Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participant Selection and Patient Samples
2.2. Laboratory Measurements
2.3. Data Analysis/Statistics
3. Results
3.1. Cohort Characteristics
3.2. COVID-19 Patients: Retinol-Binding Protein 4 and Vitamin A Plasma Levels
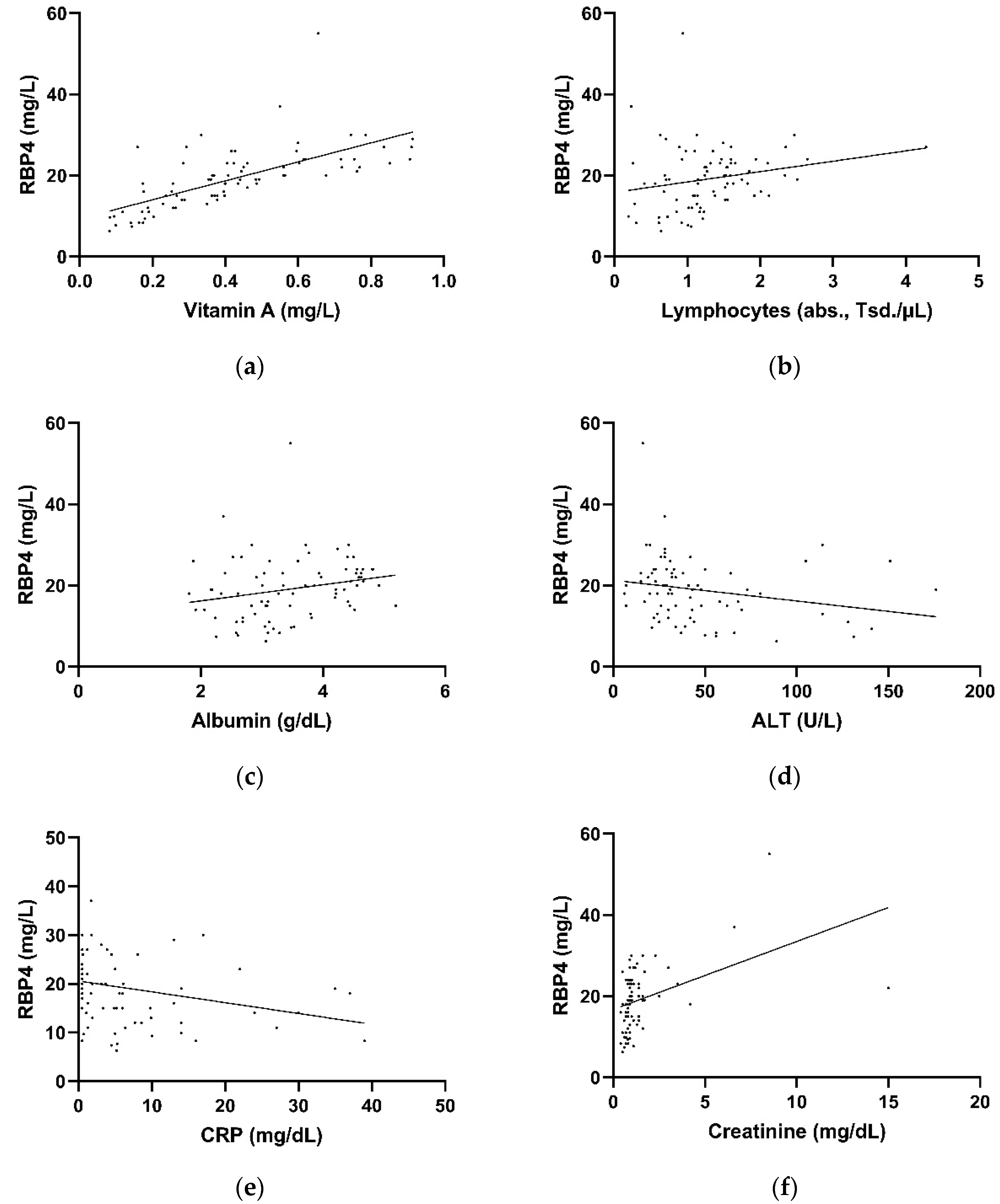
3.3. Correlation of Retinol-Binding Protein 4 Plasma Levels with Laboratory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Vollenberg, R.; Matern, P.; Nowacki, T.; Fuhrmann, V.; Padberg, J.-S.; Ochs, K.; Schütte-Nütgen, K.; Strauß, M.; Schmidt, H.; Tepasse, P.-R. Prone Position in Mechanically Ventilated COVID-19 Patients: A Multicenter Study. J. Clin. Med. 2021, 10, 1046. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Tepasse, P.-R.; Hafezi, W.; Lutz, M.; Kühn, J.; Wilms, C.; Wiewrodt, R.; Sackarnd, J.; Keller, M.; Schmidt, H.H.; Vollenberg, R. Persisting SARS-CoV-2 viraemia after rituximab therapy: Two cases with fatal outcome and a review of the literature. Br. J. Haematol. 2020, 190, 185–188. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Kessel, C.; Vollenberg, R.; Masjosthusmann, K.; Hinze, C.; Wittkowski, H.; Debaugnies, F.; Nagant, C.; Corazza, F.; Vély, F.; Kaplanski, G.; et al. Discrimination of COVID-19 From Inflammation-Induced Cytokine Storm Syndromes Using Disease-Related Blood Biomarkers. Arthritis Rheumatol. 2021, 73, 1791–1799. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta 2012, 1821, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Raverdeau, M.; Mills, K.H.G. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [Green Version]
- Tepasse, P.-R.; Vollenberg, R.; Fobker, M.; Kabar, I.; Schmidt, H.; Meier, J.A.; Nowacki, T.; Hüsing-Kabar, A. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients 2021, 13, 2173. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Lietz, G. Vitamin A in resistance to and recovery from infection: Relevance to SARS-CoV2. Br. J. Nutr. 2021, 126, 1663–1672. [Google Scholar] [CrossRef]
