Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis
Abstract
1. Introduction
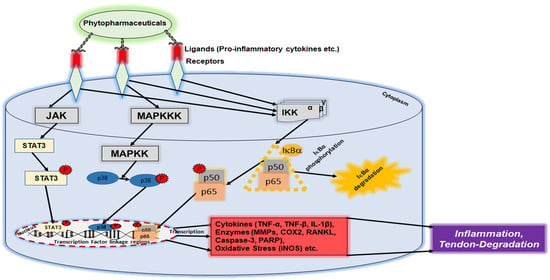
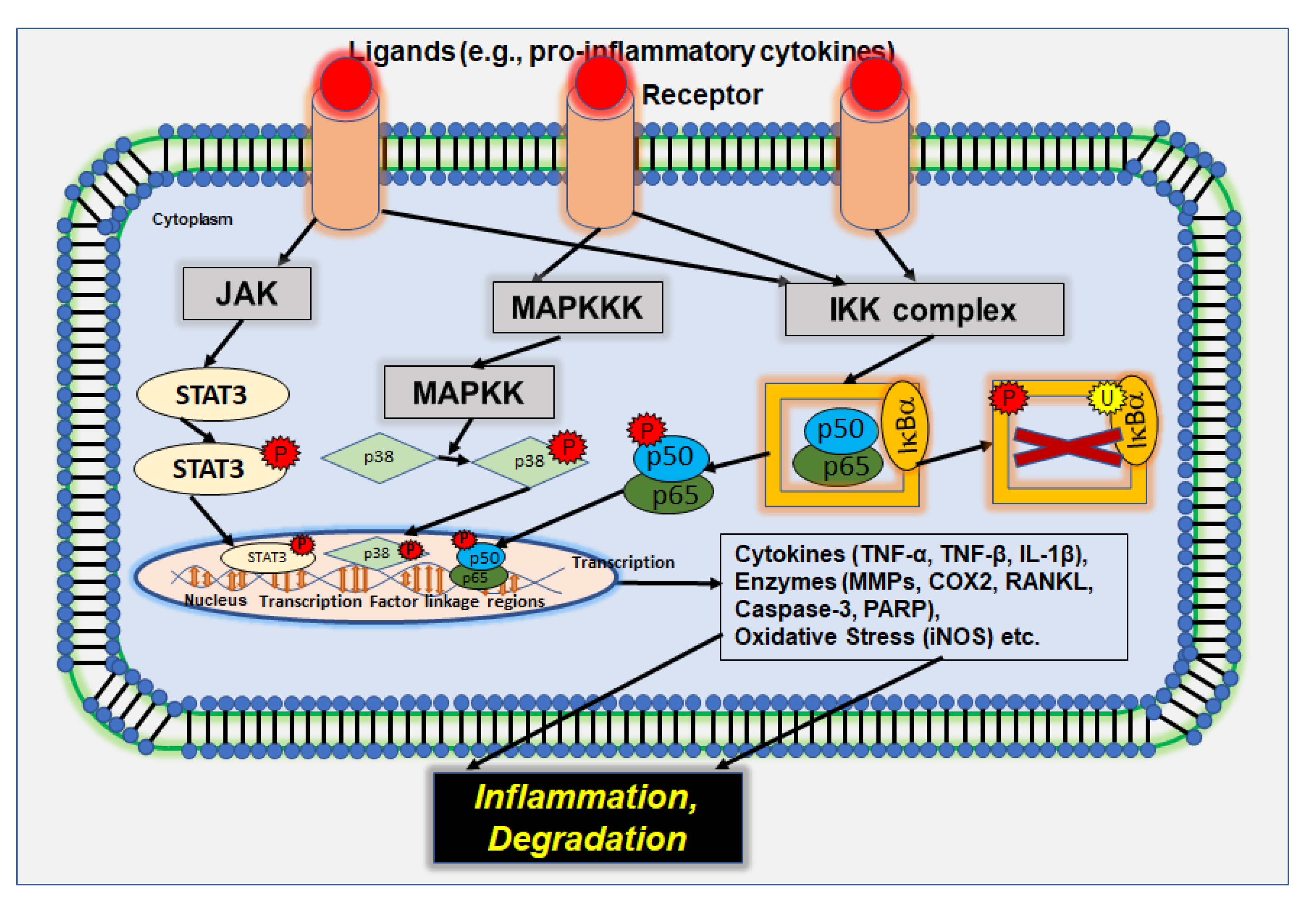
2. Signaling Pathways in Inflammation and Inflammation-Associated Diseases
2.1. p38/MAPK Signaling Pathway
2.2. IL-6/JAK/STAT3 Signaling Pathway
2.3. PI3K/Akt Signaling Pathway
2.4. NF-κB Signaling Pathway
3. Plant-Derived Nutraceuticals and Inflammation
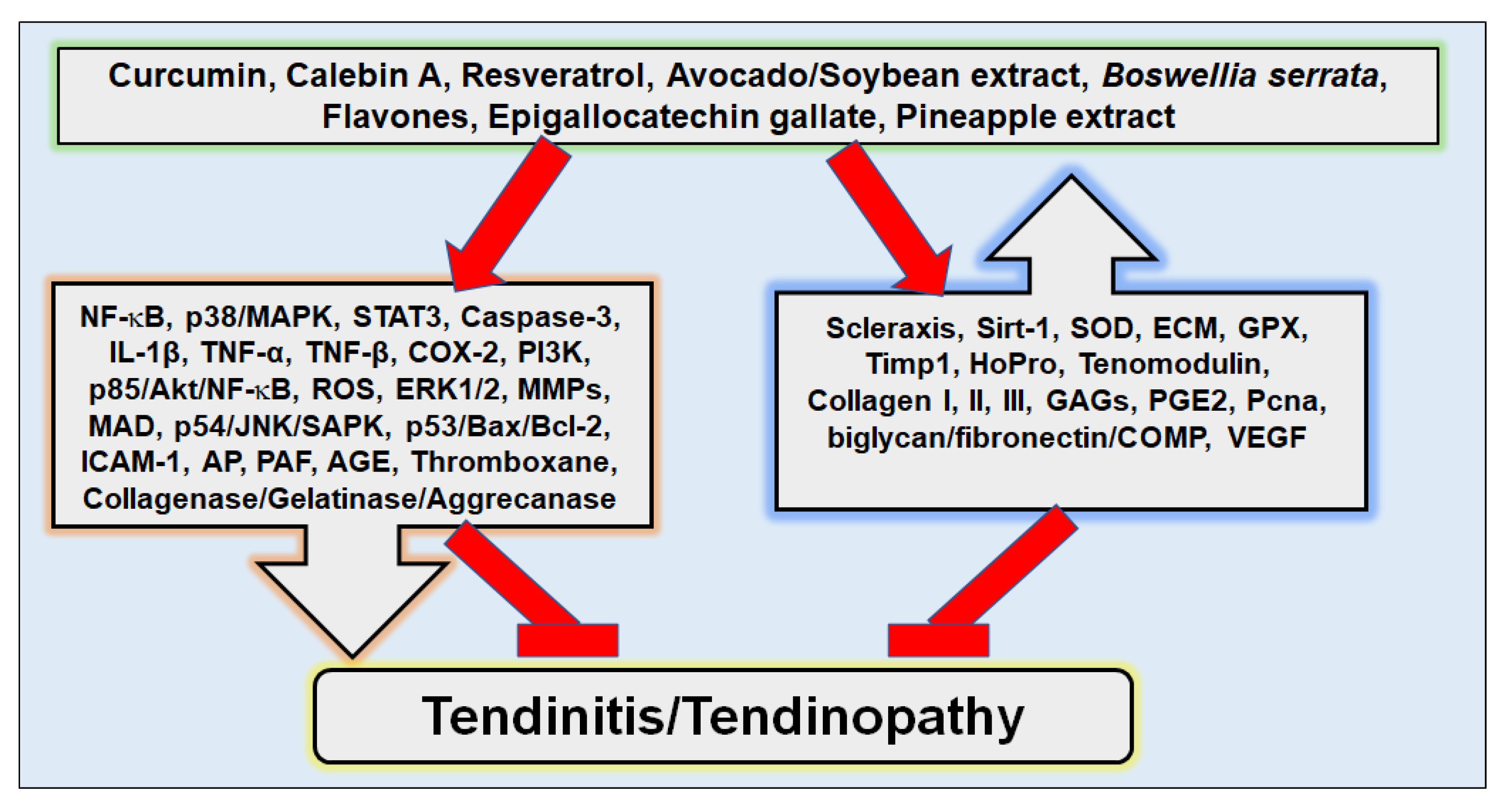
4. Plant-Derived Nutraceuticals in the Treatment of Tendinitis
4.1. Modulation of Inflammatory Pathways in Tendinitis
4.2. Plant-Derived Compounds Proposed for Tendinitis Treatment
4.2.1. Avocado/Soybean Unsaponifiables
4.2.2. Bromelain
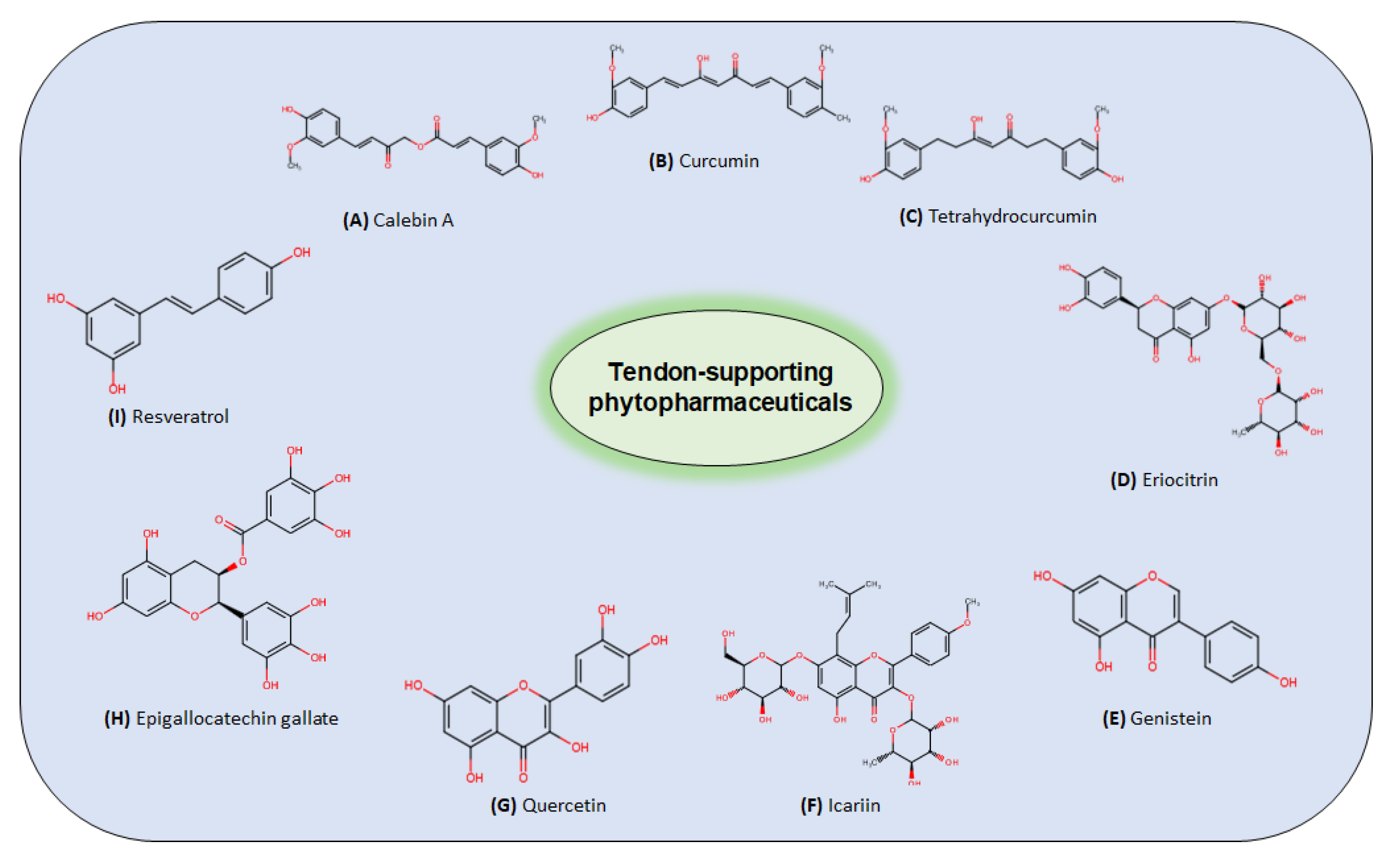
4.2.3. Curcuminoids
Curcumin
Calebin A
4.2.4. Green Tea Extracts (Epigallocatechin Gallate)
4.2.5. Flavonoids/Flavones
Anthocyanin
Eriocitrin
Genistein
Icariin
Quercetin
4.2.6. Resveratrol
| Agent | Origin | Type of Trial | Presumed Modulation | Mode of Action | Concentration/Dose Range | Reference |
|---|---|---|---|---|---|---|
| Avocado/Soybean unsaponifiables (ASU) | avocado and soybean oils | in vitro, horse tenocytes | IL-1β COX-2 PGE2 | Avocado/soybean unsaponifiables significantly inhibited inflammation response, such as combination therapy with glucosamine and chondroitin sulfate. | 8.3 μg/mL of ASU | [157] |
| Boswellia acid | Boswellia serrata | in vivo, Achilles tendinitis patients | - | Boswellia acid (as Casperome®) showed pain reduction on a visual analogical scale when Casperome® was administered in addition to physical therapy in patients with Achilles tendinitis. | 250 mg of Casperome® for 15 and 30 days | [210] |
| in vivo, joint inflammation patients | - | Boswellia acid (as Casperome®) supplementation accompanied by standard therapy reduced pain and inflammation in knee joints and tendon of rugby players. | 500 mg of Casperome® for 5 days, then 250 mg for 23 days | [211] | ||
| in vivo, supraspinatus injury patients | - | Boswellia serrata and Curcuma longa extracts (as Tendisulfur®) reduced pain after arthroscopic supraspinatus tendon repair compared to placebo treatment. | 2 daily sachets Tendisulfur® for 15 days, then 1 daily sachet for 45 days | [212] | ||
| in vivo, tendinopathy patients | - | Boswellia serrata and Curcuma longa extracts alleviated the symptoms (pain and functional limitation) of patients with tendon disease when applied as combinational therapy. | 2 tablets twice a day for 1 month | [65] | ||
| in vivo, rotator cuff tendinopathy, Achilles tendinopathy, and lateral epicondylitis patients | - | Boswellia serrata, Curcuma longa and bromelain extracts (with methyl-sulfonyl-methane, hydrolysed collagen I and II, L-arginine, L-lysin, vitamin C, chondroitin sulfate, glucosamine, and myrrh as Tendisulfur® Forte), in combination with extracorporeal shock wave therapy, accelerated pain relief and remarkably reduced NSAID intake of patients. | 2 daily tablets of Tendisulfur® Forte for 1 month, then once a day for a month | [213] | ||
| Bromelain | pineapple extracts, Ananas cosmosus | in vivo, Sprague–Dawley rats | MDA ROS | Pineapple flesh extract stimulated tenoblast proliferation and thus tendon healing after Achilles tendon injury. | 30 mg/kg of pineapple flesh axtract for 14 days | [161] |
| in vivo, Sprague–Dawley rats | ROS PAF | Pineapple extract bromelain shifted the thromboxane–prostacyclin ratio towards prostacyclin and increased the tenocyte population after Achilles tendon injury. | 7 mg/kg of bromelain for 14 days | [130,163] | ||
| in vivo, Achilles tendinopathy patients | - | Pineapple extract bromelain (as dietary supplement Tenosan with arginine, collagen, vitamin C, methyl-sulfonyl-methane, VinitroxTM) boosted the efficacy of extracorporeal shock wave therapy, resulting in better functional and clinical outcome, compared to placebo treatment. | 2 daily drug sachets containing 50 mg of bromelain for 60 days | [214] | ||
| in vivo, rotator cuff tendinopathy patients | - | Pineapple extract bromelain (as dietary supplement Tenosan with arginine L-alpha-ketoglutarate, methyl-sulfonyl-methane and hydrolysed collagen I) reduced pain and improved repair integrity of rotator cuff repair. | 2 daily drug sachets containing 50 mg of bromelain for 3 months | [215] | ||
| in vivo, rotator cuff tendinopathy, Achilles tendinopathy, and lateral epicondylitis patients | - | Pineapple extract bromelain (with methyl-sulfonyl-methane, hydrolysed collagen I and II, L-arginine, L-lysin, vitamin C, chondroitin sulfate, glucosamine, Curcuma longa, Boswellia serrata, and myrrh as Tendisulfur® Forte), in combination with extracorporeal shock wave therapy, accelerated pain relief and remarkably reduced NSAID intake of patients. | 2 daily tablets of Tendisulfur® Forte for 1 month, then once a day for an additional month | [213] | ||
| Curcuminoids | turmeric, Curcuma longa | in vitro, canine tenocytes | NF-κB scleraxis TNF-α TNF-β | Calebin A suppressed inflammation and exhibited potential as preventive and therapeutic treatment of tendinitis by suppressing down-regulation of tenomodulin and collagen I. | 1–10 µM of calebin A | [36] |
