Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Clinical and Psychological Evaluation of the Treatment Effect
2.4. Biochemical Analysis
2.5. Statistics
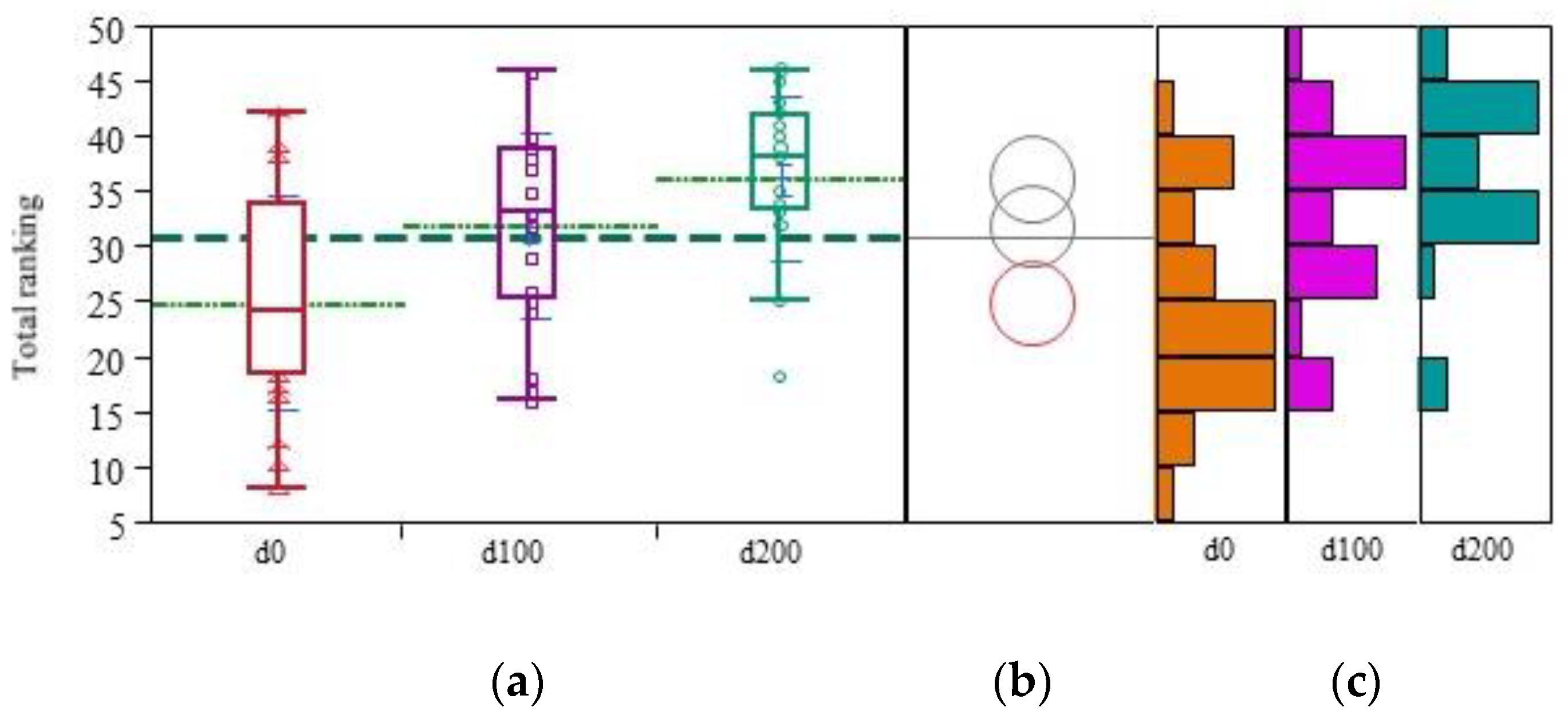
3. Results
3.1. A Syrup Form of Methylcobalamin
3.2. Clinical and Psychological Features
3.3. Biochemical Parameters
3.4. Clinical and Psychological Features vs. Biochemical Parameters
4. Discussion
4.1. Syrup Form of Methylcobalamin Enhanced Patient Compliance
4.2. Orally Administered Methylcobalamin Alleviated Autistic Symptoms
4.3. Mechanism of Action of Methylcobalamin
4.4. Oral Methylcobalamin Is Safe
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, M.-C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet Lond. Engl. 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. Off. J. Int. Soc. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Divan, G.; Koh, Y.-J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Res. Off. J. Int. Soc. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmar, F.R.; Pauls, D. Autism. Lancet Lond. Engl. 2003, 362, 1133–1141. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-5-TRTM): Washington, DC, USA; London, UK, 2013; ISBN 978-0-89042-576-3. [Google Scholar]
- DiCicco-Bloom, E.; Lord, C.; Zwaigenbaum, L.; Courchesne, E.; Dager, S.R.; Schmitz, C.; Schultz, R.T.; Crawley, J.; Young, L.J. The Developmental Neurobiology of Autism Spectrum Disorder. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 6897–6906. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Ababon, M.R.; Soliman, M.; Lin, Y.; Brzustowicz, L.M.; Matteson, P.G.; Millonig, J.H. Autism Associated Gene, engrailed2, and Flanking Gene Levels Are Altered in Post-Mortem Cerebellum. PLoS ONE 2014, 9, e87208. [Google Scholar] [CrossRef]
- Miles, J.H. Autism Spectrum Disorders—A Genetics Review. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Bruining, H.; Eijkemans, M.J.; Kas, M.J.; Curran, S.R.; Vorstman, J.A.; Bolton, P.F. Behavioral Signatures Related to Genetic Disorders in Autism. Mol. Autism 2014, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Gadad, B.; Hewitson, L.; Young, K.A.; Gemran, D. Neuropathology and Animal Models of Autism: Genetic and Environmental Factors. Autism Res. Treat. 2013, 2013, 731935. [Google Scholar] [CrossRef]
- Ecker, C.; Murphy, D. Neuroimaging in Autism—From Basic Science to Translational Research. Nat. Rev. Neurol. 2014, 10, 82–91. [Google Scholar] [CrossRef]
- Tyzio, R.; Nardou, R.; Ferrari, D.C.; Tsintsadze, T.; Shahrokhi, A.; Eftekhari, S.; Khalilov, I.; Tsintsadze, V.; Brouchoud, C.; Chazal, G.; et al. Oxytocin-Mediated GABA Inhibition during Delivery Attenuates Autism Pathogenesis in Rodent Offspring. Science 2014, 343, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A. Treatments for Biomedical Abnormalities Associated with Autism Spectrum Disorder. Front. Pediatr. 2014, 2, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eapen, V.; Crnčec, R.; Walter, A. Clinical Outcomes of an Early Intervention Program for Preschool Children with Autism Spectrum Disorder in a Community Group Setting. BMC Pediatr. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canitano, R.; Scandurra, V. Psychopharmacology in Autism: An Update. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 18–28. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative Stress in Autism. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Chauhan, A.; Gu, F.; Essa, M.M.; Wegiel, J.; Kaur, K.; Brown, W.T.; Chauhan, V. Brain Region-Specific Deficit in Mitochondrial Electron Transport Chain Complexes in Children with Autism. J. Neurochem. 2011, 117, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain Region-Specific Glutathione Redox Imbalance in Autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.-A.; Waly, M. How Environmental and Genetic Factors Combine to Cause Autism: A Redox/Methylation Hypothesis. Neurotoxicology 2008, 29, 190–201. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative Stress-Related Biomarkers in Autism: Systematic Review and Meta-Analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Akhondzadeh, S.; Hormozi, M.; Makarem, A.; Abotorabi-Zarchi, M.; Firoozabadi, A. Glutathione-Related Factors and Oxidative Stress in Autism, a Review. Curr. Med. Chem. 2012, 19, 4000–4005. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic Endophenotype and Related Genotypes Are Associated with Oxidative Stress in Children with Autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.J.; Jill James, S.; Melnyk, S.; Jernigan, S.; Hubanks, A.; Rose, S.; Gaylor, D.W. Abnormal Transmethylation/Transsulfuration Metabolism and DNA Hypomethylation among Parents of Children with Autism. J. Autism Dev. Disord. 2008, 38, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Jones, A.M. Evidence of Toxicity, Oxidative Stress, and Neuronal Insult in Autism. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Main, P.A.; Angley, M.T.; O’Doherty, C.E.; Thomas, P.; Fenech, M. The Potential Role of the Antioxidant and Detoxification Properties of Glutathione in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Nutr. Metab. 2012, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic Imbalance Associated with Methylation Dysregulation and Oxidative Damage in Children with Autism. J. Autism Dev. Disord. 2012, 42, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, D.A.; Frye, R.E. A Review of Research Trends in Physiological Abnormalities in Autism Spectrum Disorders: Immune Dysregulation, Inflammation, Oxidative Stress, Mitochondrial Dysfunction and Environmental Toxicant Exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Villagonzalo, K.-A.; Dodd, S.; Dean, O.; Gray, K.; Tonge, B.; Berk, M. Oxidative Pathways as a Drug Target for the Treatment of Autism. Expert Opin. Ther. Targets 2010, 14, 1301–1310. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and Metabolic Status of Children with Autism vs. Neurotypical Children, and the Association with Autism Severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Al-Yafee, Y.A.; Al-Ayadhi, L.Y.; Haq, S.H.; El-Ansary, A.K. Novel Metabolic Biomarkers Related to Sulfur-Dependent Detoxification Pathways in Autistic Patients of Saudi Arabia. BMC Neurol. 