- Hoffmann, M.; Begon, M.; Lafon, Y.; Duprey, S. Influence of glenohumeral joint muscle insertion on moment arms using a finite element model. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 1117–1126. [Google Scholar] [CrossRef]
- Nawijn, M.C.; Timens, W. Can ACE2 expression explain SARS-CoV-2 infection of the respiratory epithelia in COVID-19? Mol. Syst. Biol. 2020, 16, e9841. [Google Scholar] [CrossRef]
- Hamming, I.; Cooper, M.E.; Haagmans, B.L.; Hooper, N.M.; Korstanje, R.; Osterhaus, A.D.M.E.; Timens, W.; Turner, A.J.; Navis, G.; van Goor, H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007, 212, 1–11. [Google Scholar] [CrossRef]
- Gagliardi, M.C.; Tieri, P.; Ortona, E.; Ruggieri, A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020, 6, 37. [Google Scholar] [CrossRef]
- Zhong, J.-C.; Huang, D.-Y.; Yang, Y.-M.; Li, Y.-F.; Liu, G.-F.; Song, X.-H.; Du, K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension 2004, 44, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.-B.; Wu, W.-F.; Qin, Y.-H.; Yin, S.-S. Association of all-trans retinoic acid treatment with the renin-angiotensin aldosterone system expression in glomerulosclerosis rats. J. Renin Angiotensin Aldosterone Syst. 2013, 14, 299–307. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Aksoy, H.; Karadag, A.S.; Wollina, U. Angiotensin II receptors: Impact for COVID-19 severity. Dermatol. Ther. 2020, 33, e13989. [Google Scholar] [CrossRef]
- Méry, G.; Epaulard, O.; Borel, A.-L.; Toussaint, B.; Le Gouellec, A. COVID-19: Underlying Adipokine Storm and Angiotensin 1-7 Umbrella. Front. Immunol. 2020, 11, 1714. [Google Scholar] [CrossRef]
- Porzionato, A.; Emmi, A.; Barbon, S.; Boscolo-Berto, R.; Stecco, C.; Stocco, E.; Macchi, V.; De Caro, R. Sympathetic activation: A potential link between comorbidities and COVID-19. FEBS J. 2020, 287, 3681–3688. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Krasinski, S.D.; Cohn, J.S.; Russell, R.M.; Schaefer, E.J. Postprandial plasma vitamin A metabolism in humans: A reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism 1990, 39, 357–365. [Google Scholar] [CrossRef]