| in vitro, human tenocytes | NF-κB IL-1βPI3K/p85/Akt MMPs COX-2 caspase-3 Bax/Bcl-2 | Curcumin inhibited inflammation and apoptosis and showed potential for treatment of tendon inflammation. | 5 µM of curcumin | [10] | ||
| in vivo, diabetic rats | ROS AGE | Curcumin reduced oxidative stress by inhibiting lipid peroxidation and prevented glycation and crosslinking of advanced glycated collagen in tail tendon and skin. | 200 mg/kg of curcumin for 8 weeks | [168] | ||
| in vivo, Sprague–Dawley rats | MDA HOPro SOD | Curcumin improved the healing quality of tendon ruptures by promoting well-organized collagen filaments and biomechanical traits. | 100 mg/kg of curcumin for 14 days | [64] | ||
| in vivo, Sprague–Dawley rats | adhesion of inflammatory products | Curcumin (as loaded nanoparticle) promoted the healing process of Achilles tendon rupture. | 1 injection containing 0.44 mg of curcumin/kg | [169] | ||
| in vivo, Wistar albino rats | - | Curcumin showed biomechanical and histological healing (collagen I and III) promotion after surgically treated Achilles tendon ruptures. | 200 mg/kg of curcumin for 28 days | [170] | ||
| in vivo, rats | AP | Curcumin prevented tendon calcification and improved tendon regeneration by tendon stem/progenitor cells. | 3 μg of curcumin every 3 days for up to 4 weeks | [131] | ||
| in vivo, Sprague–Dawley rats | ROS IL-1β TNF-α MMPs | Curcumin showed anti-oxidative and anti-inflammatory properties as part of Cur&Mg-QCS/PF hydrogel application. | 1 injection with 50 µL of hydrogel | [146] | ||
| in vivo, diabetic rats | AGE HOPro | Curcumin’s metabolite tetrahydrocurcumin reduced accumulation and crosslinking of advanced glycated collagen. | 80 mg/kg of tetrahydrocurcumin for 45 days | [172] | ||
| in vivo, tendinopathy patients | - | Curcuma longa and Boswellia serrata extracts alleviated the symptoms (pain and functional limitation) of patients with tendon disease as combinational therapy. | 2 tablets twice a day for 1 month | [65] | ||
| in vivo, rotator cuff tendinopathy, Achilles tendinopathy, and lateral epicondylitis patients | - | Curcuma longa, Boswellia serrata and bromelain extracts (with methyl-sulfonyl-methane, hydrolysed collagen I and II, L-arginine, L-lysin, vitamin C, chondroitin sulfate, glucosamine, and myrrh as Tendisulfur® Forte), in combination with extracorporeal shock wave therapy, accelerated pain relief and remarkably reduced NSAID intake of patients. | 2 daily tablets of Tendisulfur® Forte for 1 month, then once a day for an additional month | [213] | ||
| in vivo, supraspinatus injury patients | - | Curcuma longa and Boswellia serrata extracts (as Tendisulfur®) reduced pain after arthroscopic supraspinatus tendon repair compared to placebo treatment. | 2 daily sachets of Tendisulfur® for 15 days and 1 daily sachet for the next 45 days | [212] | ||
| EGCG (epigallocatechin gallate) | green tea extracts | in vitro, human tendon-derived fibroblasts | IL-1β MMPs p54/JNK/ SAPK collagenases/ gelatinases/ aggrecanases | Green tea’s epigallocatechin gallate targeted extracellular matrix breakdown. | 2.5–25 µM of epigallocatechin gallate | [148] |
| in vivo, diabetic rats | AGE | Green tea extract reduced collagen glycation and crosslinking in the tail tendon. | 300 mg/kg of green tea extract for 4 weeks | [145] | ||
| in vivo, Wistar rats | MMPs HOPro | Green tea promoted the synthesis of ECM components and glycosaminoglycans, and thus the recovery process after Achilles tendinitis in combination with a glycin diet. | 700 mg/kg of green tea extract for 21 days | [177] | ||
| in vivo, Wistar rats | IL-1β MMPs | Green tea modulated inflammatory action and promoted synthesis of recovery elements after Achilles tendinitis, in combination with a glycin diet. | 700 mg/kg of green tea extract for 7 days | [132] | ||
| in vivo, C57BL/6 mice | ROS | Green tea extract slowed collagen aging by inhibiting crosslinking. | 21.2 mL (young mice) and 27.2 mL (adult mice) of green tea extract for 14 days | [176] | ||
| Echinaceae angustifolia extracts | Echinaceae angustifolia | in vivo, carpal tunnel syndrome patients | - | Echinaceae angustifolia extract (as a dietary supplement mainly composed of alpha lipoic acid and conjugated linoleic acid) showed significant improvement in pain, symptoms, and functionality. | 2 capsules containing 250 mg of echinacea extract for 40 days, then 1 capsule for 80 days | [216] |
| Flavones/Flavonoids | celery, parsley, red peppers, chamomile, mint and gingko bilboa, citrus fruit | in vitro, rat tenofibroblast | ROS ERK1/2 JNK | Flavonoid anthocyanin acted as an anti-apoptotic and showed the therapeutic potential of rotator cuff tendon. | 10–200 µg/mL of anthocyanins | [133] |
| in vitro, tendon stem cells | caspase-3 | Flavonoid eriocitrin inhibited apoptosis and scar formation (biglycan, fibronectin, COMP) and improved woundhealing by stimulating proliferation and migration of tendon stem cells. | 25–75 of µM eriocitrin | [147] | ||
| in vivo, Sprague–Dawley rats | - | Flavone genistein protected ovariectomy-induced collagen reduction in Achilles tendon. | 300 mg/kgof genistein for 6 weeks | [184] | ||
