Abstract
The Japanese dietary pattern has long been discussed as one of the factors behind the longevity of Japanese people. However, the health benefits of the Japanese dietary pattern have not been fully elucidated. We published the first report in the world regarding the relation between the Japanese dietary pattern and cardiovascular disease mortality in 2007 using cohort studies including Japanese residents of Ohsaki City, Miyagi Prefecture, Japan. Since then, we have developed the Japanese Diet Index (JDI) that was based on previous findings to assess the degree of the Japanese dietary pattern and to advance the evidence on the health effects of the Japanese dietary pattern. So far, we have explored the associations between the JDI score (in quartiles) and various outcomes. For all-cause mortality, in comparison to Q1 (the lowest), the multivariable hazard ratios (HRs) and 95% confidence intervals (95%CIs) were 0.92 (0.85–1.00) for Q2, 0.91 (0.83–0.99) for Q3, and 0.91 (0.83–0.99) for Q4 (the highest). For functional disability, the multivariable HRs (95%CIs) were 0.94 (0.81–1.09) for Q2, 0.90 (0.77–1.05) for Q3, and 0.79 (0.68–0.92) for Q4. For dementia, the multivariable HRs (95%CIs) were 0.88 (0.74–1.05) for Q2, 0.87 (0.73–1.04) for Q3, 0.79 (0.66–0.95) for Q4. In addition, people with higher adherence to the Japanese dietary pattern also showed decreases in disability and dementia risks. The purpose of this article was to review all six papers, summarize the health effects of the Japanese dietary pattern, and discuss implications for future research.
1. Introduction
Why does the Japanese population not only have the longest life expectancy (84.3 years for both sex) but also have the longest healthy life expectancy (74.1 years for both sex) worldwide [1]? The Japanese dietary pattern has long been discussed as one of the factors behind the longevity of Japanese people [2]. However, the health benefits of the Japanese dietary pattern have not been fully elucidated, and with respect to this, in the Dietary Guidelines for Americans 2010 it is stated that “evidence is very limited” [3].
We published the first report in the world regarding the relation between the Japanese dietary pattern and cardiovascular disease (CVD) mortality in 2007. In this study, we prospectively followed a cohort of 40,547 Japanese people for seven years, and results showed that the Japanese dietary pattern had a significant association with decreased CVD mortality. [4] However, the Japanese dietary pattern used as the exposure in this study was simply one dietary pattern derived by factor analysis, so it was unclear whether greater conformity with the Japanese dietary pattern had an association with reduced CVD mortality. According to a previous review, the Japanese dietary pattern is usually demonstrated as a higher intake of soybean products, fish, vegetables, rice, seaweed, miso soup, pickles, and green tea [5]. Such foods as fish, and vegetables are in common with components included in the well-known healthy dietary patterns such as the Mediterranean diet [6], Dietary Approaches to Stop Hypertension (DASH) [7], and Healthy Eating Index (HEI) [8], which have been proven to be protective against a variety of chronic diseases [9,10,11]. Meanwhile, the Japanese dietary pattern also includes such unique components as seaweed, soybean, and green tea, each of which has been suggested to have potential benefits on health [12,13,14]. Thus, it is expected that the Japanese dietary pattern per se could play a critical role in health.
Our team has developed the Japanese Diet Index (JDI) including rice, miso soup, pickles, seaweeds, fish, green and yellow vegetables, green tea, beef and pork, and coffee, which is a priori diet index from predefined algorithms based on previous findings to quantify the adherence to the Japanese dietary pattern and to advance the evidence on its health benefits [15]. So far, we have published six articles regarding the relation between the JDI and such health outcomes as mortality, disability, and dementia [15,16,17,18,19,20]. In this article, we review all six papers, summarize the health benefits of the Japanese diet, and discuss possible implications for the future.
2. Materials and Methods
2.1. Development of the Japanese Diet Index
We have described the details of the JDI in a previous study [15]. In brief, nine food items were identified to form the JDI: rice, miso soup, pickles, seaweeds, fish, green and yellow vegetables, green tea, beef and pork, and coffee. These items were suggested to have higher absolute factor scores in the traditional Japanese dietary pattern [21], and were the main characteristic of the traditional Japanese diet [22].
For each of the seven adhering components (rice, miso soup, pickles, seaweeds, fish, green and yellow vegetables, and green tea), participants were assigned one point if their intake was above or equal to the sex-specific median. For each of the two non-adhering components (beef and pork, and coffee), participants were assigned one point if their intake was lower than the sex-specific median. The JDI score was generated by adding the scores in the nine components (0–9 points), and a higher score indicated higher adherence to the Japanese dietary pattern. Participants were categorized in quartiles, but the way of categorization in our studies was not the same due to differences in the number of study participants included in each study. For the associations with all-cause mortality and disability-free life expectancy, participants were categorized as Q1 (0–4), Q2 (5), Q3 (6), and Q4 (7–9) [16,18], while for the associations with functional disability and dementia, participants were categorized as Q1 (0–3), Q2 (4), Q3 (5), and Q4 (6–9) [15,19].