- Muenzner, M.; Tuvia, N.; Deutschmann, C.; Witte, N.; Tolkachov, A.; Valai, A.; Henze, A.; Sander, L.E.; Raila, J.; Schupp, M. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol. Cell. Biol. 2013, 33, 4068–4082. [Google Scholar] [CrossRef] [Green Version]
- Blaner, W.S.; Li, Y.; Brun, P.-J.; Yuen, J.J.; Lee, S.-A.; Clugston, R.D. Vitamin A Absorption, Storage and Mobilization. Subcell. Biochem. 2016, 81, 95–125. [Google Scholar] [CrossRef]
- Flach, H.; Basten, T.; Schreiner, C.; Dietmann, P.; Greco, S.; Nies, L.; Roßmanith, N.; Walter, S.; Kühl, M.; Kühl, S.J. Retinol binding protein 1 affects Xenopus anterior neural development via all-trans retinoic acid signaling. Dev. Dyn. 2021, 250, 1096–1112. [Google Scholar] [CrossRef]
- Blaner, W.S.; Brun, P.-J.; Calderon, R.M.; Golczak, M. Retinol-binding protein 2 (RBP2): Biology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 197–218. [Google Scholar] [CrossRef]
- den Hollander, A.I.; McGee, T.L.; Ziviello, C.; Banfi, S.; Dryja, T.P.; Gonzalez-Fernandez, F.; Ghosh, D.; Berson, E.L. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1864–1872. [Google Scholar] [CrossRef] [Green Version]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. 2020, 11, 644–666. [Google Scholar] [CrossRef]
- Zabetian-Targhi, F.; Mahmoudi, M.J.; Rezaei, N.; Mahmoudi, M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv. Nutr. 2015, 6, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef]
- Frey, S.K.; Nagl, B.; Henze, A.; Raila, J.; Schlosser, B.; Berg, T.; Tepel, M.; Zidek, W.; Weickert, M.O.; Pfeiffer, A.F.H.; et al. Isoforms of retinol binding protein 4 (RBP4) are increased in chronic diseases of the kidney but not of the liver. Lipids Health Dis. 2008, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, C.; Okuno, M.; Tannous, L.; Piantedosi, R.; Allan, M.; Goodman, D.S.; Blaner, W.S. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 1992, 267, 1805–1810. [Google Scholar]