| in vivo, Sprague–Dawley rats | Pcna Timp1 | Flavone genistein enhanced tendon function at an estrogen-deficit through the modulation of tenomodulin. | 6 mg/kg of genistein for 6 weeks | [185] | ||
| in vivo, Sprague–Dawley rats | AP CD31 VEGF | Flavonoid icariin supported healing and angiogenesis after rotator cuff reconstruction through promoting collagen I/II. | 0.125 mg/g of icariin for 2 and 4 weeks | [189] | ||
| in vivo, Wistar rats | ROS MDA SOD GPX | Flavonoid quercetin prevented the adhesion of tendon tissue. | 50–100 mg/kg for 4 weeks | [195] | ||
| in vivo, Sprague–Dawley rats | MMPs ICAM-1 | Flavonoid quercetin prevented collagenase-induced tendon damage at Achilles tendinopathy. | 25–50 mg/kg for 7 days | [149] | ||
| in vivo, rats | - | Flavonoid quercetin, kaempferol, and isorhamnetin (Hippophae rhamnoides’ flavones) improved fibre alignment, collagen deposition, healing, and recovery after patellar tendon injury. | 1 injection with 0.1 mg of Hippophae rhamnoides’ flavones | [144] | ||
| Resveratrol | red grapes, Vitis vinifera | in vitro, human tenocytes | NF-κB p53 Sirt-1 IL-1β COX-2 MMPs Akt/scleraxis Bax/caspase-3 | Resveratrol regulated tenocytes homeostatic and inhibited inflammation of cascades and apoptosis. | 5 µM of resveratrol | [62] |
| in vitro, human tenocytes | Sirt-1 | Resveratrol averted dexamethasone-induced senescence despite glucocorticoid treatment. | 30 µM of resveratrol | [207] | ||
| in vitro, human tenocytes | NF-κB PI3K IL-1β scleraxis | Resveratrol inhibited inflammation cascades; prevented apoptosis; and promoted collagen I, collagen III, and tenomodulin expression. | 0.1–20 µM of resveratrol | [14] | ||
| in vitro, Wistar rat tail tendon | ROS | Resveratrol’s derivate polydatin protected from advanced glycation as an anti-oxidant property. | 50–500 µg of polydatin | [209] | ||
| in vivo, Sprague–Dawley rats | - | Resveratrol promoted the collagens and the healing process of Achilles tendinopathy, despite diabetic condition. | 10 mg/kg of resveratrol for 14 days | [61] |
5. Anti-Inflammatory Effect of Nutraceuticals on Tendinitis in Clinical Trials
6. Discussion and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | advanced glycation end-products |
| Akt | serine/threonine kinase B |
| AP | alkaline phosphatase |
| ASU | avocado and soybean unsaponifiables |
| BA | Boswellia acid |
| COPD | chronic destructive pulmonary disease |
| COX | cyclooxygenase |
| CS | chondroitin sulfate |
| ECM | extracellular matrix |
| EGCG | epigallochatechin-3-gallate |
| ESWT | extracorporeal shock wave therapy |
| FOXO1 | forkhead box protein O1 |
| GLU | glucosamine |
| GPCR | G-protein-coupled receptor |
| GPX | glutathione peroxidase |
| GSK3β | glycogen synthase kinase 3 beta |
| HOPro | hydroxyproline |
| IBD | inflammatory bowel disease |
| IκBα | NF-κB inhibitor alpha |
| IKK | IκB kinase |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JAK | Janus kinase |
| MAPK | mitogen-activated protein kinase |
| MAPKK | MAPK kinase |
| MAPKKK | MAPKK kinase |
| MDA | malondialdehyde |
| MMP | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| PAF | platelet activating factor |
| Pcna | proliferating cell nuclear antigen |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide-3-kinase |
| PIP3 | phosphatidylinositol 3,4,5-triphosphate |
| RA | rheumatoid arthritis |
| RANKL | receptor activator of NF-κB ligand |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| STAT3 | signal transducer and activator of transcription 3 |
| Timp1 | tissue inhibitor of MMP1 |
| TNF | tumor necrosis factor |
| TLR | toll-like receptor |
| VEGF | vascular endothelial growth factor |
References
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: Development, repair, regeneration, and healing. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.A.; Beutler, A. Management of chronic tendon injuries. Am. Fam. Physician 2013, 87, 486–490. [Google Scholar] [PubMed]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surg. J. R. Coll. Surg. Edinb. Irel. 2017, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Sendzik, J.; Shakibaei, M.; Schäfer-Korting, M.; Lode, H.; Stahlmann, R. Synergistic effects of dexamethasone and quinolones on human-derived tendon cells. Int. J. Antimicrob. Agents 2010, 35, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Wu, W.; Cortes, D.; Rochon, P.A. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. 2013, 36, 709–721. [Google Scholar] [CrossRef]
- Rolf, C.; Movin, T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997, 18, 565–569. [Google Scholar] [CrossRef]
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Wells, G.; Appleton, L.; et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367. [Google Scholar] [CrossRef]
