The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder
Abstract
:1. Introduction
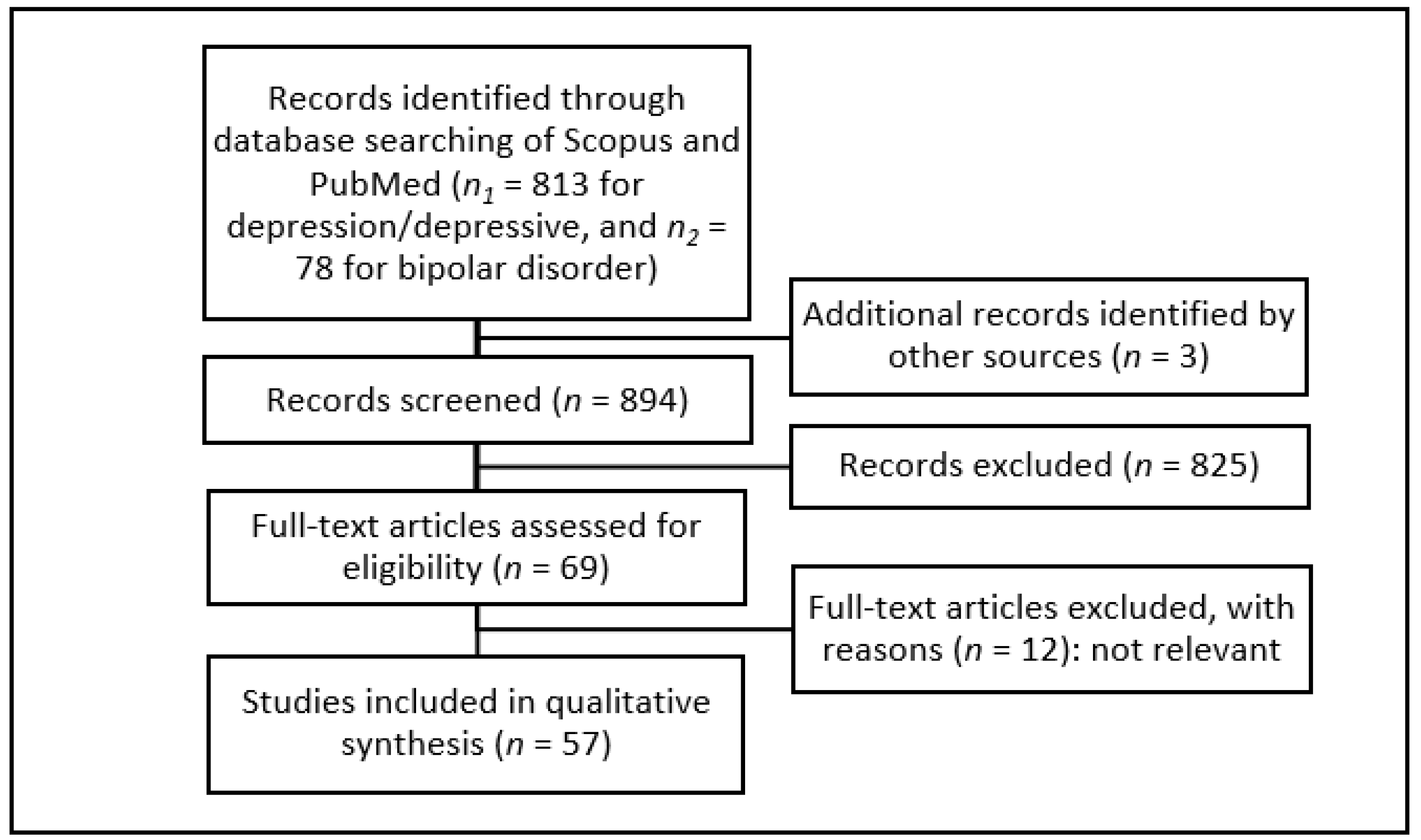
2. Materials and Methods
- -
- Studies were written in English
- -
- Studies were conducted with human subjects
- -
- Studies at least partly focused on depression or depressive symptoms, and their correlation with the intestinal microbiota
- -
- Diseased and healthy subjects were analyzed in the study
3. Results
3.1. Diversity
| Source | Shannon | Ace | Chao1 | Nr. OTUs | UniFrac | PLS-DA |
|---|---|---|---|---|---|---|
| [41] | D = | D sign. | ||||
| [42] | aMDD ↑, rMDD = | MDD = | MDD = | MDD = | ||
| [43] | MDD = | |||||
| [44] | MDD = | MDD = | ||||
| [46] | BD ↓, MDD = | BD ↓, MDD = | BD, MDD, C sign. | |||
| [47] | P = | P = | ||||
| [48] | P ↓ | P ↓ | ||||
| [49] | MDD = | MDD = | MDD sign. | |||
| [50] | MDD =, BD = | MDD ↓, BD = | ||||
| [51] | MDD = | MDD = | MDD = | MDD sign. | ||
| [52] | BD = | BD = | BD = | BD = | ||
| [53] | D = | D ↓ | D = | |||
| [54] | BD ↓ * | BD ↓ * | BD sign. | |||
| [55] | D = IBS | D = IBS | D, IBS sign. | |||
| [56] | IBS ↓ | IBS sign. | ||||
| [57] | MDD = | MDD = | MDD sign. | |||
| [58] | MDD ↓ | MDD ↓ | MDD ↓ | MDD sign. | ||
| [59] | pm = | |||||
| [60] | D ↓, IBS ↓ | |||||
| [61] | D ↓ | D ↓ | ||||
| [62] | MDD sign. | |||||
| [63] | MDD = | MDD sign. | ||||
| [64] | BD = | BD = | BD sign. | |||
| [65] | BD = | BD sign. |
3.2. Phylum
| Source | Mean Age (Years) | Bacteroidetes | Firmicutes | Proteobacteria | Actinobacteria | |
|---|---|---|---|---|---|---|
| [44] | MDD 21.9; C 22.1 | MDD ↑ | MDD ↓ | |||
| [49] | MDD 24.0; C 25.0 | MDD ↑ | MDD ↓ | |||
| [66] | BD 24.2; C 36.3 | BD ↑ | BD ↓ | BD ↓ * | ||
| [42] | aMDD 25.3; rMDD 27.1; C 26.8 | MDD ↑ | MDD ↓ | MDD ↑ | MDD ↑ | |
| [46] | MDD 26.5; BD 25.6; C 26.9 | MDD ↑ | BD ↓ | BD ↑ | ||
| [69] | non-D 33.4 | ↓ | ||||
| [48] | P 35.7 | ↓ | ||||
| [62] | MDD 36.2; C 38.1 | MDD ↓ | M ↑ | MDD ↑ * | ||
| [63] | MDD 40.6; C 41.8 | MDD ↓ | MDD ↑ | |||
| [52] | BD 41.3; C 31.4 | BD ↑ | ||||
| [57] | MDD 41.5; C 44.0 | MDD ↓ | MDD ↑ | |||
| [50] | MDD 41.6; BD 38.4; C 39.5 | MDD ↓; BD ↓ | MDD ↑; BD ↑ | BD ↑ | MDD ↑; BD ↑ | |
| [43] | MDD 43.7, C 39.4 | MDD ↓ | MDD ↑ | |||
| [67] | MDD 43.9; C 39.6 | MDD ↓ | M ↑ | MDD ↓ | MDD ↑ | |
| [60] | D 44.8; IBS 38.5; D + IBS 39.0; C 43.9 | D ↑ | D ↓ | |||
| [55] | IBS + Di 45.0; IBS (non-Di) 33.0 | ↑ | ||||
| [49] | MDD 45.0; C 47.2 | MDD ↓ | MDD ↑ | |||
| [51] | MDD 45.8; C 41.2 | MDD ↓ | MDD ↑ | MDD ↓ | MDD ↑ | |
| [58] | MDD 48.7; C 42.3 | MDD ↓ | ||||
| [41] | D 49.2; C 46.1 | D ↓ | ||||
3.3. Bacteroidetes
