Effects of a Formula with scGOS/lcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Stool Collection
2.3. Fecal DNA Extraction and Metagenomic Sequencing
2.4. Taxonomic and Functional Profiling
2.5. Statistical Analysis
2.6. Data Availability
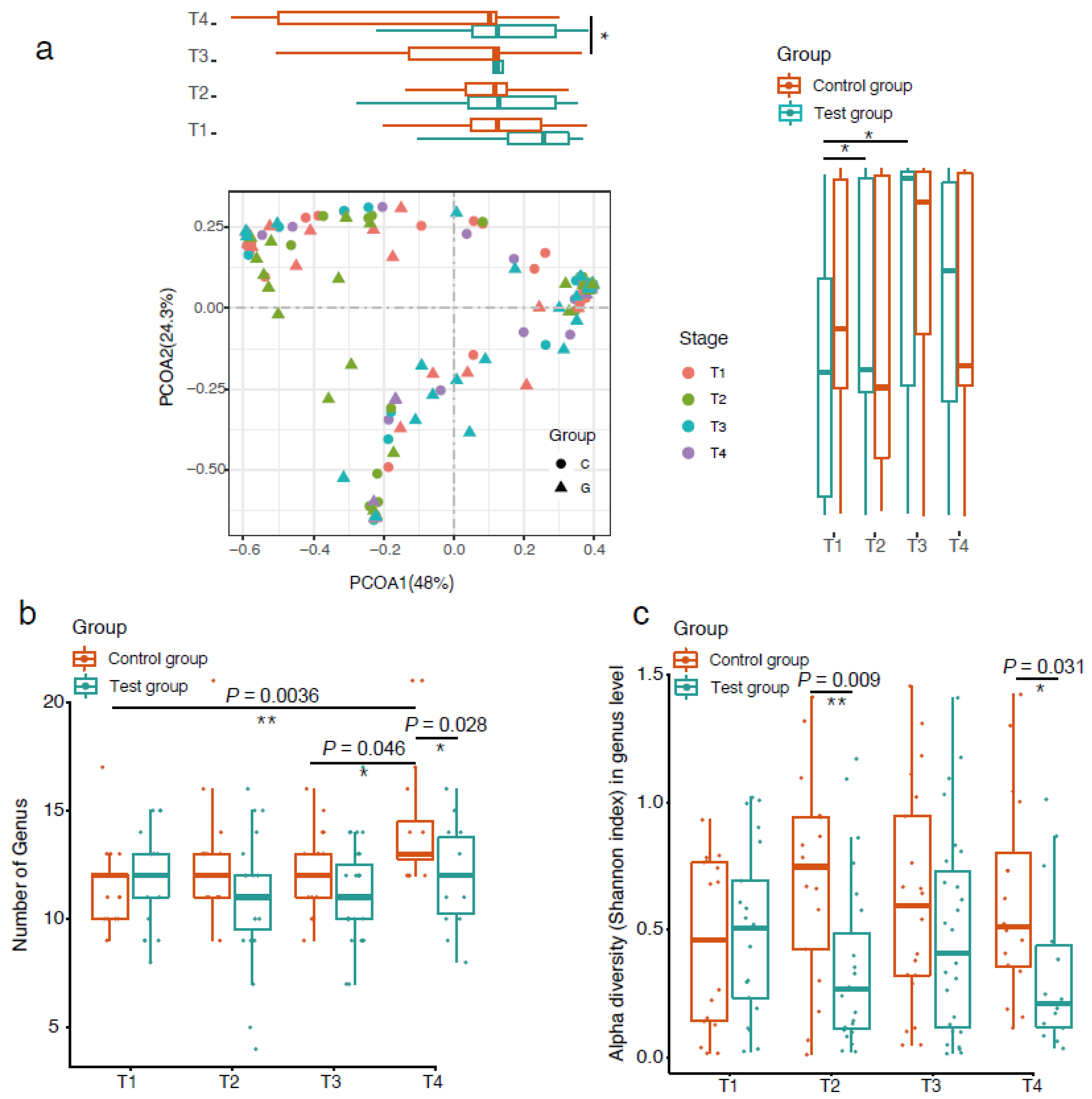
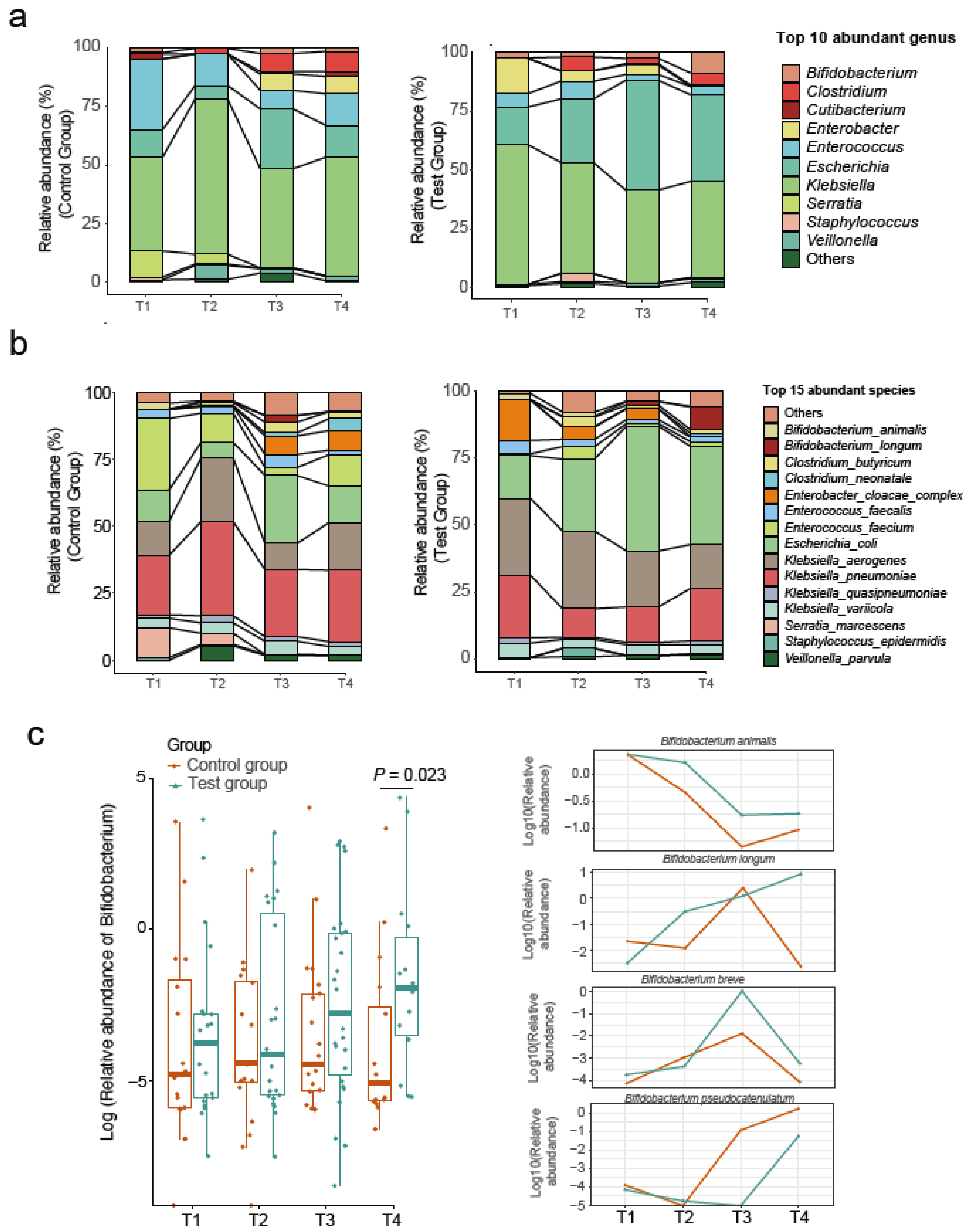
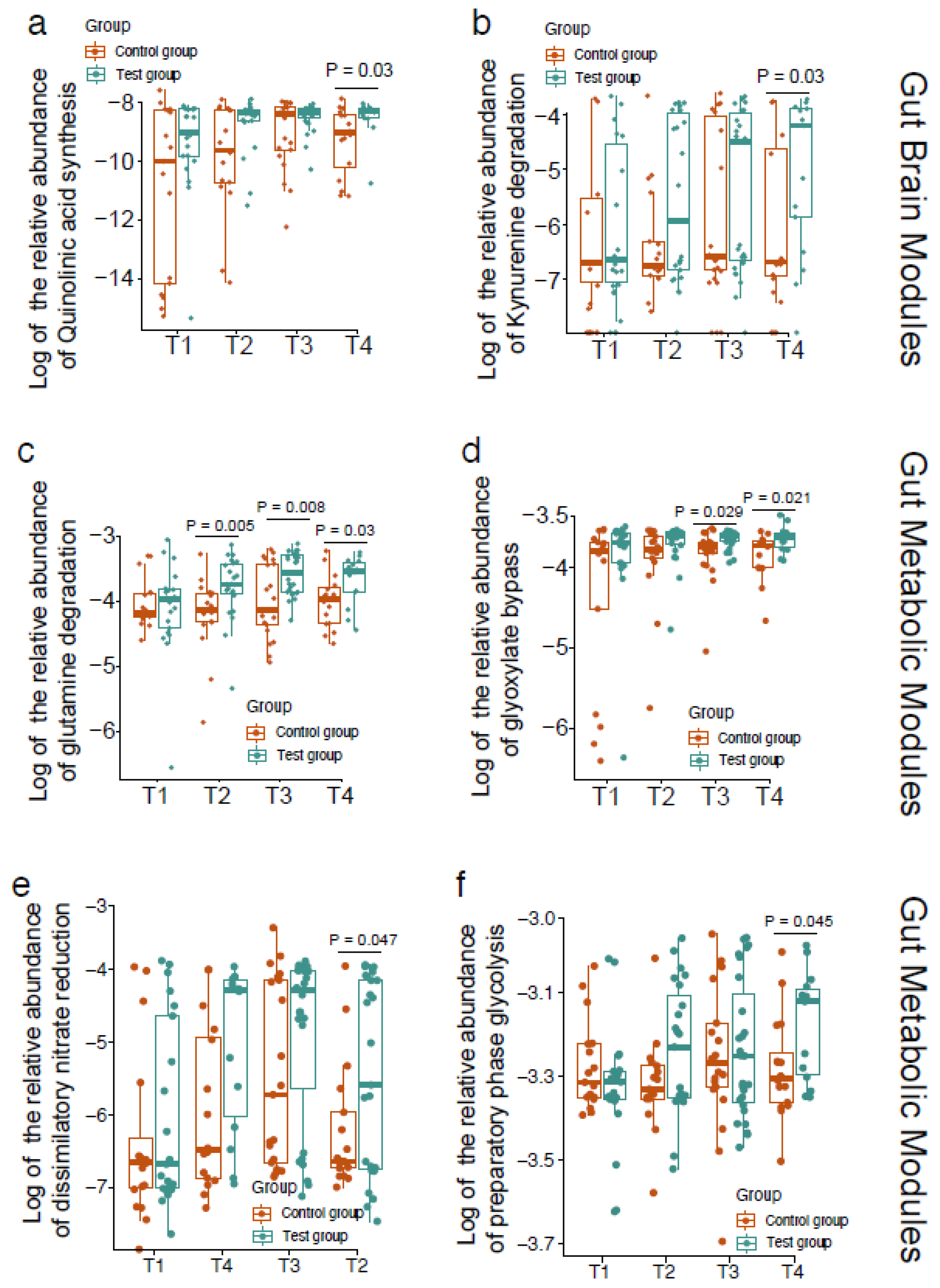
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pr. Res. Clin. Obs. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.J. Outcomes in preterm infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Lopez, M.; Dinsmoor, A.M.; Ho, T.T.B.; Donovan, S.M. A systematic review of the factors influencing microbial colonization of the preterm infant gut. Gut Microbes 2021, 13, 1–33. [Google Scholar] [CrossRef]
- Groer, M.W.; Gregory, K.E.; Louis-Jacques, A.; Thibeau, S.; Walker, W.A. The very low birth weight infant microbiome and childhood health. Birth Defects Res. C Embryo Today 2015, 105, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Bellanger, A.; Boudry, G.; Gangneux, J.P.; Yverneau, M.; Beuchée, A.; Blat, S.; Le Huërou-Luron, I. New Insights Into Microbiota Modulation-Based Nutritional Interventions for Neurodevelopmental Outcomes in Preterm Infants. Front. Microbiol. 2021, 12, 676622. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.J.; Crofts, T.S.; Gibson, M.K.; Tarr, P.I.; Warner, B.B.; Dantas, G. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes 2016, 7, 443–449. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in nutrition of the newborn infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef]