We then modified the JDI and developed the 8-item Japanese Diet Index (JDI8), by excluding coffee, which was included in the original JDI [23], as a previous meta-analysis has indicated that coffee intake may have benefits on reducing all-cause and CVD mortality [24].
2.2. Study Setting
2.2.1. The Ohsaki Cohort 1994 Study (the 1994 Survey)
The Ohsaki Cohort 1994 Study was part of the Ohsaki National Health Insurance (NHI) Cohort Study, the detail of which was described in a previous study [25]. The NHI system covers all self-employed individuals, pensioners, and farmers and their dependents. The source population for the baseline survey comprised 54,996 individuals (40–79 years old), and all of them were NHI beneficiaries who lived in 14 municipalities within the catchment area of the Ohsaki Public Health Center, Miyagi Prefecture, Japan in 1994. In 2006, seven of fourteen municipalities were merged into the new Ohsaki City. Since then, the subjects of the seven municipalities (32,978 men and women in 1994) of the original Ohsaki NHI Cohort were followed in the name of the Ohsaki Cohort 1994 Study.
The 1994 survey was conducted from October to December 1994. Participants were asked to answer the questionnaires themselves and to return them within a week.
In 1994, the questionnaires were distributed to 32,126 of 32,978 eligible individuals, and of those, 30,552 with valid responses formed the study cohort. Then, we excluded 60 individuals who had died or moved out before follow-up and 806 individuals not identified by the Residential Registry of Ohsaki City. A total of 29,686 participants were eligible for follow-up, which was from 1 January 1995, to 30 November 2019 (Figure 1).
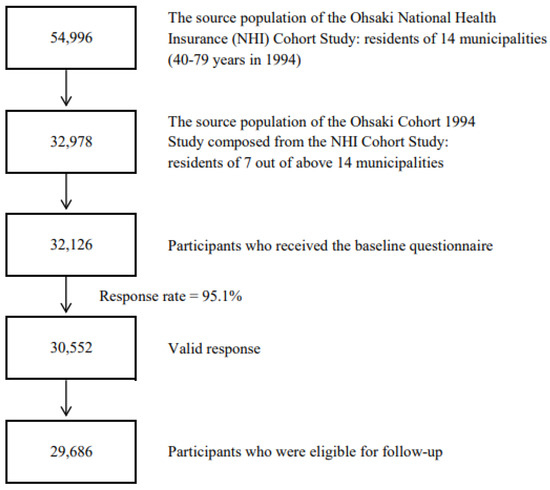
Figure 1.
Flowchart of study participants of the Ohsaki Cohort 1994 Study.
2.2.2. The Ohsaki Cohort 2006 Study (the 2006 Survey)
We have described the design in detail elsewhere [26]. The source population for the 2006 survey comprised all 31,694 older residents aged 65 years or older on 1 December 2006 living in Ohsaki City.
The 2006 survey was conducted from 1 December 2006 to 15 December 2006. Of all the older citizens, 23,091 of them provided valid answers and formed the study cohort. We then excluded 6333 persons without written consent for review of their Long-term Care Insurance (LTCI) information, 1979 individuals who had been certified as disabled by the LTCI before the start of follow-up, and five individuals who had died or moved before the start of follow-up. A total of 14,774 individuals were eligible for follow-up, and the follow-up started from 16 December 2006, until 30 November 2019. (Figure 2).
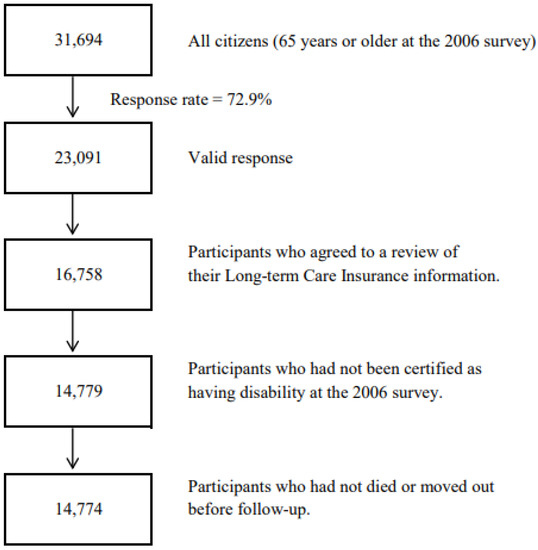
Figure 2.