- Zhong, J.; Shi, G. Editorial: Regulation of Inflammation in Chronic Disease. Front. Immunol. 2019, 10, 737. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB Signaling by Calebin A, a Compound of Turmeric, in Multicellular Tumor Microenvironment: Potential Role of Apoptosis Induction in CRC Cells. Biomedicines 2020, 8, 236. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Shabnam, B.; Girisa, S.; Harsha, C.; Banik, K.; Devi, T.B.; Choudhury, R.; Sahu, H.; Parama, D.; Sailo, B.L.; et al. Inflammation, NF-κB, and Chronic Diseases: How are They Linked? Crit. Rev. Immunol. 2020, 40, 1–39. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. NF-κB in cancer: A matter of life and death. Cancer Discov. 2011, 1, 469–471. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Kannappan, R.; Reuter, S.; Dougherty, P.M.; Aggarwal, B.B. Role of nuclear factor κB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp. Biol. Med. 2011, 236, 658–671. [Google Scholar] [CrossRef]
- Bernard-Beaubois, K.; Hecquet, C.; Houcine, O.; Hayem, G.; Adolphe, M. Culture and characterization of juvenile rabbit tenocytes. Cell Biol. Toxicol. 1997, 13, 103–113. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator cuff calcific tendinopathy: From diagnosis to treatment. Acta Bio-Med. Atenei Parm. 2018, 89, 186–196. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Reviews. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. TEM 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. PR 2007, 59, 247–258. [Google Scholar]
- Grosser, T.; Ricciotti, E.; FitzGerald, G.A. The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol. Sci. 2017, 38, 733–748. [Google Scholar] [CrossRef]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. J. Int. Soc. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Riley, G.P.; Cox, M.; Harrall, R.L.; Clements, S.; Hazleman, B.L. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J. Hand Surg. 2001, 26, 224–228. [Google Scholar] [CrossRef]
- Tillander, B.; Franzén, L.E.; Karlsson, M.H.; Norlin, R. Effect of steroid injections on the rotator cuff: An experimental study in rats. J. Shoulder Elb. Surg. 1999, 8, 271–274. [Google Scholar] [CrossRef]
- Akpinar, S.; Hersekli, M.A.; Demirors, H.; Tandogan, R.N.; Kayaselcuk, F. Effects of methylprednisolone and betamethasone injections on the rotator cuff: An experimental study in rats. Adv. Ther. 2002, 19, 194–201. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Brockmueller, A.; Kunnumakkara, A.B.; Shakibaei, M. Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling. Int. J. Mol. Sci. 2022, 23, 1695. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-Cancer Effects of Green Tea Polyphenols Against Prostate Cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflam. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Antioxidant therapies in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed]
- Van Iersel, L.E.J.; Beijers, R.; Gosker, H.R.; Schols, A. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: A systematic review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef]
- Biswas, S.; Hwang, J.W.; Kirkham, P.A.; Rahman, I. Pharmacological and dietary antioxidant therapies for chronic obstructive pulmonary disease. Curr. Med. Chem. 2013, 20, 1496–1530. [Google Scholar] [CrossRef]
- Nardo, V.D.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of Curcumin in Psoriasis. Open Access Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef]
- Maioli, E.; Valacchi, G. Rottlerin: Bases for a possible usage in psoriasis. Curr. Drug Metab. 2010, 11, 425–430. [Google Scholar] [CrossRef]
- Dimitris, D.; Ekaterina-Michaela, T.; Christina, K.; Ioannis, S.; Ioanna, S.K.; Aggeliki, L.; Sophia, H.; Michael, R.; Helen, S. Melissa officinalis ssp. altissima extracts: A therapeutic approach targeting psoriasis in mice. J. Ethnopharmacol. 2020, 246, 112208. [Google Scholar] [CrossRef]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed]
- Vahdat-Lasemi, F.; Aghaee-Bakhtiari, S.H.; Tasbandi, A.; Jaafari, M.R.; Sahebkar, A. Targeting interleukin-β by plant-derived natural products: Implications for the treatment of atherosclerotic cardiovascular disease. Phytother. Res. PTR 2021, 35, 5596–5622. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Athanassiou, P.; Athanassiou, L.; Kostoglou-Athanassiou, I. Nutritional Pearls: Diet and Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2020, 31, 319–324. [Google Scholar] [CrossRef]
- Bellavia, D.; Caradonna, F.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Giavaresi, G. Non-flavonoid polyphenols in osteoporosis: Preclinical evidence. Trends Endocrinol. Metab. TEM 2021, 32, 515–529. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
- Li, X.; Lin, F.; Wu, Y.; Liu, N.; Wang, J.; Chen, R.; Lu, Z. Resveratrol attenuates inflammation environment-induced nucleus pulposus cell senescence in vitro. Biosci. Rep. 2019, 39, BSR20190126. [Google Scholar] [CrossRef]
- Zeytin, K.; Ciloğlu, N.S.; Ateş, F.; Vardar Aker, F.; Ercan, F. The effects of resveratrol on tendon healing of diabetic rats. Acta Orthop. Et Traumatol. Turc. 2014, 48, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Mobasheri, A.; Shayan, P.; Stahlmann, R.; Shakibaei, M. Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J. Biol. Chem. 2012, 287, 25770–25781. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Muralidharan, C.; Jayakumar, R. Study on the stabilisation of collagen with vegetable tannins in the presence of acrylic polymer. Biomaterials 2002, 23, 2841–2847. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, P.; Lin, H.; Geng, H. Curcumin improves tendon healing in rats: A histological, biochemical, and functional evaluation. Connect. Tissue Res. 2016, 57, 20–27. [Google Scholar] [CrossRef]