2011, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Bertoglio, K.; Jill James, S.; Deprey, L.; Brule, N.; Hendren, R.L. Pilot Study of the Effect of Methyl B12 Treatment on Behavioral and Biomarker Measures in Children with Autism. J. Altern. Complement. Med. 2010, 16, 555–560. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.; Geier, M.R. A Prospective Study of Transsulfuration Biomarkers in Autistic Disorders. Neurochem. Res. 2009, 34, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.; Nataf, R.; Geier, M.R. Biomarkers of Environmental Toxicity and Susceptibility in Autism. J. Neurol. Sci. 2009, 280, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired Synthesis and Antioxidant Defense of Glutathione in the Cerebellum of Autistic Subjects: Alterations in the Activities and Protein Expression of Glutathione-Related Enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic Biomarkers of Increased Oxidative Stress and Impaired Methylation Capacity in Children with Autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- James, S.J.; Rose, S.; Melnyk, S.; Jernigan, S.; Blossom, S.; Pavliv, O.; Gaylor, D.W. Cellular and Mitochondrial Glutathione Redox Imbalance in Lymphoblastoid Cells Derived from Children with Autism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2374–2383. [Google Scholar] [CrossRef]
- Mostafa, G.A.; El-Hadidi, E.S.; Hewedi, D.H.; Abdou, M.M. Oxidative Stress in Egyptian Children with Autism: Relation to Autoimmunity. J. Neuroimmunol. 2010, 219, 114–118. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of Oxidative Damage and Inflammation Associated with Low Glutathione Redox Status in the Autism Brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a Vitamin/Mineral Supplement on Children and Adults with Autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Derakhshan, N. N-Acetylcysteine for Treatment of Autism, a Case Report. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 985–987. [Google Scholar]
- Frye, R.E.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W.; Walters, L.; James, S.J. Effectiveness of Methylcobalamin and Folinic Acid Treatment on Adaptive Behavior in Children with Autistic Disorder Is Related to Glutathione Redox Status. Autism Res. Treat. 2013, 2013, 609705. [Google Scholar] [CrossRef] [PubMed]
- Hendren, R.L.; James, S.J.; Widjaja, F.; Lawton, B.; Rosenblatt, A.; Bent, S. Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism. J. Child Adolesc. Psychopharmacol. 2016, 26, 774–783. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W. Efficacy of Methylcobalamin and Folinic Acid Treatment on Glutathione Redox Status in Children with Autism. Am. J. Clin. Nutr. 2009, 89, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; LeCouteur, A.; Lord, C. (ADITM-R) Autism Diagnostic InterviewTM, 4th ed.; Western Psychological Services: Los Angeles, CA, USA, 2010. [Google Scholar]
- Schopler, E.; Van Bourgondien, M.E.; Wellman, J.; Love, S.R. CARS-2. Childhood Autism Rating Scale, 4th ed.; Western Psychological Services: Los Angeles, CA, USA, 2011. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Wing, L. Autistic Continuum Disorders, 2nd ed.; The National Autistic Society: London, UK, 1993. [Google Scholar]