Flowchart of study participants of the Ohsaki Cohort 2006 Study.
2.3. Study Outcomes and Measurement
We examined three outcomes: all-cause death, disability, and dementia. The all-cause deaths were identified via the review of death certificates, with approval from the Ministry of Health, Labour, and Welfare of Japan and the Ministry of Internal Affairs and Communications of Japan.
Incident functional disability was defined as Support Level 1 or higher by LTCI certification. The LTCI is a mandatory form of national social insurance to assist activities of daily living among the disabled and older adults [27,28,29]. People aged ≥40 years paying premiums, and those aged ≥65 years are eligible for formal caregiving services under a uniform standard of disability certification [30]. The procedure for disability certification comprises two parts: assessment of the degree of functional disability using a questionnaire, and reference to the Doctor’s Opinion Paper prepared by the attending physician [31].
Incident dementia was defined as incident functional disability with dementia by the LTCI, using the Dementia Scale (Degree of Independence in Daily Living for Elderly with Dementia) entered on the Doctor’s Opinion Paper [32]. The Dementia Scale includes six ranks: 0, I–IV, and M. Rank M means severe behavioral and psychological symptoms requiring medical care. A rank ≥II is usually applied as the cutoff for definition of dementia [32,33,34]. It has been reported that the Dementia Scale showed a sensitivity of 73% and a specificity of 96% against clinical diagnoses [35].
A dataset was obtained annually in December, including information on the care level of LTCI certification, date of LTCI certification, date of death, and date of emigration from the Ohsaki City Government based on an agreement about the secondary use of data. The dataset was transferred under the agreement related to Epidemiologic Research and Privacy Protection.
2.4. Covariates and Measurement
Data on sociodemographic, lifestyle, and health-related factors were obtained in both 1994 and 2006. In detail, education level was measured with the question, “How old were you when you left school?” and we categorized participants as junior high school or less (<16 years), high school (16–18 years) or college or higher (≥19 years). Body mass index (BMI) was calculated as the self-reported body weight (kg) divided by the square of the self-reported body height (m), and participants were categorized into <18.5 kg/m2, 18.5–24.9 kg/m2, and ≥25.0 kg/m2. Time spent walking was assessed with the question, ‘How long on average do you walk per day?’, and participants were categorized into <0.5 h, 0.5–1 h or ≥1 h. Smoking status was divided as follows: never smokers; former smokers; and current smokers. Similarly, alcohol drinking status was divided as: never drinkers; former drinkers; and current drinkers. We asked participants whether they had suffered from the following diseases: hypertension; stroke, myocardial infarction; diabetes; osteoporosis; arthritis; falling/fracture; or cancer. In both 1994 and 2006, the consumption volume of each food item was calculated and energy intake and protein intake were estimated using a food composition table that corresponded to all items in the FFQ.
Psychological distress was only evaluated in 2006 by the Kessler 6-item Psychological Distress Scale [36,37]. Participants were asked about their mental status over the past four weeks with six questions (0–24 points). A score ≥ 13 was classified as psychological distress [37].
The Kihon Checklist was used in 2006, which was developed to predict functional decline in community-dwelling older adults. Regarding the motor function score in the Kihon Checklist, respondents were asked about their current motor function status with five questions (0–5 points). A score of <3 were classified as better motor function. Regarding the cognitive function score, respondents were asked about their current subjective memory complaints with three questions (0–3 points). A score of zero was classified as better cognitive function [38].
3. Results
3.1. Japanese Diet, Mortality, and Length of Survival
To investigate the associations between the JDI score and mortality and the length of survival, we used the setting of the Ohsaki Cohort 1994 Study (Study No. 1 in Table 1) [16]. A total of 14,764 participants were categorized in quartiles (Q1: 0–4 points [n = 4782]; Q2: 5 points [n = 3243]; Q3: 6 points [n = 3219]; Q4: 7–9 points [n = 3520]).

Table 1.
Outline of published articles.
Multivariable Cox proportional hazard models were applied to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality, which adjusted for sex, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, and energy intake, using age as the time scale. Results showed that higher JDI scores were related to lower all-cause mortality. In comparison to participants in Q4 (the lowest), the multivariable HRs (95% CIs) for all-cause mortality were 0.92 (0.85–1.00) in Q2, 0.91 (0.83–0.99) in Q3, and 0.91 (0.83–0.99) in Q4 (Table 2).

Table 2.
Summary of published results.
We performed Laplace regression for estimating differences in survival time between the quartiles of the JDI score [16]. Laplace regression model estimates the percentile of the time variable of concern [39,40]. Differences in median (50th PDs) age at death and their 95% CIs were calculated. Results suggested that people with higher JDI scores had a longer survival time. The multivariable 50th PDs (95% CIs) of age at death were 8.9 (2.6–15.2) months longer in Q2, 10.4 (3.4–17.3) months longer in Q3, and 10.2 (3.2–17.2) months longer in Q4, in comparison with participants in Q1 (the lowest) (Table 2).