3.4. Firmicutes
3.5. Proteobacteria
3.6. Actinobacteria
3.7. Human Interventional Trials in Depression
| Source | Subjects; Pre-/Syn-/Probiotics | Influence on Depression/Depressive Symptoms |
|---|---|---|
| [84] | MDD; C. butyricum | +(treatment response, remission) |
| [99] | D; Lactobacilli, Bifidobacteria | =(BDI, BAI, DASS) |
| [114] | H; L. gasseri | +(depression, anxiety, sleep) |
| [115] | H; L. gasseri | +(HADS, fatigue, mental state) |
| [116] | H; L. gasseri | +(depressive mood, anxiety, sleep, stress) |
| [118] | C; any probiotics/supplementation | =(PHQ-9) |
| [117] | Dialysis; L. acidophilus, Bifidobacteria; fiber | +with synbiotics (HADS, BDNF)=with probiotics (HADS, BDNF) |
| [119] | MDD; galacto-oligosaccaride; L. helveticus, B. longum | =with prebiotics (BDI)+with probiotics (BDI) |
| [120] | MDD; L. plantarum | =(HDRS, PSS)+(attention, perceptivity, verbal learning) |
| [121] | D; L. helveticus, B. longum | =(MADRS, DASS) |
| [122] | H; L. rhamnosus | +(depression, anxiety) |
| [123] | IBS with anx. or depr.; B. longum | +(HADS, QoL, brain activity) |
| [124] | H; Lactobacilli, Bifidobacteria | =(BDI, BAI)+(cognitive reactivity to sad mood) |
| [125] | MDD; Lactobacilli, B. bifidum | +(BDI, serum hs-CPR) |
| [126] | BD; L. acidophilus, Bifidobacteria | =(YMRS, HDRS) |
3.8. Studies Involving Twins and Their Relatives
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotrophin |
| BAI | Beck anxiety inventory |
| BD | Bipolar disorder |
| BDI | Beck depression inventory |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| DASS | Depression anxiety stress scales |
| FMT | Fecal microbiota transplantation |
| FGID | Functional gastrointestinal disorders |
| GABA | Gamma-aminobutyric acid |
| HADS | Hospital anxiety and depression scale |
| HC | Healthy controls |
| HDRS | Hamilton depression rating scale |
| HPA axis | Hypothalamic-pituitary-adrenal axis |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IgM | Immune globulin M |
| LPS | Lipopolysaccarides |
| MADRS | Montgomery-Åsberg depression rating scale |
| MDD | Major depressive disorder |
| OTU | Operational taxonomic unit |
| PANDAS | Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections |
| PHQ-9 | Patient health questionnaire |
| PLS-DA | Partial least squares discriminant analysis |
| PSS | Perceived stress scale |
| PUFA | Polyunsaturated fatty acids |
| QoL | Quality of life |
| SCFA | Short-chain fatty acids |
| spp. | Species |
| YMRS | Young mania rating scale |
References
- Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Licence: CC BY-NC-SA 3.0 IGO.