- Boquien, C.Y. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front. Pediatr. 2018, 6, 295. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Wicinski, M.; Sawicka, E.; Gebalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; De Greef, E.; Veereman, G. Prebiotics in infant formula. Gut Microbes 2014, 5, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.M.; Wells, J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef]
- Foisy Sauvé, M.; Spahis, S.; Delvin, E.; Levy, E. Glycomacropeptide: A Bioactive Milk Derivative to Alleviate Metabolic Syndrome Outcomes. Antioxid. Redox Signal. 2021, 34, 201–222. [Google Scholar] [CrossRef]
- Fang, C.; Zhong, H.; Lin, Y.; Chen, B.; Han, M.; Ren, H.; Lu, H.; Luber, J.M.; Xia, M.; Li, W.; et al. Assessment of the cPAS-based BGISEQ-500 platform for metagenomic sequencing. Gigascience 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Xie, H.; Guo, R.; Zhong, H.; Feng, Q.; Lan, Z.; Qin, B.; Ward, K.J.; Jackson, M.A.; Xia, Y.; Chen, X.; et al. Shotgun Metagenomics of 250 Adult Twins Reveals Genetic and Environmental Impacts on the Gut Microbiome. Cell Syst. 2016, 3, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira-Silva, S.; Falony, G.; Darzi, Y.; Lima-Mendez, G.; Garcia Yunta, R.; Okuda, S.; Vandeputte, D.; Valles-Colomer, M.; Hildebrand, F.; Chaffron, S.; et al. Species-function relationships shape ecological properties of the human gut microbiome. Nat. Microbiol. 2016, 1, 16088. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, M.K.; Wang, B.; Ahmadi, S.; Burnham, C.A.; Tarr, P.I.; Warner, B.B.; Dantas, G. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat. Microbiol. 2016, 1, 16024. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Smith, B.; Mortensen, M.S.; Krych, L.; Sørensen, S.J.; Greisen, G.; Krogfelt, K.A.; Nielsen, D.S. The effect of early probiotic exposure on the preterm infant gut microbiome development. Gut Microbes 2021, 13, 1951113. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.L.; Lohmann, P.; Preidis, G.A.; Gordon, P.S.; O'Donnell, A.; Hagan, J.; Venkatachalam, A.; Balderas, M.; Luna, R.A.; Hair, A.B. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother's own milk compared with donor breast milk. Am. J. Clin. Nutr. 2019, 109, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Minoli, I.; Mosca, M.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 291–295. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, M.; Muñoz, F.C.; Cervantes-García, D.; Cervantes, M.M.; Hernández-Mercado, A.; Barrón-García, B.; Moreno Hernández-Duque, J.L.; Rodríguez-Carlos, A.; Rivas-Santiago, B.; Salinas, E. Protective Effect of Glycomacropeptide on the Atopic Dermatitis-Like Dysfunctional Skin Barrier in Rats. J. Med. Food 2020, 23, 1216–1224. [Google Scholar] [CrossRef]
- Brück, W.M.; Redgrave, M.; Tuohy, K.M.; Lönnerdal, B.; Graverholt, G.; Hernell, O.; Gibson, G.R. Effects of bovine alpha-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, I.M.; Tannock, G.W. Galacto- and Fructo-oligosaccharides Utilized for Growth by Cocultures of Bifidobacterial Species Characteristic of the Infant Gut. Appl. Environ. Microbiol. 2020, 86, e00214-20. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Sánchez, B.; Solís, G.; Fernández, N.; Suárez, M.; Hernández-Barranco, A.M.; Milani, C.; Margolles, A.; de Los Reyes-Gavilán, C.G.; Ventura, M.; et al. Impact of Prematurity and Perinatal Antibiotics on the Developing Intestinal Microbiota: A Functional Inference Study. Int. J. Mol. Sci. 2016, 17, 649. [Google Scholar] [CrossRef] [PubMed]