- Henrotin, Y.; Dierckxsens, Y.; Delisse, G.; Seidel, L.; Albert, A. Curcuminoids and Boswellia serrata extracts combination decreases tendinopathy symptoms: Findings from an open-label post-observational study. Curr. Med. Res. Opin. 2021, 37, 423–430. [Google Scholar] [CrossRef]
- Schindler, J.F.; Monahan, J.B.; Smith, W.G. p38 pathway kinases as anti-inflammatory drug targets. J. Dent. Res. 2007, 86, 800–811. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 2018, 35, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Bournazou, E.; Bromberg, J. Targeting the tumor microenvironment: JAK-STAT3 signaling. Jak-Stat 2013, 2, e23828. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Hawkins, P.T.; Stephens, L.R. PI3K signalling in inflammation. Biochim. Biophys. Acta 2015, 1851, 882–897. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Yadav, S.S.; Tewari, A.K. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014, 114, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Aggarwal, B.B. Nuclear factor-kappaB: A friend or a foe in cancer? Biochem. Pharmacol. 2004, 68, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Li, L.; Aggarwal, B.B.; Shishodia, S.; Abbruzzese, J.; Kurzrock, R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 2004, 101, 2351–2362. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Castilho, P.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Die Pharm. 2013, 68, 689–694. [Google Scholar]
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That Calebin A, a Component of Curcuma Longa Suppresses NF-B Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-β (Lymphotoxin). Nutrients 2019, 11, 2904. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Jensen, G.S.; Wu, X. Phenolic acids of the two major blueberry species in the US Market and their antioxidant and anti-inflammatory activities. Plant Foods Hum. Nutr. 2015, 70, 56–62. [Google Scholar] [CrossRef]
- Aranaz, P.; Romo-Hualde, A.; Zabala, M.; Navarro-Herrera, D.; Ruiz de Galarreta, M.; Gil, A.G.; Martinez, J.A.; Milagro, F.I.; González-Navarro, C.J. Freeze-dried strawberry and blueberry attenuates diet-induced obesity and insulin resistance in rats by inhibiting adipogenesis and lipogenesis. Food Funct. 2017, 8, 3999–4013. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Kuner, P.; Schubenel, R.; Hertel, C. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J. Neurosci. Res. 1998, 54, 798–804. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Q.; Duan, P.; Yang, L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2018, 40, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, Y.; Li, A.; Yu, P.; Song, L.; Liang, J.; Cao, N.; Gao, J.; Xu, R.; Ma, Y.; et al. Curcumin reverses hepatic epithelial mesenchymal transition induced by trichloroethylene by inhibiting IL-6R/STAT3. Toxicol. Mech. Methods 2021, 31, 589–599. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: A novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012, 21, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Cai, G.H.; Chen, Y.; Shi, X.C.; Zhang, C.C.; Xia, B.; Xie, B.C.; Liu, H.; Zhang, R.X.; et al. A Potential Nutraceutical Candidate Lactucin Inhibits Adipogenesis through Downregulation of JAK2/STAT3 Signaling Pathway-Mediated Mitotic Clonal Expansion. Cells 2020, 9, 331. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, C.Y.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Jung, C.; Sohn, H.Y.; Lee, T.J. Lactucin induces apoptosis through reactive oxygen species-mediated BCL-2 and CFLAR(L) downregulation in Caki-1 cells. Genes Genom. 2021, 43, 1199–1207. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-eIF2α-CHOP Pathway through Promoting SIRT1 Expression in Oxidative Stress-induced Rat Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Oxidative Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ye, Y.; Yuan, W.; Tan, Y.; Zhang, S.; Meng, Q. Curcumin exerts a protective effect on murine knee chondrocytes treated with IL-1β through blocking the NF-κB/HIF-2α signaling pathway. Ann. Transl. Med. 2021, 9, 940. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomed. Int. J. Phytother. Phytopharm. 2019, 63, 153002. [Google Scholar] [CrossRef]