3.2. Japanese Diet, Disability, and the Length of Disability-Free Survival
First, we examined the association between the JDI score and incident functional disability, using the setting of the Ohsaki Cohort 2006 Study (Study No. 2 in Table 1) [15]. 10,148 individuals were categorized in quartiles (Q1: 0–3 points [n = 2247]; Q2: 4 points [n = 2096]; Q3: 5 points [n = 2400]; Q4: 6–9 points [n = 3405]). Multivariable Cox proportional hazard models were applied to estimate the HRs (95%CIs) for incident functional disability, which were adjusted for sex, age, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, psychological distress, motor function, energy intake and protein intake. Results suggested that people in a higher quartile of the JDI score showed lower incident risk of functional disability. The multivariable HRs (95% CIs) for incident functional disability were 0.94 (0.81–1.09) in Q2, 0.90 (0.77–1.05) in Q3, and 0.79 (0.68–0.92) in Q4, in comparison with Q1 (Table 2).
Second, the settings of both the Ohsaki Cohort 1994 Study and the Ohsaki Cohort 2006 Study were applied to investigate the effect of changes in the JDI score on incident risk of functional disability (Study No.3 in Table 1) [17]. We categorized a total of 2923 individuals into five groups according to changes in the JDI score between 1994 and 2006 (great decrease [≤–2 points, n = 462], moderate decrease [–1 point, n = 513], no change [=0, n = 648], moderate increase [+1 point, n = 642], and great increase [≥+2 points, n = 658]). Multivariable Cox proportional hazard models were applied to estimate the HRs (95%CIs) for incident functional disability, which were adjusted for sex, age, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, psychological distress, and energy intake in 2006 and smoking, alcohol drinking, time spent walking, body mass index, history of disease, the JDI score and energy intake in 1994. Results showed that participants with an increase in the JDI score had a decreased risk of functional disability. Compared to a great decrease (≤–2 points), the multivariable HRs (95% CIs) for incident functional disability were 0.82 (0.66–1.01) for moderate decrease, 0.80 (0.65–0.98) for no change, 0.76 (0.61–0.95) for moderate increase, and 0.77 (0.61–0.98) for great increase (Table 2).
Third, we also examined the relation of the JDI score to disability-free survival time, using the Ohsaki Cohort 2006 Study (Study No. 4 in Table 1) [18]. A total of 9456 participants were categorized in quartiles (Q1: 0–4 points [n = 3426]; Q2: 5 points [n = 2009]; Q3: six points [n = 1983]; Q4: 7–9 points [n = 2038]). Laplace regression was performed to calculate differences in median age at disability or death (50th PDs in age at incident disability or death) and their 95% CIs, adjusted for sex, age, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, psychological distress, number of remaining teeth, motor function, cognitive function, energy intake and protein intake. Results indicated that people with greater JDI scores showed longer disability-free survival time. In comparison to people in Q1, the disability-free survival time were 2.0 (–2.7–6.8) months longer in Q2, 5.9 (1.4–10.3) months longer in Q3, and 7.1 (1.8–12.4) months longer in Q4 (Table 2).
3.3. Japanese Diet and Dementia
We examined the relation between the JDI score and dementia, using the setting of the Ohsaki Cohort 2006 Study (Study No.5 in Table 1) [19]. We categorized 14,402 participants in quartiles (Q1: 0–3 points [n = 2921]; Q2: 4 points [n = 2644]; Q3: 5 points [n = 3130]; Q4: 6–9 points [n = 5707]). Multivariable Cox proportional hazard models were applied to estimate the HRs 95%CIs for incident dementia, which were adjusted for sex, age, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, psychological distress, number of remaining teeth, motor function, cognitive function, energy intake and protein intake. Results suggested that the JDI score had an inverse association with incident risk of dementia. Compared to Q1, the multivariable HRs (95% CIs) for incident dementia were 0.88 (0.74–1.05) in Q2, 0.87 (0.73–1.04) in Q3, and 0.79 (0.66–0.95) in Q4 (Table 2).