- Kand’ár, R.; Záková, P.; Lotková, H.; Kucera, O.; Cervinková, Z. Determination of Reduced and Oxidized Glutathione in Biological Samples Using Liquid Chromatography with Fluorimetric Detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar] [CrossRef]
- Vilaseca, M.A.; Moyano, D.; Ferrer, I.; Artuch, R. Total Homocysteine in Pediatric Patients. Clin. Chem. 1997, 43, 690–692. [Google Scholar] [CrossRef]
- Li, Y.-J.; Li, Y.-M.; Xiang, D.-X. Supplement Intervention Associated with Nutritional Deficiencies in Autism Spectrum Disorders: A Systematic Review. Eur. J. Nutr. 2018, 57, 2571–2582. [Google Scholar] [CrossRef]
- Carmel, R. How I Treat Cobalamin (Vitamin B12) Deficiency. Blood 2008, 112, 2214–2221. [Google Scholar] [CrossRef] [Green Version]
- Kozyraki, R.; Cases, O. Vitamin B12 Absorption: Mammalian Physiology and Acquired and Inherited Disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Kim, H.-I.; Hyung, W.J.; Song, K.J.; Choi, S.H.; Kim, C.-B.; Noh, S.H. Oral Vitamin B12 Replacement: An Effective Treatment for Vitamin B12 Deficiency after Total Gastrectomy in Gastric Cancer Patients. Ann. Surg. Oncol. 2011, 18, 3711–3717. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Butler, C.C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čorejová, A.; Jánošiková, D.; Pospíšilová, V.; Rauová, D.; Kyselovič, J.; Hrabovská, A. Cessation of Nocturnal Enuresis after Intervention with Methylcobalamin in an 18-Year-Old Patient with Autism. J. Child Adolesc. Psychopharmacol. 2015, 25, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Gravel, R.A. Genetic Disorders of Vitamin B12 Metabolism: Eight Complementation Groups—Eight Genes. Expert Rev. Mol. Med. 2010, 12, e37. [Google Scholar] [CrossRef] [Green Version]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primer 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Michalska, M.; Rynkowski, J. Vitamin Supplementation Reduces the Level of Homocysteine in the Urine of Autistic Children. Nutr. Res. 2011, 31, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Paşca, S.P.; Nemeş, B.; Vlase, L.; Gagyi, C.E.; Dronca, E.; Miu, A.C.; Dronca, M. High Levels of Homocysteine and Low Serum Paraoxonase 1 Arylesterase Activity in Children with Autism. Life Sci. 2006, 78, 2244–2248. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Trusty, T.A.; Pavliv, O.; Seidel, L.; Li, J.; Nick, T.; James, S.J. Intracellular and Extracellular Redox Status and Free Radical Generation in Primary Immune Cells from Children with Autism. Autism Res. Treat. 2012, 2012, 986519. [Google Scholar] [CrossRef] [Green Version]
- Delhey, L.M.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; Frye, R.E. Comparison of Treatment for Metabolic Disorders Associated with Autism:Reanalysis of Three Clinical Trials. Front. Neurosci. 2018, 12, 19. [Google Scholar] [CrossRef]
- Dietary Reference Values for Cobalamin (Vitamin B12). Available online: http://www.efsa.europa.eu/en/efsajournal/pub/4150 (accessed on 7 August 2018).
- Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- Bailey, A.; Phillips, W.; Rutter, M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J. Child Psychol. Psychiatry 1996, 37, 89–126. [Google Scholar] [CrossRef]