- Soberón, M.; González, A. Physiological role of glutaminase activity in Saccharomyces cerevisiae. J. Gen. Microbiol. 1987, 133, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Németh, B.; Doczi, J.; Csete, D.; Kacso, G.; Ravasz, D.; Adams, D.; Kiss, G.; Nagy, A.M.; Horvath, G.; Tretter, L.; et al. Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. FASEB J. 2016, 30, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Jung, J.; Jang, I.A.; Madsen, E.L.; Park, W. Role of Glyoxylate Shunt in Oxidative Stress Response. J. Biol. Chem. 2016, 291, 11928–11938. [Google Scholar] [CrossRef] [Green Version]
- Allison, C.; Macfarlane, G.T. Dissimilatory nitrate reduction by Propionibacterium acnes. Appl. Environ. Microbiol. 1989, 55, 2899–2903. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.A. A history of research on yeasts 5: The fermentation pathway. Yeast 2003, 20, 509–543. [Google Scholar] [CrossRef]
- Cohen Kadosh, K.; Muhardi, L.; Parikh, P.; Basso, M.; Jan Mohamed, H.J.; Prawitasari, T.; Samuel, F.; Ma, G.; Geurts, J.M. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children-An Update and Novel Insights. Nutrients 2021, 13, 199. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Coyte, K.Z.; Bainter, W.; Geha, R.S.; Martin, C.R.; Rakoff-Nahoum, S. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature 2021, 591, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.C. Principles of research: Limitations of non-randomized studies. Surgery 2008, 26, 120–124. [Google Scholar]

| Test Group (n = 37) | Control Group (n = 35) | p | |
|---|---|---|---|