In addition, to investigate the effect of changes in the JDI8 score on incident dementia, we used both the Ohsaki Cohort 1994 Study and the Ohsaki Cohort 2006 Study (Study No. 6 in Table 1) [20]. A total of 3146 individuals were divided into five groups according to changes in the JDI8 score (great decrease [≤–2 points, n = 584], moderate decrease [–1 point, n = 599], no change [=0e, n = 784], moderate increase [+1 point, n = 635], and great increase [≥+2 points, n = 544]). Multivariable Cox proportional hazard models were applied to estimate the HRs 95%CIs for incident dementia, which were adjusted for sex, age, education level, alcohol drinking, smoking, time spent walking, body mass index, history of diseases, psychological distress, and energy intake in 2006 and alcohol drinking, smoking, time spent walking, body mass index, history of diseases, the JDI8 score, and energy intake in 1994. Results showed that people with an increase in the JDI8 score from 1994 to 2006 showed decreased risk of incident dementia. In comparison with no change group, the multivariable HRs (95% CIs) for incident dementia was 1.72 (1.13–2.62) in great decrease, 1.10 (0.73–1.66) in moderate decrease, 0.82 (0.54–1.25) in moderate increase, and 0.62 (0.38–1.02) in great increase (Table 2).
4. Discussion
Our findings have demonstrated that the Japanese dietary pattern has association with multiple health outcomes, such as longer survival and lower risks of disability and dementia. The Japanese dietary pattern was also significantly related to reduced CVD mortality. In Japan, both incident disability and dementia are largely due to CVD, so we consider that the reduction in CVD risk and the reductions in incident disability and dementia risk are consistent.
As these studies were observational, we cannot determine the causality of the Japanese dietary pattern with mortality, disability, and dementia. However, we also reported that people with an increase in the JDI score showed decreased risks of disability and dementia; thus, this may suggest a causal relationship of the Japanese dietary pattern with health benefits.
Various beneficial components are contained in the Japanese dietary pattern such as vegetables, fish, miso (made from soybean), seaweeds, pickles, and green tea. These unique foods may have helped reduce the risks of mortality, disability, and dementia, as it has been reported that these foods are related to decreased risks of all-cause and CVD mortality, CVD morbidity, incident disability, cognitive decline, and dementia [41,42,43,44,45,46,47]. In addition, the healthy foods that compose the Japanese dietary pattern include various beneficial nutrients, for instance, vegetables contain potassium, carotenoids, vitamin C, fiber, antioxidants, and flavonoids [48,49], fish contain DHA, EPA, and very-long-chain fatty acids [50,51,52], soybean products contain isoflavane [53,54], seaweeds contain dietary fiber [44], and green tea contains polyphenol catechins with antioxidative activity [30,55]. These nutrients have been reported to have preventive effects on non-communicable diseases that may cause disability and dementia. Therefore, it is considered that the reductions in risks of mortality, disability, and dementia were related to not only individual foods, but also the Japanese dietary pattern as a whole.
However, there is a concern about the higher amount of sodium contained in the Japanese dietary pattern. Regarding this issue, a previous study reported that people with greater adherence to the Japanese dietary pattern consumed not only sodium, but also such beneficial nutrients as protein, calcium, iron, magnesium, potassium, vitamins A, C, and E, and fiber [23,56]. Therefore, in the Japanese dietary pattern, the adverse health effects of sodium may be counteracted by other beneficial nutrients.
5. Implications for Future Research
So far, our studies have revealed associations between the Japanese dietary pattern and various health outcomes and consistently reported potential health benefits of the Japanese dietary pattern. These findings may be partly attributable to the cumulative effects of individual food items of the Japanese dietary pattern. Due to the complex biological interactions between various components included in the Japanese dietary pattern, using a whole diet approach instead of individual food groups or nutrients may contribute to understanding the role played by the Japanese dietary pattern in health and longevity [57].
Since our JDI was established in a region of northeast Japan, Ohsaki City, its generalizability must be verified. We have already examined the association between the JDI8 and mortality using the Japan Public Health Center-based Prospective Study dataset, participants of which were from 11 public health center areas in Japan [7]. A total of 92,969 participants were categorized in quartiles of the JDI8 score (Q1: 0–2 points [n = 16,838]; Q2: 3 points [n = 15,461]; Q3: 4–5 points [n = 36,196]; Q4: 6–8 points [n = 24,474]). Results suggested that people with a higher JDI8 score showed lower all-cause mortality. The multivariate-adjusted HRs (95% CIs) for all-cause mortality were 0.95 (0.90–0.99) in Q2, 0.91 (0.87–0.95) in Q3, and 0.86 (0.81–0.90) in Q4, compared to Q1. This result suggests that our findings may be generalizable to the whole Japanese population.
In addition, we believe that interventional studies remain a critical and irreplaceable tool in elucidating the health effects of the Japanese diet. Several interventional studies have been conducted on the Mediterranean diet, which is known to be a healthy diet. To promote discussions that can include the drawing of causal relationships between diet and health outcomes, it is necessary to conduct interventional studies in addition to observational studies.