- Du, M.; Yang, S.; Liu, M.; Liu, J. COVID-19 and liver dysfunction: Epidemiology, association and potential mechanisms. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101793. [Google Scholar] [CrossRef]
- Erikstrup, C.; Mortensen, O.H.; Nielsen, A.R.; Fischer, C.P.; Plomgaard, P.; Petersen, A.M.; Krogh-Madsen, R.; Lindegaard, B.; Erhardt, J.G.; Ullum, H.; et al. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 204–212. [Google Scholar] [CrossRef]
- Fu, Y.; Gaelings, L.; Jalovaara, P.; Kakkola, L.; Kinnunen, M.T.; Kallio-Kokko, H.; Valkonen, M.; Kantele, A.; Kainov, D.E. Protein profiling of nasopharyngeal aspirates of hospitalized and outpatients revealed cytokines associated with severe influenza A(H1N1)pdm09 virus infections: A pilot study. Cytokine 2016, 86, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Roma, E.; Krini, M.; Hantzi, E.; Sakka, S.; Panayiotou, I.; Margeli, A.; Papassotiriou, I.; Kanaka-Gantenbein, C. Retinol Binding Protein 4 in children with Inflammatory Bowel Disease: A negative correlation with the disease activity. Hippokratia 2012, 16, 360–365. [Google Scholar]
- Gieng, S.H.; Green, M.H.; Green, J.B.; Rosales, F.J. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 2007, 48, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Aklamati, E.K.; Mulenga, M.; Dueker, S.R.; Buchholz, B.A.; Peerson, J.M.; Kafwembe, E.; Brown, K.H.; Haskell, M.J. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J. Nutr. 2010, 140, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Sarohan, A.R.; Kızıl, M.; İnkaya, A.Ç.; Mahmud, S.; Akram, M.; Cen, O. A novel hypothesis for COVID-19 pathogenesis: Retinol depletion and retinoid signaling disorder. Cell. Signal. 2021, 87, 110121. [Google Scholar] [CrossRef]
- Shoemark, D.K.; Colenso, C.K.; Toelzer, C.; Gupta, K.; Sessions, R.B.; Davidson, A.D.; Berger, I.; Schaffitzel, C.; Spencer, J.; Mulholland, A.J. Molecular Simulations suggest Vitamins, Retinoids and Steroids as Ligands of the Free Fatty Acid Pocket of the SARS-CoV-2 Spike Protein**. Angew. Chem. Int. Ed. Engl. 2021, 60, 7098–7110. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, M.; Bedi, O.; Gupta, M.; Kumar, S.; Jaiswal, G.; Rahi, V.; Yedke, N.G.; Bijalwan, A.; Sharma, S.; et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021, 29, 1001–1016. [Google Scholar] [CrossRef]