| Male sex (n [%]) | 14 (37.8) | 20 (58.8) | 0.077 |
| Siblings at birth (n [% yes]) | 11 (29.7) | 13 (37.1) | 0.505 |
| Cesarean delivery (n [%]) | 23 (62.2) | 22 (64.7) | 0.824 |
| Maternal antibiotics at delivery (n [% yes]) | 11 (29.7) | 10 (30.3) | 0.958 |
| Infant antibiotics after inclusion (n [% yes]) | 12 (32.4) | 14 (40.0) | 0.504 |
| Maternal age (year) | 32.2 ± 4.8 | 32.8 ± 4.7 | 0.589 |
| Birth weight (g) | 1329.2 ± 268.1 | 1265.1 ± 257.1 | 0.305 |
| Birth length (cm) | 38.3 ± 3.3 | 37.7 ± 2.5 | 0.384 |
| Head circumference (cm) | 27.6 ± 1.7 | 26.9 ± 1.7 | 0.060 |
| 1-min Apgar score | 8.6 ± 2.0 | 8.2 ± 2.1 | 0.356 |
| 5-min Apgar score | 9.4 ± 1.1 | 9.0 ± 1.2 | 0.227 |
| 10-min Apgar score | 9.5 ± 0.8 | 9.4 ± 0.8 | 0.562 |
| Gestational age at birth (week) | 30.4 ± 1.8 | 29.5 ± 1.6 | 0.040 |
| Corrected age at the beginning of intervention (week) | 32.4 ± 1.3 | 31.8 ± 1.5 | 0.095 |
| Hospitalization (day) | 41.4 ± 15.2 | 49.4 ± 16.9 | 0.039 |
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| Infant feeding volume (mL/day) | ||||
| Test | 175.1 ± 63.5 | 214.7 ± 69.5 | 258.4 ± 55.1 | 297.5 ± 38.0 |
| Control | 171.9 ± 78.3 | 199.0 ± 64.2 | 249.7 ± 59.7 | 293.9 ± 36.8 |
| p value | 0.136 | 0.719 | 0.217 | 0.314 |
| Weight (g) | ||||
| Test | 1420.9 ± 199.9 | 1459.2 ± 186.4 | 1634.6 ± 199.5 | 1900.0 ± 197.2 |
| Control | 1393.1 ± 210.1 | 1456.8 ± 222.8 | 1599.1 ± 235.3 | 1900.0 ± 129.2 |
| pvalue | 0.582 | 0.966 | 0.583 | 0.699 |
| Length (cm) | ||||
| Test | 39.8 ± 2.9 | 40.3 ± 2.1 | 41.3 ± 2.3 | 44.7 ± 2.5 |
| Control | 38.8 ± 2.7 | 40.0 ± 2.6 | 41.1 ± 2.7 | 44.4 ± 2.8 |
| p value | 0.231 | 0.736 | 0.814 | 0.794 |
| Head circumference (cm) | ||||
