekwer, S.; Desiderio, A.; Farges, M.-C.; Rougé, S.; Le Naour, A.; Le Guennec, D.; Goncalves-Mendès, N.; Mille-Hamard, L.; Momken, I.; Rossary, A.; et al. Vitamin D supplementation associated with physical exercise promotes a tolerogenic immune environment without effect on mammary tumour growth in C57BL/6 mice. Eur. J. Nutr. 2021, 60, 2521–2535. [Google Scholar] [CrossRef]
- Li, R.; Guo, E.; Yang, J.; Li, A.; Yang, Y.; Liu, S.; Liu, A.; Jiang, X. 1,25(OH)2D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity 2017, 25, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Geldenhuys, S.; Hart, P.H.; Endersby, R.; Jacoby, P.; Feelisch, M.; Weller, R.B.; Matthews, V.; Gorman, S. Ultraviolet Radiation Suppresses Obesity and Symptoms of Metabolic Syndrome Independently of Vitamin D in Mice Fed a High-Fat Diet. Diabetes 2014, 63, 3759–3769. [Google Scholar] [CrossRef] [Green Version]
- Guareschi, Z.M.; Valcanaia, A.C.; Ceglarek, V.M.; Hotz, P.; Amaral, B.K.; de Souza, D.W.; de Souza, T.A.; Nardelli, T.; Ferreira, T.R.; Leite, N.C.; et al. The effect of chronic oral vitamin D supplementation on adiposity and insulin secretion in hypothalamic obese rats. Br. J. Nutr. 2019, 121, 1334–1344. [Google Scholar] [CrossRef]
- Park, C.Y.; Kim, T.Y.; Yoo, J.S.; Seo, Y.; Pae, M.; Han, S.N. Effects of 1,25-Dihydroxyvitamin D3 on the Inflammatory Responses of Stromal Vascular Cells and Adipocytes from Lean and Obese Mice. Nutrients 2020, 12, 364. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-J.; Wang, B.-W.; Zhang, C.; Xia, M.-Z.; Chen, Y.-H.; Hu, C.-Q.; Wang, H.; Chen, X.; Xu, D.-X. Vitamin D Deficiency Attenuates High-Fat Diet-Induced Hyperinsulinemia and Hepatic Lipid Accumulation in Male Mice. Endocrinology 2015, 156, 2103–2113. [Google Scholar] [CrossRef]
- Bastie, C.C.; Gaffney-Stomberg, E.; Lee, T.-W.A.; Dhima, E.; Pessin, J.E.; Augenlicht, L.H. Dietary Cholecalciferol and Calcium Levels in a Western-Style Defined Rodent Diet Alter Energy Metabolism and Inflammatory Responses in Mice. J. Nutr. 2012, 142, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.; Noolu, B.; Qadri, S.S.; Ismail, A. Vitamin D deficiency decreases adiposity in rats and causes altered expression of uncoupling proteins and steroid receptor coactivator3. J. Steroid Biochem. Mol. Biol. 2014, 144, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Gliniak, C.; Zhang, Z.; Xue, Y.; Smith, K.B.; McCreedy, R.; Adedokun, S.A. Serum Metabolite Profiles and Target Tissue Gene Expression Define the Effect of Cholecalciferol Intake on Calcium Metabolism in Rats and Mice. J. Nutr. 2008, 138, 1114–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Dalifard, J.; Defoort, C.; Svilar, L.; Martin, J.-C.; Tourniaire, F.; Landrier, J.-F. Gene Expression Pattern in Response to Cholecalciferol Supplementation Highlights Cubilin as a Major Protein of 25(OH)D Uptake in Adipocytes and Male Mice White Adipose Tissue. Endocrinology 2017, 159, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; Ping-Delfos, W.L.C.S.; Piers, L.S. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes. Rev. 2012, 13, 592–605. [Google Scholar] [CrossRef]
- Schutkowski, A.; Max, D.; Bönn, M.; Brandsch, C.; Grundmann, S.M.; Hirche, F.; Staege, M.S.; Stangl, G.I. Vitamin D Does Not Play a Functional Role in Adipose Tissue Development in Rodent Models. Mol. Nutr. Food Res. 2018, 62, 1700726. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Chanet, A.; Salles, J.; Guillet, C.; Giraudet, C.; Berry, A.; Patrac, V.; Domingues-Faria, C.; Tagliaferri, C.; Bouton, K.; Bertrand-Michel, J.; et al. Vitamin D supplementation restores the blunted muscle protein synthesis response in deficient old rats through an impact on ectopic fat deposition. J. Nutr. Biochem. 2017, 46, 30–38. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Chanet, A.; Salles, J.; Berry, A.; Giraudet, C.; Patrac, V.; Denis, P.; Bouton, K.; Goncalves-Mendes, N.; Vasson, M.-P.; et al. Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutr. Metab. 2014, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH)2D3. Cell Reports 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuth, M.M.; Mahapatra, D.; Jima, D.; Wan, D.; Hammock, B.D.; Law, M.; Kullman, S.W. Vitamin D deficiency serves as a precursor to stunted growth and central adiposity in zebrafish. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Influence of the Mediterranean Diet on 25-Hydroxyvitamin D Levels in Adults. Nutrients 2020, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Boucher, B.J. Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutr. Rev. 2018, 76, 678–692. [Google Scholar] [CrossRef]