Author Contributions
S.M.: Conceptualization, Writing—original draft. T.S.: Writing—review and editing. Y.T.: Writing—review and editing. S.Z.: Writing—review and editing. S.A.: Writing—review and editing. Y.L.: Writing—review and editing. I.T.: Conceptualization, Funding acquisition, Resources, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Foundation for Health, Labour and Welfare Policy Research Grants (19FA2001 and 22FA2001) from the Ministry of Health, Labour and Welfare of Japan and the Project of the NARO Bio-oriented Technology Research Advancement Institution (advanced integration research for agriculture and interdisciplinary fields) (17943029).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tohoku University Graduate School of Medicine (approval No. 2014-1-839 and 2016-1-586).
Informed Consent Statement
We considered the return of completed questionnaires to imply consent to participate in the study involving the baseline survey data and subsequent follow-up. We also confirmed information regarding LTCI certification status after obtaining written consent.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made publicly available because private information of participants was included but are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank Yoshiko Nakata for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CI | confidence interval |
| CVD | cardiovascular disease |
| HR | hazard ratio |
| JDI | Japanese diet Index |
| JDI8 | 8-item Japanese Diet Index |
| LTCI | Long-term Care Insurance |
| NHI | National Health Insurance |
References
- World Health Organization. Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/life-expectancy-and-healthy-life-expectancy (accessed on 29 April 2022).
- Ikeda, N.; Saito, E.; Kondo, N.; Inoue, M.; Ikeda, S.; Satoh, T.; Wada, K.; Stickley, A.; Katanoda, K.; Mizoue, T.; et al. What Has Made the Population of Japan Healthy? Lancet 2011, 378, 1094–1105. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2010, 7th ed.; U.S. Government Printing Office: Washington, DC, USA, 2010. [Google Scholar]
- Shimazu, T.; Kuriyama, S.; Hozawa, A.; Ohmori, K.; Sato, Y.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Dietary Patterns and Cardiovascular Disease Mortality in Japan: A Prospective Cohort Study. Int. J. Epidemiol. 2007, 36, 600–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the Japanese Diet Described in Epidemiologic Publications: A Qualitative Systematic Review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; Predimed Investigators. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. Int. Rev. J. 2016, 7, 889–904. [Google Scholar] [CrossRef] [Green Version]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e15. [Google Scholar] [CrossRef]
- Omoni, A.O.; Aluko, R.E. Soybean Foods and Their Benefits: Potential Mechanisms of Action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Watanabe, T.; Sugawara, Y.; Chou, W.-T.; Kakizaki, M.; Tsuji, I. Dietary Patterns and Incident Functional Disability in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. Ser. A 2014, 69, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Zhang, S.; Tomata, Y.; Tsuduki, T.; Sugawara, Y.; Tsuji, I. Japanese Diet and Survival Time: The Ohsaki Cohort 1994 Study. Clin. Nutr. 2020, 39, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, S.; Zhang, S.; Tomata, Y.; Abe, S.; Tanji, F.; Sugawara, Y.; Tsuji, I. Association between Improved Adherence to the Japanese Diet and Incident Functional Disability in Older People: The Ohsaki Cohort 2006 Study. Clin. Nutr. 2019, 39, 2238–2245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tomata, Y.; Sugawara, Y.; Tsuduki, T.; Tsuji, I. The Japanese Dietary Pattern Is Associated with Longer Disability-Free Survival Time in the General Elderly Population in the Ohsaki Cohort 2006 Study. J. Nutr. 2019, 149, 1245–1251. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Dietary Patterns and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. Ser. A 2016, 71, 1322–1328. [Google Scholar] [CrossRef]
- Lu, Y.; Matsuyama, S.; Sugawara, Y.; Sone, T.; Tsuji, I. Changes in a Specific Dietary Pattern and Incident Dementia: A Prospective Cohort Study. Clin. Nutr. 2021, 40, 3495–3502. [Google Scholar] [CrossRef]
- Okubo, H.; Murakami, K.; Sasaki, S.; Kim, M.K.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Relative Validity of Dietary Patterns Derived from a Self-Administered Diet History Questionnaire Using Factor Analysis among Japanese Adults. Public Health Nutr. 2010, 13, 1080–1089. [Google Scholar] [CrossRef]