- Jones, B.G.; Oshansky, C.M.; Bajracharya, R.; Tang, L.; Sun, Y.; Wong, S.S.; Webby, R.; Thomas, P.G.; Hurwitz, J.L. Retinol binding protein and vitamin D associations with serum antibody isotypes, serum influenza virus-specific neutralizing activities and airway cytokine profiles. Clin. Exp. Immunol. 2016, 183, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, M.; Pichlmair, A.; Martínez-Sobrido, L.; Cros, J.; García-Sastre, A.; Haller, O.; Weber, F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005, 79, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Frieman, M.; Yount, B.; Heise, M.; Kopecky-Bromberg, S.A.; Palese, P.; Baric, R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007, 81, 9812–9824. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ye, F.; Zhu, N.; Wang, W.; Deng, Y.; Zhao, Z.; Tan, W. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci. Rep. 2015, 5, 17554. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Xiao, F.; Hu, D.; Ge, W.; Tian, M.; Wang, W.; Pan, P.; Wu, K.; Wu, J. SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-β Production. Viruses 2020, 13, 47. [Google Scholar] [CrossRef]
- Cooper, A.D. Hepatic clearance of plasma chylomicron remnants. Semin. Liver Dis. 1992, 12, 386–396. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Bajzová, M.; Kováčiková, M.; Vítková, M.; Klimčáková, E.; Polák, J.; Kováčová, Z.; Viguerie, N.; Vedral, T.; Mikulášek, L.; Šrámková, P.; et al. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiol. Res. 2008, 57, 927–934. [Google Scholar] [CrossRef]
- Janke, J.; Engeli, S.; Boschmann, M.; Adams, F.; Böhnke, J.; Luft, F.C.; Sharma, A.M.; Jordan, J. Retinol-binding protein 4 in human obesity. Diabetes 2006, 55, 2805–2810. [Google Scholar] [CrossRef] [Green Version]