| Test | 28.0 ± 2.0 | 28.3 ± 2.0 | 29.1 ± 2.1 | 31.2 ± 3.1 |
| Control | 27.5 ± 1.8 | 28.2 ± 2.3 | 28.6 ± 2.3 | 29.7 ± 2.5 |
| p value | 0.323 | 0.861 | 0.488 | 0.187 |
| Stool consistency (Bristol stool score) | ||||
| Test | 3.9 ± 0.5 | 4.0 ± 0.0 | 3.9 ± 0.4 | 4.0 ± 0.0 |
| Control | 3.8 ± 0.6 | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.4 |
| p value | 0.359 | 0.103 | 0.865 | 0.253 |
| Stool frequency (times/day) | ||||
| Test | 1.7 ± 1.5 | 1.7 ± 1.3 | 2.2 ± 1.5 | 2.3 ± 1.1 |
| Control | 1.9 ± 1.2 | 2.1 ± 1.4 | 2.2 ± 1.3 | 1.7 ± 1.3 |
| p value | 0.643 | 0.268 | 0.908 | 0.778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xing, Y.; Liu, H.; Chang, Y.; You, Y.; Dou, Y.; Liu, B.; Wang, Q.; Ma, D.; Chen, L.; et al. Effects of a Formula with scGOS/lcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants. Nutrients 2022, 14, 1901. https://doi.org/10.3390/nu14091901
Yu X, Xing Y, Liu H, Chang Y, You Y, Dou Y, Liu B, Wang Q, Ma D, Chen L, et al. Effects of a Formula with scGOS/lcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants. Nutrients. 2022; 14(9):1901. https://doi.org/10.3390/nu14091901
Chicago/Turabian StyleYu, Xue, Yan Xing, Hui Liu, Yanmei Chang, Yanxia You, Yuqi Dou, Bin Liu, Qi Wang, Defu Ma, Lijun Chen, and et al. 2022. "Effects of a Formula with scGOS/lcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants" Nutrients 14, no. 9: 1901. https://doi.org/10.3390/nu14091901
APA StyleYu, X., Xing, Y., Liu, H., Chang, Y., You, Y., Dou, Y., Liu, B., Wang, Q., Ma, D., Chen, L., & Tong, X. (2022). Effects of a Formula with scGOS/lcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants. Nutrients, 14(9), 1901. https://doi.org/10.3390/nu14091901