- Filipek, P.A.; Accardo, P.J.; Ashwal, S.; Baranek, G.T.; Cook, E.H.; Dawson, G.; Gordon, B.; Gravel, J.S.; Johnson, C.P.; Kallen, R.J.; et al. Practice parameter: Screening and diagnosis of autism: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000, 55, 468–479. [Google Scholar] [CrossRef]
- Happé, F.; Frith, U. The neuropsychology of autism. Brain J. Neurol. 1996, 119, 1377–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozonoff, S.; Goodlin-Jones, B.L.; Solomon, M. Evidence-based assessment of autism spectrum disorders in children and adolescents. J. Clin. Child Adolesc. Psychol. 2005, 34, 523–540. [Google Scholar] [CrossRef] [PubMed]



| Ranking Variable | Rv(d0) (±sR) | Rv(d100) (±sR) | Rv(d200) (±sR) | Rv(d200-d0) (±sR) | Absolute Treatment Effect Cohen Delta | Rv(d200-d0)/R(d0) (±s R) | Relative Treatment Effect Cohen Delta | Gain of Treatment Normalized for Scale Length (%) |
|---|---|---|---|---|---|---|---|---|
| Social (scale length = 13) | 5.16 (±2.94) | 8.2 (±2.65) | 9.28 (±2.69) | 4.12 (±3.60) | 1.08 +++ | 1.01 (±1.06) | 0.89 +++ | 31.69 |
| Behavioral (scale length = 14) | 6.16 (±3.30) | 6.72 (±2.89) | 8.36 (±2.50) | 2.20 (±4.00) | 0.51 ++ | 1.01 (±1.99) | 0.47 ++ | 15.71 |
| Communication (scale length = 10) | 4.44 (±1.66) | 5.36 (±2.02) | 5.84 (±1.68) | 1.40 (±1.63) | 0.80 +++ | 0.44 (±0.60) | 0.68 ++ | 14.00 |
| Cognitive (scale length = 17) | 9.08 (±3.09) | 11.6 (±2.84) | 12.64 (±2.14) | 3.56 (±3.14) | 1.05 +++ | 0.57 (±0.67) | 0.78 ++ | 20.94 |
| Total (scale length = 54) | 24.84 (±9.69) | 31.88 (±8.29) | 36.12 (±7.43) | 11.28 (±10.84) | 0.97 +++ | 0.70 (±0.89) | 0.73 ++ | 20.89 |
| Parameter | Bio(d0) (±sR) | Bio(d100) (±sR) | Bio(d200) (±sR) | Bio(d200-d0) (±sR) | Absolute Treatment Effect Cohen delta | Bio(d200-d0)/Bio(d0) (±s Bio) | Relative Treatment Effect Cohen Delta |
|---|---|---|---|---|---|---|---|
| GSH | 0.36 (±0.40) | 0.48 (±0.44) | 0.78 (±0.65) | 0.42 (±0.66) | 0.58 ++ | 2.74 (±3.48) | 0.73 ++ |
| GSSG | 1.48 (±0.54) | 1.57 (±1.34) | 1.36 (±0.66) | −0.11 (±0.73) | −0.14 | 0.05 (±0.69) | 0.06 |
| GSH/GSSG | 0.26 (±0.26) | 0.39 (±0.33) | 0.66 (±0.59) | 0.40 (±0.51) | 0.73 ++ | 3.15 (±4.26) | 0.69 ++ |
| Vit. B12 | 408.48 (±474.9) | 601.33 (±404.92) | 628.64 (±209.95) | 211.33 (±423.46) | 0.46 ++ | 1.03 (±0.93) | 1.02 +++ |
| Homocysteine | 3.69 (±1.21) | 3.47 (±0.87) | 4.02 (±1.17) | 0.29 (±1.14) | 0.23 + | 0.14 (±0.38) | 0.34 + |
| Cysteine | 95.29 (±39.79) | 85.35 (±32.01) | 115.68 (±30.58) | 19.69 (±44.40) | 0.42 ++ | 0.43 (±0.91) | 0.44 ++ |
| Biochemical Parameter | Statistics Descriptor | Social Ranking Statistics | Behavioral Ranking Statistics | Communication Ranking Statistics | Cognitive Ranking Statistics | Total Ranking Statistics |
|---|---|---|---|---|---|---|
| GSH | Fisher exact OR (CI) CMH | 0.0002 * 7.4388 (2.5967; 21.3103) 0.0040 * | 0.0381 * 2.8000 (1.0945; 7.1629) 0.2225 | 0.0091 * 3.8961 (1.4748; 10.2927) 0.0338 * | 0.0002 * 7.0000 (2.4993;19.6048) 0.0148 * | 0.0056 * 3.9487 (1.5073; 10.3441) 0.0515 |
| GSSG | Fisher exact OR (CI) CMH | 0.6440 0.7519 (0.3018; 1.8732) 0.8187 | 0.8188 0.8571 (0.3459; 2.1236) 0.9052 | 0.2412 1.9000 (0.7458; 4.8402) 0.0244 * | 1.0000 0.9418 (0.3803; 2.3324) 0.3369 | 1.0000 0.9580 (0.3864; 2.3752) 0.6335 |