- Marquina, C.; Mousa, A.; Scragg, R.; De Courten, B. Vitamin D and cardiometabolic disorders: A review of current evidence, genetic determinants and pathomechanisms. Obes. Rev. 2018, 20, 262–277. [Google Scholar] [CrossRef]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, Cardiometabolic Risk, and Vitamin D Status: The Framingham Heart Study. Diabetes 2009, 59, 242–248. [Google Scholar] [CrossRef] [Green Version]
- McGill, A.-T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Xu, C.; Shu, Y.; Xie, Z.; Lu, C.; Mo, X. Serum 25-hydroxyvitamin D is associated with obesity and metabolic parameters in US children. Public Health Nutr. 2019, 23, 1214–1222. [Google Scholar] [CrossRef]
- Perna, S. The enigma of vitamin D supplementation in aging with obesity. Minerva Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Parikh, S.J.; Edelman, M.; Uwaifo, G.I.; Freedman, R.J.; Semega-Janneh, M.; Reynolds, J.; Yanovski, J. The Relationship between Obesity and Serum 1,25-Dihydroxy Vitamin D Concentrations in Healthy Adults. J. Clin. Endocrinol. Metab. 2004, 89, 1196–1199. [Google Scholar] [CrossRef] [Green Version]
- Konradsen, S.; Ag, H.; Lindberg, F.; Hexeberg, S.; Jorde, R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur. J. Nutr. 2008, 47, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Goossens, G.H.; Blaak, E.E. Vitamin D release across abdominal adipose tissue in lean and obese men: The effect of ß-adrenergic stimulation. Physiol. Rep. 2019, 7, e14308. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric Dilution, Rather Than Sequestration Best Explains the Low Vitamin D Status of Obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int. J. Obes. 2012, 37, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, L.F.; de Azevedo, L.G.; da Mota Santana, J.; de Sales, L.P.C.; Pereira-Santos, M. Obesity and overweight decreases the effect of vitamin D supplementation in adults: Systematic review and meta-analysis of randomized controlled trials. Rev. Endocr. Metab. Disord. 2019, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Diamond, D.; Baylin, A.; Mora, M.; Marin, C.; Arsenault, J.E.; Hughes, M.D.; Willett, W.C.; Villamor, E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: A prospective study. Am. J. Clin. Nutr. 2010, 92, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Mai, X.-M.; Chen, Y.; Camargo, C.A.; Langhammer, A. Cross-Sectional and Prospective Cohort Study of Serum 25-Hydroxyvitamin D Level and Obesity in Adults: The HUNT Study. Am. J. Epidemiol. 2012, 175, 1029–1036. [Google Scholar] [CrossRef]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutierrez, C.; Rubio, E.; Pérez-Valero, V.; Esteva, I.; De Adana, M.S.R.; Almaraz, M.C.; Colomo, N.; et al. Hypovitaminosis D and incidence of obesity: A prospective study. Eur. J. Clin. Nutr. 2013, 67, 680–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, E.S.; Rizzo, J.H.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.; Hochberg, M. Associations Between 25-Hydroxyvitamin D and Weight Gain in Elderly Women. J. Women’s Health 2012, 21, 1066–1073. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Ebeling, P.R.; Daly, R.M. Low Serum 25-Hydroxyvitamin D Is Associated with Increased Risk of the Development of the Metabolic Syndrome at Five Years: Results from a National, Population-Based Prospective Study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamycheva, E.; Joakimsen, R.M.; Jorde, R. Intakes of Calcium and Vitamin D Predict Body Mass Index in the Population of Northern Norway. J. Nutr. 2003, 133, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ochs-Balcom, H.M.; Chennamaneni, R.; Millen, A.E.; Shields, P.G.; Marian, C.; Trevisan, M.; Freudenheim, J.L. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am. J. Clin. Nutr. 2010, 93, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bienertová-Vašků, J.; Zlámal, F.; Pohořalá, A.; Mikeš, O.; Goldbergová-Pávková, M.; Novák, J.; Šplíchal, Z.; Pikhart, H. Allelic variants in vitamin D receptor gene are associated with adiposity measures in the central-European population. BMC Med. Genet. 2017, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.Z.; Reis, A.F.; Dubois-Laforgue, D.; Bellanné-Chantelot, C.; Timsit, J.; Velho, G. Vitamin D receptor gene polymorphisms are associated with obesity in type 2 diabetic subjects with early age of onset. Eur. J. Endocrinol. 2001, 145, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Xiong, D.-H.; Xu, F.-H.; Zhang, Y.-Y.; Lei, S.-F.; Deng, H.