- Yao-Borengasser, A.; Varma, V.; Bodles, A.M.; Rasouli, N.; Phanavanh, B.; Lee, M.-J.; Starks, T.; Kern, L.M.; Spencer, H.J.; Rashidi, A.A.; et al. Retinol binding protein 4 expression in humans: Relationship to insulin resistance, inflammation, and response to pioglitazone. J. Clin. Endocrinol. Metab. 2007, 92, 2590–2597. [Google Scholar] [CrossRef] [Green Version]
- Broch, M.; Vendrell, J.; Ricart, W.; Richart, C.; Fernández-Real, J.-M. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care 2007, 30, 1802–1806. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.M.; Youn, B.-S.; Lee, H.; Lee, N.; Min, S.-S.; Kwak, S.H.; Lee, H.K.; Park, K.S. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006, 29, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Ulgen, F.; Herder, C.; Kühn, M.C.; Willenberg, H.S.; Schott, M.; Scherbaum, W.A.; Schinner, S. Association of serum levels of retinol-binding protein 4 with male sex but not with insulin resistance in obese patients. Arch. Physiol. Biochem. 2010, 116, 57–62. [Google Scholar] [CrossRef]
- Kotnik, P.; Fischer-Posovszky, P.; Wabitsch, M. RBP4: A controversial adipokine. Eur. J. Endocrinol. 2011, 165, 703–711. [Google Scholar] [CrossRef] [Green Version]
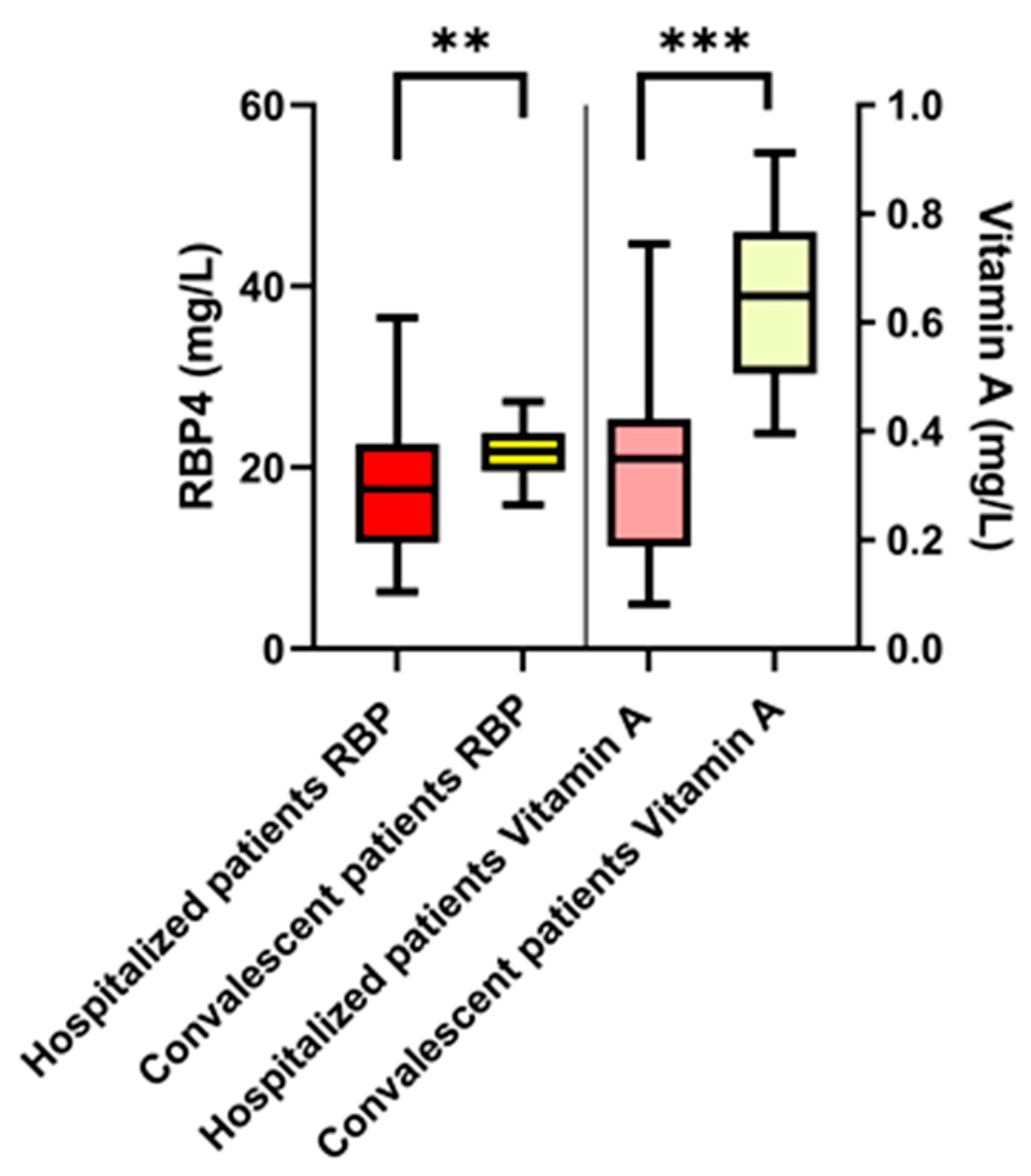
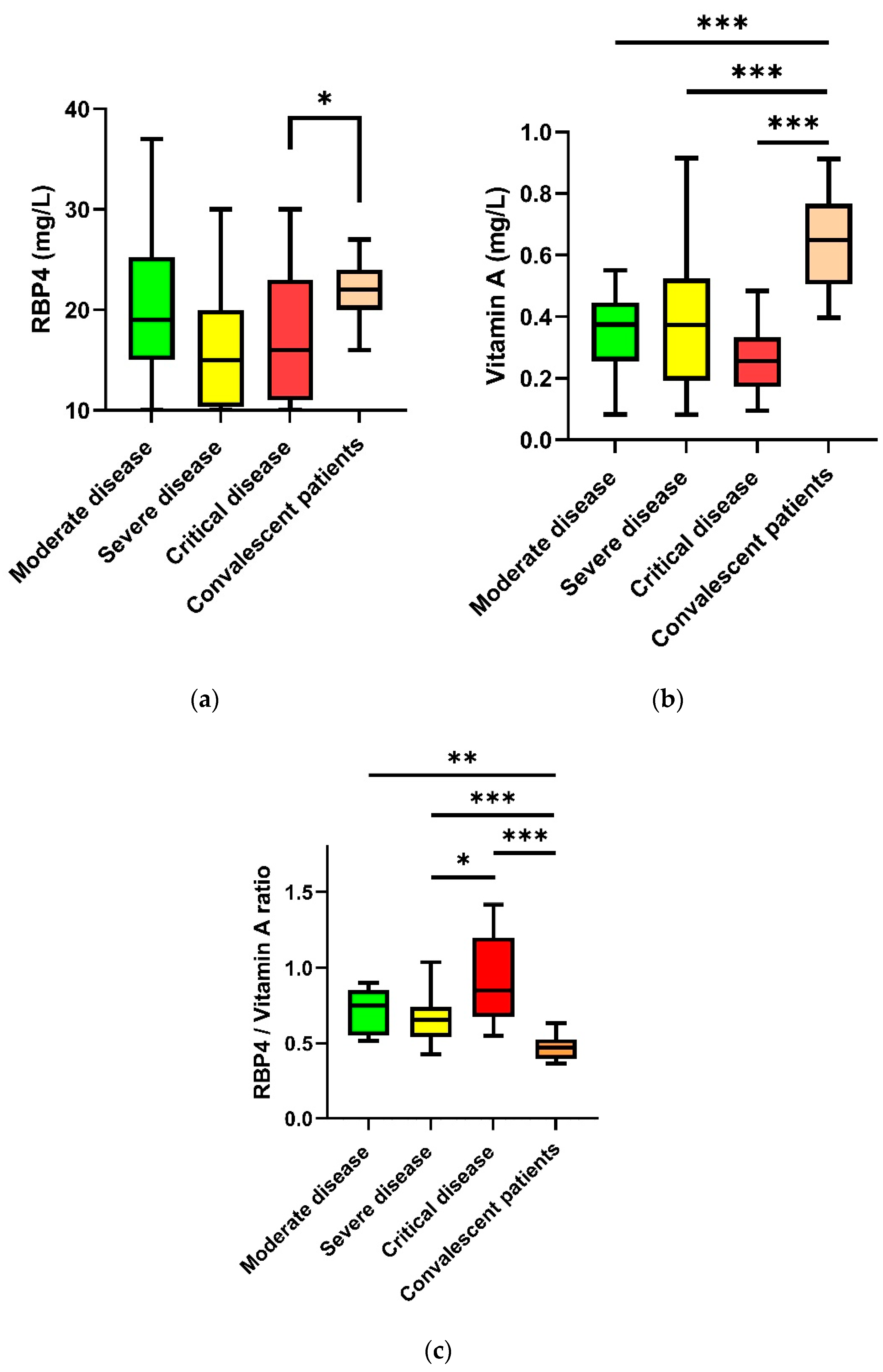
| Moderate Disease (n = 20) | Severe Disease (n = 20) | Critical Disease (n = 19) | Convalescent Patients (n = 20) | p-Value | |
|---|---|---|---|---|---|
| Age, years, median (min–max) | 56 (21–81) | 60 (44–85) | 59 (41–88) | 58 (50–70) | 0.54 |
| Gender, male (%) | 85 | 70 | 84 | 90 | 0.38 |
| BMI (kg/m2), median (IQR) | 23 (21–24) | 24 (22–27) | 28 (25–29) | 27 (25–30) | <0.001 |
| Interval from first symptom to acquisition of blood sample, days, median (IQR) | 4 (2–9) | 7 (3–12) | 13 (9–22) | 46 (42–53) | 0.003 |
| Cardiovascular disease (abs.) | 2 | 5 | 3 | 0 | 0.11 |
| Respiratory disease (abs.) | 3 | 6 | 1 | 0 | 0.03 |
| Kidney insufficiency (abs.) | 3 | 3 | 0 | 0 | 0.1 |
| Neoplasm (abs.) | 3 | 1 | 2 | 0 | 0.02 |
| Diabetes (abs.) | 2 | 4 | 2 | 0 | 0.22 |
| Death (abs.) | 0 | 0 | 6 | 0 | <0.001 |