- Aiyegbusi, A.I.; Duru, F.I.; Anunobi, C.C.; Noronha, C.C.; Okanlawon, A.O. Bromelain in the early phase of healing in acute crush Achilles tendon injury. Phytother. Res. PTR 2011, 25, 49–52. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Liu, M.; Hu, J.; Tang, C.; Huang, J.; Qin, T.; Chen, X.; Chen, W.; Shen, W.; et al. Controlled-release curcumin attenuates progression of tendon ectopic calcification by regulating the differentiation of tendon stem/progenitor cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109711. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; De Oliveira, L.P.; Da Ré Guerra, F.; Marcondes, M.C.; Pimentel, E.R. Green Tea and Glycine Modulate the Activity of Metalloproteinases and Collagen in the Tendinitis of the Myotendinous Junction of the Achilles Tendon. Anat. Rec. 2016, 299, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Hah, Y.S.; Yang, J.W.; Nam, J.B.; Cho, S.H.; Jeong, S.T. Antiapoptotic effects of anthocyanins on rotator cuff tenofibroblasts. J. Orthop. Res. 2010, 28, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Sodagari, H.R.; Esfahani, S.A.; Rezaei, N. A mechanistic review on plant-derived natural compounds as dietary supplements for prevention of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 745–758. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Olivares-Vicente, M.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Segura-Carretero, A.; Joven, J.; Encinar, J.A.; Micol, V. Plant-Derived Polyphenols in Human Health: Biological Activity, Metabolites and Putative Molecular Targets. Curr. Drug Metab. 2018, 19, 351–369. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Speed, C. Inflammation in Tendon Disorders. Adv. Exp. Med. Biol. 2016, 920, 209–220. [Google Scholar] [CrossRef]
- McHugh, J. Targeting NF-κB in tendinopathy. Nat. Rev. Rheumatol. 2019, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Hui, C.W.; Li, L.C.; Cheuk, Y.C.; Qin, L.; Gao, J.; Chan, K.M. Total flavones of Hippophae rhamnoides promotes early restoration of ultimate stress of healing patellar tendon in a rat model. Med. Eng. Phys. 2005, 27, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.; Sabitha, K.E.; Shyamaladevi, C.S. Effect of green tea extract on advanced glycation and cross-linking of tail tendon collagen in streptozotocin induced diabetic rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, Y.; Zhang, J.; Bai, L.; Xu, M.; Han, Q.; Han, X.; Xiu, J.; Li, M.; Zhou, X.; et al. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg2+/curcumin from injectable self-healing hydrogels. Theranostics 2021, 11, 5911–5925. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Tian, Z.; Li, G.; Liu, G.; Zhang, H. Effect of Eriocitrin on Cell Proliferation, Apoptosis, Migration, and Scar Formation-Related Genes Expression in Tendon Stem Cells. Doklady. Biochem. Biophys. 2021, 500, 402–407. [Google Scholar] [CrossRef]
- Corps, A.N.; Curry, V.A.; Buttle, D.J.; Hazleman, B.L.; Riley, G.P. Inhibition of interleukin-1beta-stimulated collagenase and stromelysin expression in human tendon fibroblasts by epigallocatechin gallate ester. Matrix Biol. J. Int. Soc. Matrix Biol. 2004, 23, 163–169. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kandemir, F.M.; Kaynar, O. Evaluation of Therapeutic Effects of Quercetin Against Achilles Tendinopathy in Rats via Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Metalloproteinases. Am. J. Sports Med. 2022, 50, 486–498. [Google Scholar] [CrossRef]
- Davis, M.E.; Gumucio, J.P.; Sugg, K.B.; Bedi, A.; Mendias, C.L. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J. Appl. Physiol. 2013, 115, 884–891. [Google Scholar] [CrossRef]
- Diniz-Fernandes, T.; Godoy-Santos, A.L.; Santos, M.C.; Pontin, P.; Pereira, C.A.A.; Jardim, Y.J.; Velosa, A.P.P.; Maffulli, N.; Teodoro, W.R.; Capelozzi, V.L. Matrix metalloproteinase-1 (MMP-1) and (MMP-8) gene polymorphisms promote increase and remodeling of the collagen III and V in posterior tibial tendinopathy. Histol. Histopathol. 2018, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, A.E.; Bragdon, G.A.; Jacobson, J.A.; Hasslund, S.; Cortes, Z.E.; Schwarz, E.M.; Mitten, D.J.; Awad, H.A.; O’Keefe, R.J. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J. Orthop. Res. 2009, 27, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Dowlati Beirami, A.; et al. Avocado-Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Avocado-soybean unsaponifiables (ASU) for osteoarthritis—A systematic review. Clin. Rheumatol. 2003, 22, 285–288. [Google Scholar] [CrossRef]
- Angermann, P. Avocado/soybean unsaponifiables in the treatment of knee and hip osteoarthritis. Ugeskr. Laeger 2005, 167, 3023–3025. [Google Scholar]
- Al-Afify, A.S.A.; El-Akabawy, G.; El-Sherif, N.M.; El-Safty, F.E.A.; El-Habiby, M.M. Avocado soybean unsaponifiables ameliorates cartilage and subchondral bone degeneration in mono-iodoacetate-induced knee osteoarthritis in rats. Tissue Cell 2018, 52, 108–115. [Google Scholar] [CrossRef]
- Grzanna, M.W.; Au, R.Y.; Au, A.Y.; Rashmir, A.M.; Frondoza, C.G. Avocado/Soybean Unsaponifiables, Glucosamine and Chondroitin Sulfate Combination Inhibits Proinflammatory COX-2 Expression and Prostaglandin E2 Production in Tendon-Derived Cells. J. Med. Food 2020, 23, 139–146. [Google Scholar] [CrossRef]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef]
- Orsini, R.A. Bromelain. Plast. Reconstr. Surg. 2006, 118, 1640–1644. [Google Scholar] [CrossRef]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef]