-W. Association between VDR ApaI Polymorphism and Hip Bone Mineral Density Can Be Modified by Body Mass Index: A Study on Postmenopausal Chinese Women. Acta Biochim. Biophys. Sin. 2005, 37, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Xiong, D.-H.; Guo, Y.-F.; Shen, H.; Xiao, P.; Yang, F.; Chen, Y.; Zhang, F.; Recker, R.R.; Deng, H.-W. Association analysis of vitamin D-binding protein gene polymorphisms with variations of obesity-related traits in Caucasian nuclear families. Int. J. Obes. 2007, 31, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Vimaleswaran, K.S.; The Genetic Investigation of Anthropometric Traits (GIANT) Consortium; Cavadino, A.; Berry, D.J.; Whittaker, J.; Power, C.; Jarvelin, M.-R.; Hypponen, E. Genetic association analysis of vitamin D pathway with obesity traits. Int. J. Obes. 2013, 37, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Dorjgochoo, T.; Shi, J.; Gao, Y.-T.; Long, J.; Delahanty, R.; Xiang, Y.-B.; Cai, Q.; Shu, X.O. Genetic variants in vitamin D metabolism-related genes and body mass index: Analysis of genome-wide scan data of approximately 7000 Chinese women. Int. J. Obes. 2011, 36, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Mallard, S.R.; Howe, A.S.; Houghton, L.A. Vitamin D status and weight loss: A systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am. J. Clin. Nutr. 2016, 104, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannu, P.K.; Zhao, Y.; Soares, M.J. Reductions in body weight and percent fat mass increase the vitamin D status of obese subjects: A systematic review and metaregression analysis. Nutr. Res. 2015, 36, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Soares, M.J.; Calton, E.K.; Zhao, Y.; Hallett, J. Vitamin D supplementation and body weight status: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Wagner, C.L.; Shab-Bidar, S. Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55, 368. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.J. Why do so many trials of vitamin D supplementation fail? Endocr. Connect. 2020, 9, R195–R206. [Google Scholar] [CrossRef]
- Shin, J.; Choi, M.; Longtine, M.; Nelson, D. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of Calcitriol Biosynthesis and Activity: Focus on Gestational Vitamin D Deficiency and Adverse Pregnancy Outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef] [Green Version]
- Larqué, E.; Morales, E.; Leis, R.; Blanco-Carnero, J.E. Maternal and Foetal Health Implications of Vitamin D Status during Pregnancy. Ann. Nutr. Metab. 2018, 72, 179–192. [Google Scholar] [CrossRef]
- Ideraabdullah, F.Y.; Belenchia, A.M.; Rosenfeld, C.S.; Kullman, S.W.; Knuth, M.; Mahapatra, D.; Bereman, M.; Levin, E.D.; Peterson, C.A. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J. Endocrinol. 2019, 241, R65–R80. [Google Scholar] [CrossRef] [Green Version]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.E.; McGrath, J.; Eyles, D.; Burne, T.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.-C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2014, 212, 511.e1–511.e7. [Google Scholar] [CrossRef] [PubMed]
- Amraei, M.; Mohamadpour, S.; Sayehmiri, K.; Mousavi, S.F.; Shirzadpour, E.; Moayeri, A. Effects of Vitamin D Deficiency on Incidence Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Endocrinol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gernand, A.D.; Simhan, H.N.; Klebanoff, M.A.; Bodnar, L.M. Maternal Serum 25-Hydroxyvitamin D and Measures of Newborn and Placental Weight in a U.S. Multicenter Cohort Study. J. Clin. Endocrinol. Metab. 2013, 98, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiao, Y.; Zhang, L.; Gao, Q. Maternal early pregnancy vitamin D status in relation to low birth weight and small-for-gestational-age offspring. J. Steroid Biochem. Mol. Biol. 2018, 175, 146–150. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Chen, H.; Föller, M.; Slowinski, T.; Li, J.; Chen, Y.-P.; Lang, F.; Hocher, B. Maternal Vitamin D Deficiency and Fetal Programming—Lessons Learned from Humans and Mice. Kidney Blood Press. Res. 2014, 39, 315–329. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Zmuda, J.M.; Cooper, M.E.; Parrott, M.S.; Roberts, J.M.; Marazita, M.L.; Simhan, H.N. Maternal Serum 25-Hydroxyvitamin D Concentrations Are Associated with Small-for-Gestational Age Births in White Women. J. Nutr. 2010, 140, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women’s Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Boyle, V.T.; Thorstensen, E.B.; Thompson, J.M.D.; McCowan, L.M.E.; A Mitchell, E.; Godfrey, K.M.; Poston, L.; Wall, C.R.; Murphy, R.; Cutfield, W.; et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: An observational study. Int. J. Obes. 2017, 41, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Miliku, K.; Felix, J.F.; Voortman, T.; Tiemeier, H.; Eyles, D.; Burne, T.; McGrath, J.; Jaddoe, V.W. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern. Child Nutr. 2018, 15, e12672. [Google Scholar] [CrossRef] [Green Version]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tint, M.T.; Chong, M.F.; Aris, I.; Godfrey, K.M.; Quah, P.L.; Kapur, J.; Saw, S.M.; Gluckman, P.D.; Rajadurai, V.S.; Yap, F.; et al. Association between maternal mid-gestation vitamin D status and neonatal abdominal adiposity. Int. J. Obes. 2018, 42, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iñiguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jiménez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int. J. Obes. 2014, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rytter, D.; Bech, B.H.; Halldorsson, T.; Henriksen, T.B.; Grandström, C.; Cohen, A.; Olsen, S. Maternal Vitamin D Status at Week 30 of Gestation and Offspring Cardio-Metabolic Health at 20 Years: A Prospective Cohort Study over Two Decades. PLoS ONE 2016, 11, e0164758. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 26, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wei, S.; Bi, W.; Weiler, H.; Wen, S. Effect of Vitamin D Supplementation in Early Life on Children’s Growth and Body Composition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 524. [Google Scholar] [CrossRef]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Wang, X.; Ji, C.; et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Nascimento, F.A.M.; Ceciliano, T.C.; Aguila, M.B.; Mandarim-De-Lacerda, C. Transgenerational Effects on the Liver and Pancreas Resulting from Maternal Vitamin D Restriction in Mice. J. Nutr. Sci. Vitaminol. 2013, 59, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Schoenrock, S.A.; Valdar, W.; Tarantino, L.M.; Ideraabdullah, F.Y. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenet. 2016, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chu, X.; Huang, Y.; Li, G.; Wang, Y.; Li, Y.; Sun, C. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014, 57, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Johnson, S.A.; Ellersieck, M.R.; Rosenfeld, C.S.; A Peterson, C. In utero vitamin D deficiency predisposes offspring to long-term adverse adipose tissue effects. J. Endocrinol. 2017, 234, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Seipelt, E.M.; Tourniaire, F.; Couturier, C.; Astier, J.; Loriod, B.; Vachon, H.; Pucéat, M.; Mounien, L.; Landrier, J. Prenatal maternal vitamin D deficiency sex-dependently programs adipose tissue metabolism and energy homeostasis in offspring. FASEB J. 2020, 34, 14905–14919. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2013, 72, 48–54. [Google Scholar] [CrossRef]
| In Rodents | References | |
|---|---|---|
| 25(OH)D plasma levels | Lack of clear-cut results (decrease or no effect reported) | [27,28,29,55,56,57,58,59,60,61] |
| Free 25(OH)D plasma levels | Reduced in obese mice | [29,55] |
| 1,25(OH)2D plasma levels | Lack of clear-cut results (increase, decrease or no effect reported) | [28,29,55,56,59] |
| VDR−/− mice | Reduced obesity/adiposity | [62,63,64,65,66] |
| Adipose tissue human VDR overexpression | Increase obesity/adiposity | [66,67] |
| Adipose tissue VDR−/− mice | Increase obesity/adiposity | [68,69] |
| No effect on obesity/adiposity | [70] | |
| Curative effect of VD supplementation on obesity | No | [58,71] |
| Curative effect of 1,25(OH)2D supplementation on obesity | Yes | [72] |
| Preventive effect of VD supplementation on obesity | Reduction of obesity/adiposity | [60,73,74,75,76] |
| No effect on obesity/adiposity | [56,57,59,61,77,78,79] | |
| Decrease in obesity/adiposity | [80,81,82] | |
| Effect of VD supplementation on 25(OH)D plasma levels in obese rodents | Increase 25(OH) plasma levels | [58,59,60,83,84] |
| No effect | [56,57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. https://doi.org/10.3390/nu14102049
Bennour I, Haroun N, Sicard F, Mounien L, Landrier J-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients. 2022; 14(10):2049. https://doi.org/10.3390/nu14102049
Chicago/Turabian StyleBennour, Imene, Nicole Haroun, Flavie Sicard, Lourdes Mounien, and Jean-François Landrier. 2022. "Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies" Nutrients 14, no. 10: 2049. https://doi.org/10.3390/nu14102049
APA StyleBennour, I., Haroun, N., Sicard, F., Mounien, L., & Landrier, J.-F. (2022). Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients, 14(10), 2049. https://doi.org/10.3390/nu14102049