- Nanri, A.; Shimazu, T.; Ishihara, J.; Takachi, R.; Mizoue, T.; Inoue, M.; Tsugane, S. Reproducibility and Validity of Dietary Patterns Assessed by a Food Frequency Questionnaire Used in the 5-Year Follow-Up Survey of the Japan Public Health Center-Based Prospective Study. J. Epidemiol. 2012, 22, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, S.; Sawada, N.; Tomata, Y.; Zhang, S.; Goto, A.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsuji, I.; Tsugane, S.; et al. Association between Adherence to the Japanese Diet and All-Cause and Cause-Specific Mortality: The Japan Public Health Center-Based Prospective Study. Eur. J. Nutr. 2021, 60, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Sciacca, S.; Pajak, A.; Martinez-Gonzalez, M.A.; Giovannucci, E.L.; Galvano, F. Coffee Consumption and Risk of All-Cause, Cardiovascular, and Cancer Mortality in Smokers and Non-smokers: A Dose-Response Meta-Analysis. Eur. J. Epidemiol. 2016, 31, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Nishino, Y.; Ohkubo, T.; Kuwahara, A.; Ogawa, K.; Watanabe, Y.; Tsubono, Y.; Bando, T.; Kanemura, S.; Izumi, Y.; et al. A Prospective Cohort Study on National Health Insurance Beneficiaries in Ohsaki, Miyagi Prefecture, Japan: Study Design, Profiles of the Subjects and Medical Cost During the First Year. J. Epidemiol. 1998, 8, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriyama, S.; Nakaya, N.; Ohmori-Matsuda, K.; Shimazu, T.; Kikuchi, N.; Kakizaki, M.; Sone, T.; Sato, F.; Nagai, M.; Sugawara, Y.; et al. The Ohsaki Cohort 2006 Study: Design of Study and Profile of Participants at Baseline. J. Epidemiol. 2010, 20, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikegami, N. Public Long-Term Care Insurance in Japan. JAMA 1997, 278, 1310–1314. [Google Scholar] [CrossRef]
- Imahashi, K.; Kawagoe, M.; Eto, F.; Haga, N. Clinical Status and Dependency of the Elderly Requiring Long-Term Care in Japan. Tohoku J. Exp. Med. 2007, 212, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, T.; Muramatsu, N. Care-Needs Certification in the Long-Term Care Insurance System of Japan. J. Am. Geriatr. Soc. 2005, 53, 522–527. [Google Scholar] [CrossRef]
- Tomata, Y.; Kakizaki, M.; Nakaya, N.; Tsuboya, T.; Sone, T.; Kuriyama, S.; Hozawa, A.; Tsuji, I. Green Tea Consumption and the Risk of Incident Functional Disability in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Clin. Nutr. 2012, 95, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, Y.; Tamiya, N.; Kamimura, A.; Sandoval, F.; Luptak, M. Doctors’ Opinion Papers in Long-term Care Need Certification in Japan: Comparison between Clinic and Advanced Treatment Hospital Settings. Public Policy Adm. Res. 2014, 4, 31–37. [Google Scholar]
- Ikeda, A.; Yamagishi, K.; Tanigawa, T.; Cui, R.; Yao, M.; Noda, H.; Umesawa, M.; Chei, C.; Yokota, K.; Shiina, Y.; et al. Cigarette Smoking and Risk of Disabling Dementia in a Japanese Rural Community: A Nested Case-Control Study. Cerebrovasc. Dis. 2008, 25, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kondo, K.; Hirai, H.; Nakade, M.; Aida, J.; Hirata, Y. Association between Self-Reported Dental Health Status and Onset of Dementia: A 4-Year Prospective Cohort Study of Older Japanese Adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom. Med. 2012, 74, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Meguro, K.; Team, K.P. The Validity of the Basic Checklist in the Old-Old Population: The Kurihara Project. Jpn. J. Geriatr. Phychiatry 2012, 23, 725–730. [Google Scholar]
- Noda, H.; Yamagishi, K.; Ikeda, A.; Asada, T.; Iso, H. Identification of Dementia Using Standard Clinical Assessments by Primary Care Physicians in Japan. Geriatr. Gerontol. Int. 2018, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Andrews, G.; Colpe, L.J.; Hiripi, E.; Mroczek, D.K.; Normand, S.-L.; Walters, E.E.; Zaslavsky, A.M. Short Screening Scales to Monitor Population Prevalences and Trends in Non-specific Psychological Distress. Psychol. Med. 2002, 32, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Barker, P.R.; Colpe, L.J.; Epstein, J.F.; Gfroerer, J.C.; Hiripi, E.; Howes, M.J.; Normand, S.-L.T.; Manderscheid, R.W.; Walters, E.E.; et al. Screening for Serious Mental Illness in the General Population. Arch. Gen. Psychiatry 2003, 60, 184–189. [Google Scholar] [CrossRef]
- Tomata, Y.; Hozawa, A.; Ohmori-Matsuda, K.; Nagai, M.; Sugawara, Y.; Nitta, A.; Kuriyama, S.; Tsuji, I. Validation of the Kihon Checklist for Predicting the Risk of 1-Year Incident Long-Term Care Insurance Certification: The Ohsaki Cohort 2006 Study. Nihon Koshu Eisei Zasshi 2011, 58, 3–13. [Google Scholar]