- Aiyegbusi, A.I.; Duru, F.I.; Awelimobor, D.; Noronha, C.C.; Okanlawon, A.O. The role of aqueous extract of pineapple fruit parts on the healing of acute crush tendon injury. Niger. Q. J. Hosp. Med. 2010, 20, 223–227. [Google Scholar]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, A.I.; Olabiyi, O.O.; Duru, F.I.; Noronha, C.C.; Okanlawon, A.O. A comparative study of the effects of bromelain and fresh pineapple juice on the early phase of healing in acute crush achilles tendon injury. J. Med. Food 2011, 14, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Sindona, C.; Bramanti, P.; Mazzon, E. A State of the Art of Antioxidant Properties of Curcuminoids in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 3168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A, a novel component of turmeric, suppresses NF-κB regulated cell survival and inflammatory gene products leading to inhibition of cell growth and chemosensitization. Phytomed. Int. J. Phytother. Phytopharm. 2017, 34, 171–181. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem. Pharmacol. 1998, 56, 1607–1614. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Comes Franchini, M.; Xu, K.; Locatelli, E.; Martin, R.C.; Monaco, I.; Li, Y.; Cui, S. Controlled release of curcumin from curcumin-loaded nanomicelles to prevent peritendinous adhesion during Achilles tendon healing in rats. Int. J. Nanomed. 2016, 11, 2873–2881. [Google Scholar] [CrossRef]
- Güleç, A.; Türk, Y.; Aydin, B.K.; Erkoçak, Ö.F.; Safalı, S.; Ugurluoglu, C. Effect of curcumin on tendon healing: An experimental study in a rat model of Achilles tendon injury. Int. Orthop. 2018, 42, 1905–1910. [Google Scholar] [CrossRef]
- Fusini, F.; Bisicchia, S.; Bottegoni, C.; Gigante, A.; Zanchini, F.; Busilacchi, A. Nutraceutical supplement in the management of tendinopathies: A systematic review. Muscles Ligaments Tendons J. 2016, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugan, P. Influence of tetrahydrocurcumin on tail tendon collagen contents and its properties in rats with streptozotocin-nicotinamide-induced type 2 diabetes. Fundam. Clin. Pharmacol. 2007, 21, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Rutter, K.; Sell, D.R.; Fraser, N.; Obrenovich, M.; Zito, M.; Starke-Reed, P.; Monnier, V.M. Green tea extract suppresses the age-related increase in collagen crosslinking and fluorescent products in C57BL/6 mice. Int. J. Vitam. Nutr. Res. 2003, 73, 453–460. [Google Scholar] [CrossRef]
- Vieira, C.P.; Guerra Fda, R.; de Oliveira, L.P.; Almeida, M.S.; Marcondes, M.C.; Pimentell, E.R. Green tea and glycine aid in the recovery of tendinitis of the Achilles tendon of rats. Connect. Tissue Res. 2015, 56, 50–58. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Manthey, J.A.; Nery, M.S.; Cesar, T.B. Pharmacokinetics and Biodistribution of Eriocitrin in Rats. J. Agric. Food Chem. 2021, 69, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Deng, B.; Zhang, B.; Luo, Q.; Song, G. Stretch-Induced Tenomodulin Expression Promotes Tenocyte Migration via F-Actin and Chromatin Remodeling. Int. J. Mol. Sci. 2021, 22, 4928. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.E.; Al-Nakkash, L.; Peterson, A.; Gump, B.S.; Janjulia, T.; Moore, M.S.; Broderick, T.L.; Carroll, C.C. The soy isoflavone genistein inhibits the reduction in Achilles tendon collagen content induced by ovariectomy in rats. Scand. J. Med. Sci. Sports 2012, 22, e108–e114. [Google Scholar] [CrossRef]
- Carroll, C.C.; Patel, S.H.; Simmons, J.; Gordon, B.D.; Olson, J.F.; Chemelewski, K.; Saw, S.; Hale, T.M.; Howden, R.; Sabbaghi, A. The Impact of Genistein Supplementation on Tendon Functional Properties and Gene Expression in Estrogen-Deficient Rats. J. Med. Food 2020, 23, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Sanches Silva, A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Hypotensive effects of genistein: From chemistry to medicine. Chem. Biol. Interact. 2017, 268, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Huang, Y.; Wismeijer, D.; Liu, Y. Icariin: Does it have an osteoinductive potential for bone tissue engineering? Phytother. Res. PTR 2014, 28, 498–509. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, W.; Wang, S.; Jiang, S.; Yu, Y.; Chen, E.; Xue, D.; Chen, J.; He, R. Icariin Promotes Tendon-Bone Healing during Repair of Rotator Cuff Tears: A Biomechanical and Histological Study. Int. J. Mol. Sci. 2016, 17, 1780. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.K.; Fu, S.C.; Lee, Y.W.; Mok, T.Y.; Chan, K.M. Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J. Bone Jt. Surgery. Am. Vol. 2013, 95, e41. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, K.; Zhang, P.; Zhang, J.; Chen, P.; He, J.; Fang, Y.; Zhou, Y.; Wang, J.; Bai, J. Quercetin reduces tendon adhesion in rat through suppression of oxidative stress. BMC Musculoskelet. Disord. 2020, 21, 608. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef]
- De Ligt, M.; Timmers, S.; Schrauwen, P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta 2015, 1852, 1137–1144. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Lin, K.; Yu, R.; Zhu, P.; Zhang, Y.; Wang, L.; Xu, J.; Chen, K. Resveratrol Protects the Myocardium in Sepsis by Activating the Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway and Inhibiting the Nuclear Factor-κB (NF-κB) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9290–9298. [Google Scholar] [CrossRef]