| GHS/GSSG | Fisher exact OR (CI) CMH | 0.0023 * 4.8148 (1.7853; 12.9855) 0.0297 * | 0.0055 * 3.9808 (1.5216; 10.4151) 0.0307 * | 0.1074 2.1991 (0.8620; 5.6103) 0.3694 | 0.0025 * 4.6429 (1.7429; 12.3678) 0.0693 | 0.0026 * 4.4722 (1.6922; 11.8191) 0.0199 * |
| B12 | Fisher exact OR (CI) CMH | 0.0519 2.7692 (1.0243; 7.4865) 0.5640 | 0.6280 1.3333 (0.5087; 3.4945) 0.5955 | 0.1411 2.2727 (0.8473; 60.959) 0.6156 | 0.0519 2.7692 (1.0243; 7.4865) 0.9010 | 0.0280 * 3.2168 (1.1811; 8.7608) 0.2469 |
| HCY | Fisher exact OR (CI) CMH | 0.6264 0.7143 (0.2738; 1.8632) 0.4040 | 0.4664 1.6071 (0.6158; 4.1938) 0.4375 | 0.6245 1.3866 (0.5282; 3.6391) 0.4836 | 1.0000 0.8947 (0.3449; 2.3206) 0.6624 | 0.6273 1.4166 (0.5445; 3.6857) 0.5156 |
| CYS | Fisher exact OR (CI) CMH | 0.6324 0.7500 (0.29042; 1.9368) 0.0817 | 0.6329 1.3359 (0.5185; 3.4421) 0.6874 | 0.6279 0.735119 (0.2816; 1.9190) 0.2110 | 0.8112 1.1912 (0.4631; 3.0645) 0.2947 | 0.6324 0.7456 (0.2891; 1.9230) 0.1119 |
| Biochemical Parameter | Statistics Descriptor | Social Ranking Statistics | Behavioral Ranking Statistics | Communication Ranking Statistics | Cognitive Ranking Statistics | Total Ranking Statistics |
|---|---|---|---|---|---|---|
| GSH | R2 p(Model) p(Intercept) p(GSH) p(Time) p(GSH × Time) | 0.7444 <0.0001 * <0.0001 * <0.0001 * 0.0144 * 0.0088 * | 0.6656 <0.0001 * <0.0001 * 0.0234 * 0.0647 0.0136 * | 0.8091 <0.0001 * <0.0001 * 0.0007 * 0.1131 0.0357 * | 0.7344 <0.0001 * <0.0001 * <0.0001 * 0.0706 0.0113 * | 0.7721 <0.0001 * <0.0001 * <0.0001 * 0.0140 * 0.0022 * |
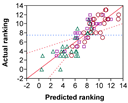 |  |  |  |  | ||
| GSH/GSSG | R2 p(Model) p(Intercept) p(GSH/GSSG) p(Time) p(GSH/GSSG × Time) | 0.7512 <0.0001 * <0.0001 * <0.0001 * 0.0149 * 0.0025 * | 0.6660 <0.0001 * <0.0001 * 0.0499 * 0.0630 0.0115 * | 0.8015 <0.0001 * <0.0001 * 0.0007 * 0.4341 0.0962 * | 0.7010 <0.0001 * <0.0001 * <0.0001 * 0.0660 0.0115 * | 0.7715 <0.0001 * <0.0001 * <0.0001 * 0.0202 * 0.0017 * |
 |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čorejová, A.; Fazekaš, T.; Jánošíková, D.; Repiský, J.; Pospíšilová, V.; Miková, M.; Rauová, D.; Ostatníková, D.; Kyselovič, J.; Hrabovská, A. Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration. Nutrients 2022, 14, 2035. https://doi.org/10.3390/nu14102035
Čorejová A, Fazekaš T, Jánošíková D, Repiský J, Pospíšilová V, Miková M, Rauová D, Ostatníková D, Kyselovič J, Hrabovská A. Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration. Nutrients. 2022; 14(10):2035. https://doi.org/10.3390/nu14102035
Chicago/Turabian StyleČorejová, Adela, Tomáš Fazekaš, Daniela Jánošíková, Juraj Repiský, Veronika Pospíšilová, Maria Miková, Drahomíra Rauová, Daniela Ostatníková, Ján Kyselovič, and Anna Hrabovská. 2022. "Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration" Nutrients 14, no. 10: 2035. https://doi.org/10.3390/nu14102035
APA StyleČorejová, A., Fazekaš, T., Jánošíková, D., Repiský, J., Pospíšilová, V., Miková, M., Rauová, D., Ostatníková, D., Kyselovič, J., & Hrabovská, A. (2022). Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration. Nutrients, 14(10), 2035. https://doi.org/10.3390/nu14102035