| Leukocytes (×109/L), median (IQR) | 4.7 (2.7–6.3) | 5.6 (3.7–6.0) | 9.6 (7.0–11.9) | 5.52 (4.9–67.2) | <0.001 |
| Lymphocytes (rel., %), median (IQR) | 25.7 (18.1–32.1) | 22.05 (18.65–27.53) | 9.1 (6.9–13.9) | 28.5 (20.7–34.5) | <0.001 |
| Creatinine (mg/dL), median (IQR) | 0.9 (0.8–1.3) | 0.4 (0.7–1.6) | 0.9 (0.7–1.7) | 0.9 (0.8–1) | 0.99 |
| Ferritin (µg/L), median (IQR) | 380 (232–735) | 682 (248–824) | 956 (688–2111) | 160 (106–432) | <0.001 |
| Interleukin-6 (pg/mL), median (IQR) | 15 (8–27) | 31 (16–82) | 95 (38–224) | 2 (2–2) | <0.001 |
| C-reactive protein (mg/dL), median (IQR) | 1.3 (0.5–3.8) | 5.1 (3.1–7.3) | 14.2 (5.6–26.9) | 0.5 (0.5–0.5) | <0.001 |
| PCHe (U/L), median (IQR) | 5302 (3928–7237) | 6247 (4933–8324) | 3446 (2766–4711) | 8962 (7853–10329) | <0.001 |
| ALT (U/L), median (IQR) | 33 (23–64) | 34 (28–45) | 48 (26–70) | 29 (22–33) | 0.09 |
| Albumin (g/dL), median (IQR) | 3.1 (2.8–3.9) | 3.5 (3.1–3.8) | 2.6 (2.2–2.9) | 4.5 (4.4–4.6) | < 0.001 |
| RBP4 (mg/L) | 19.38 (14.59–25.28) | 15.13 (10.23–20.39) | 15.5 (10.5–22.5) | 21.80 (19.56–23.84) | 0.02 |
| Vitamin A (mg/L), median (IQR) | 0.37 (0.26–0.45) | 0.37 (0.19–0.53) | 0.26 (0.17–0.33) | 0.65 (0.51–0.77) | < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollenberg, R.; Tepasse, P.-R.; Fobker, M.; Hüsing-Kabar, A. Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients. Nutrients 2022, 14, 2007. https://doi.org/10.3390/nu14102007
Vollenberg R, Tepasse P-R, Fobker M, Hüsing-Kabar A. Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients. Nutrients. 2022; 14(10):2007. https://doi.org/10.3390/nu14102007
Chicago/Turabian StyleVollenberg, Richard, Phil-Robin Tepasse, Manfred Fobker, and Anna Hüsing-Kabar. 2022. "Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients" Nutrients 14, no. 10: 2007. https://doi.org/10.3390/nu14102007
APA StyleVollenberg, R., Tepasse, P.-R., Fobker, M., & Hüsing-Kabar, A. (2022). Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients. Nutrients, 14(10), 2007. https://doi.org/10.3390/nu14102007