- Constanze, B.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β suppresses osteoblast differentiation of mesenchymal stem cells and resveratrol reverses it through modulation of NF-κB, Sirt1 and Runx2. Cell Tissue Res. 2020, 381, 83–98. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Watts, A.C.; Murphy, R.J.; Snelling, S.J.; Carr, A.J.; Hulley, P.A. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: In vivo and in vitro evidence. Ann. Rheum. Dis. 2014, 73, 1405–1413. [Google Scholar] [CrossRef]
- Mikolyzk, D.K.; Wei, A.S.; Tonino, P.; Marra, G.; Williams, D.A.; Himes, R.D.; Wezeman, F.H.; Callaci, J.J. Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J. Bone Jt. Surgery. Am. Vol. 2009, 91, 1172–1180. [Google Scholar] [CrossRef]
- Selvakumar, G.; Venu, D.; Kuttalam, I.; Lonchin, S. Inhibition of Advanced Glycation End Product Formation in Rat Tail Tendons by Polydatin and p-Coumaric acid: An In Vitro Study. Appl. Biochem. Biotechnol. 2022, 194, 339–353. [Google Scholar] [CrossRef]
- Riva, A.; Allegrini, P.; Franceschi, F.; Togni, S.; Giacomelli, L.; Eggenhoffner, R. A novel boswellic acids delivery form (Casperome®) in the management of musculoskeletal disorders: A review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5258–5263. [Google Scholar] [CrossRef]
- Franceschi, F.; Togni, S.; Belcaro, G.; Dugall, M.; Luzzi, R.; Ledda, A.; Pellegrini, L.; Eggenhoffner, R.; Giacomelli, L. A novel lecithin based delivery form of Boswellic acids (Casperome®) for the management of osteo-muscular pain: A registry study in young rugby players. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4156–4161. [Google Scholar] [PubMed]
- Merolla, G.; Dellabiancia, F.; Ingardia, A.; Paladini, P.; Porcellini, G. Co-analgesic therapy for arthroscopic supraspinatus tendon repair pain using a dietary supplement containing Boswellia serrata and Curcuma longa: A prospective randomized placebo-controlled study. Musculoskelet. Surg. 2015, 99, S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Naim Rodriguez, N.; Pironti, P.; Drossinos, A.; Di Carlo, G.; Chawla, A.; Gianfranco, F. ESWT and nutraceutical supplementation (Tendisulfur Forte) vs. ESWT-only in the treatment of lateral epicondylitis, Achilles tendinopathy, and rotator cuff tendinopathy: A comparative study. J. Drug Assess. 2019, 8, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Pesce, V.; Vicenti, G.; Tafuri, S.; Forcignanò, M.; Moretti, B. SWAAT study: Extracorporeal shock wave therapy and arginine supplementation and other nutraceuticals for insertional Achilles tendinopathy. Adv. Ther. 2012, 29, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Gumina, S.; Passaretti, D.; Gurzì, M.D.; Candela, V. Arginine L-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: A prospective randomized study. Curr. Med. Res. Opin. 2012, 28, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Tafuri, S.; Fiore, A.; Pesce, V.; Moretti, B. Comparison of shock wave therapy and nutraceutical composed of Echinacea angustifolia, alpha lipoic acid, conjugated linoleic acid and quercetin (perinerv) in patients with carpal tunnel syndrome. Int. J. Immunopathol. Pharmacol. 2015, 28, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. PTR 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Shi, Q.; Lang, W.; Wang, S.; Li, G.; Bai, X.; Yan, X.; Zhang, H. Echinacea polysaccharide attenuates lipopolysaccharide-induced acute kidney injury via inhibiting inflammation, oxidative stress and the MAPK signaling pathway. Int. J. Mol. Med. 2021, 47, 243–255. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, W.; Wang, S.; Li, B.; Li, G.; Shi, Q. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int. Immunopharmacol. 2020, 88, 106974. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Sharip, A.; Kunz, J. Understanding the Pathogenesis of Spondyloarthritis. Biomolecules 2020, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Alagheband Bahrami, A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Sari, Z.; Aydoğdu, O.; Demirbüken, İ.; Yurdalan, S.U.; Polat, M.G. A Better Way to Decrease Knee Swelling in Patients with Knee Osteoarthritis: A Single-Blind Randomised Controlled Trial. Pain Res. Manag. 2019, 2019, 8514808. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, A.-L.; Brockmueller, A.; Kunnumakkara, A.B.; Shakibaei, M. Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis. Nutrients 2022, 14, 2030. https://doi.org/10.3390/nu14102030
Mueller A-L, Brockmueller A, Kunnumakkara AB, Shakibaei M. Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis. Nutrients. 2022; 14(10):2030. https://doi.org/10.3390/nu14102030
Chicago/Turabian StyleMueller, Anna-Lena, Aranka Brockmueller, Ajaikumar B. Kunnumakkara, and Mehdi Shakibaei. 2022. "Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis" Nutrients 14, no. 10: 2030. https://doi.org/10.3390/nu14102030
APA StyleMueller, A.-L., Brockmueller, A., Kunnumakkara, A. B., & Shakibaei, M. (2022). Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis. Nutrients, 14(10), 2030. https://doi.org/10.3390/nu14102030