- Orsini, N.; Wolk, A.; Bottai, M. Evaluating Percentiles of Survival. Epidemiology 2012, 23, 770–771. [Google Scholar] [CrossRef]
- Bellavia, A.; Discacciati, A.; Bottai, M.; Wolk, A.; Orsini, N. Using Laplace Regression to Model and Predict Percentiles of Age at Death When Age Is the Primary Time Scale. Am. J. Epidemiol. 2015, 182, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and Vegetable Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of All-Cause Mortality: A Systematic Review and Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Sasatani, M.; Doi, T.; Masaki, T.; Satoh, K.; Yoshizumi, M. Protective Effects of Japanese Soybean Paste (Miso) on Stroke in Stroke-Prone Spontaneously Hypertensive Rats (SHRSP). Am. J. Hypertens. 2017, 31, 43–47. [Google Scholar] [CrossRef]
- Ikeda, K.; Kitamura, A.; Machida, H.; Watanabe, M.; Negishi, H.; Hiraoka, J.; Nakano, T. Effect of Undaria Pinnatifida (Wakame) On the Development of Cerebrovascular Diseases in Stroke-Prone Spontaneously Hypertensive Rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 44–48. [Google Scholar] [CrossRef]
- Oda, K.; Nagai, T.; Ueno, Y.; Mori, Y. Further Evidence That a New Type of Japanese Pickles Reduce the Blood Pressure of Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2015, 79, 318–324. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer, and All Causes in Japan: The Ohsaki Study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Saito, E.; Inoue, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; Noda, M.; Iso, H.; Tsugane, S. Association of Green Tea Consumption with Mortality Due to All Causes and Major Causes of Death in a Japanese Population: The Japan Public Health Center-based Prospective Study (JPHC Study). Ann. Epidemiol. 2015, 25, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Welch, A.A.; Bingham, S.A.; Surtees, P.G.; Wainwright, N.W.J.; Luben, R.N.; Wareham, N.J.; Smith, R.D.; Harvey, I.M.; Day, N.E.; et al. Fruit and Vegetable Consumption and Self-Reported Functional Health in Men and Women in the European Prospective Investigation into Cancer-Norfolk (EPIC-Norfolk): A Population-Based Cross-Sectional Study. Public Health Nutr. 2007, 10, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayedi, A.; Shab-Bidar, S.; Eimeri, S.; Djafarian, K. Fish Consumption and Risk of All-Cause and Cardiovascular Mortality: A Dose-Response Meta-Analysis of Prospective Observational Studies. Public Health Nutr. 2018, 21, 1297–1306. [Google Scholar] [CrossRef]
- Takayama, M.; Arai, Y.; Sasaki, S.; Hashimoto, M.; Shimizu, K.; Abe, Y.; Hirose, N. Association of Marine-Origin N-3 Polyunsaturated Fatty Acids Consumption and Functional Mobility in the Community-Dwelling Oldest Old. J. Nutr. Health Aging 2013, 17, 82–89. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Plourde, M.; Pifferi, F.; Bégin, M.; Féart, C.; Barberger-Gateau, P. Fish, Docosahexaenoic Acid and Alzheimer’s Disease. Prog. Lipid Res. 2009, 48, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Hozawa, A.; Sugawara, Y.; Tomata, Y.; Kakizaki, M.; Tsuboya, T.; Ohmori-Matsuda, K.; Nakaya, N.; Kuriyama, S.; Fukao, A.; Tsuji, I. Relationship between Serum Isoflavone Levels and Disability-Free Survival among Community-Dwelling Elderly Individuals: Nested Case-Control Study of the Tsurugaya Project. J. Gerontol. Ser. A. 2013, 68, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozue, M.; Shimazu, T.; Charvat, H.; Mori, N.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Kokubo, Y.; et al. Fermented Soy Products Intake and Risk of Cardiovascular Disease and Total Cancer Incidence: The Japan Public Health Center-Based Prospective Study. Eur. J. Clin. Nutr. 2021, 75, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zheng, J.S.; Fang, L.; Jin, Y.; Cai, W.; Li, D. Tea Consumption and Mortality of All Cancers, CVD and All Causes: A Meta-Analysis of Eighteen Prospective Cohort Studies. Br. J. Nutr. 2015, 114, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Zhang, S.; Kaiho, Y.; Tanji, F.; Sugawara, Y.; Tsuji, I. Nutritional Characteristics of the Japanese Diet: A Cross-Sectional Study of the Correlation between Japanese Diet Index and Nutrient Intake among Community-Based Elderly Japanese. Nutrition 2019, 57, 115–121. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.J.; Park, Y.; Subar, A.F. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).