- Klengel, T.; Binder, E.B. Gene—Environment Interactions in Major Depressive Disorder. Can. J. Psychiatry 2013, 58, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craddock, N.; Jones, I. Genetics of bipolar disorder. J. Med. Genet. 1999, 36, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.S. Genetics of Bipolar Disorder. Psychiatr. Clin. N. Am. 2016, 39, 139–155. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Filteau, M.-J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Doo, E.; Choi, J.M.; Jang, S.; Ryu, H.-S.; Lee, J.Y.; Oh, J.H.; Park, J.H.; Kim, Y.S. Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J. Neurogastroenterol. Motil. 2017, 23, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Mudyanadzo, T.A.; Hauzaree, C.; Yerokhina, O.; Architha, N.N.; Ashqar, H.M. Irritable Bowel Syndrome and Depression: A Shared Pathogenesis. Cureus 2018, 10, e3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.-L.; Chevallier, J.-M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; Preter, V.D.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Immerseel, F.V.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.N. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J. Gastroenterol. 2014, 20, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [Green Version]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.; Aminov, R.; Matthias, T. Dysbiosis May Trigger Autoimmune Diseases via Inappropriate Post-Translational Modification of Host Proteins. Front. Microbiol. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Van Nhieu, J.T.; Furet, J.P. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef]
- Belančić, A. Gut microbiome dysbiosis and endotoxemia—Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice: Commensal microbiota and stress response. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- D’Argenio, G.; Mazzacca, G. Short-Chain Fatty Acid in the Human Colon. In Advances in Nutrition and Cancer 2; Zappia, V., Della Ragione, F., Barbarisi, A., Russo, G.L., Iacovo, R.D., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 472, pp. 149–158. ISBN 978-1-4419-3331-7. [Google Scholar]
- Barrett, E.; Ross, R.P.; O’Toole, P.W. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, W.R.; Valvassori, S.S.; Réus, G.Z.; Varela, R.B.; Arent, C.O.; Ribeiro, K.F.; Bavaresco, D.V.; Andersen, M.L.; Zugno, A.I.; Quevedo, J. Effects of sodium butyrate in animal models of mania and depression: Implications as a new mood stabilizer. Behav. Pharmacol. 2013, 24, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.-C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- Vagnerová, K.; Vodička, M.; Hermanová, P.; Ergang, P.; Šrůtková, D.; Klusoňová, P.; Balounová, K.; Hudcovic, T.; Pácha, J. Interactions Between Gut Microbiota and Acute Restraint Stress in Peripheral Structures of the Hypothalamic–Pituitary–Adrenal Axis and the Intestine of Male Mice. Front. Immunol. 2019, 10, 2655. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Mihaylova, I.; Kubera, M.; Ringel, K. Activation of cell-mediated immunity in depression: Association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 169–175. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Koh, H.; Tuddenham, S.; Sears, C.L.; Zhao, N. Meta-analysis methods for multiple related markers: Applications to microbiome studies with the results on multiple α-diversity indices. Stat. Med. 2021, 40, 2859–2876. [Google Scholar] [CrossRef]
- Su, X. Elucidating the Beta-Diversity of the Microbiome: From Global Alignment to Local Alignment. mSystems 2021, 6, e00363-21. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.; Deng, W.; Xu, S.; Zhao, J.; Xu, D.; Liu, Y.; Guo, Y.; Wang, M.; He, F.; Ye, S.; et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients. Psychol. Med. 2019, 51, 90–101. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.; Sheehan, A. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain, Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, Q.; Xie, B.; Duan, L.; Zhao, W.; Hu, D.; Wu, R.; Liu, H. Gut microbes in correlation with mood: Case study in a closed experimental human life support system. Neurogastroenterol. Motil. 2016, 28, 1233–1240. [Google Scholar] [CrossRef]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef]
- Mason, B.L.; Li, Q.; Minhajuddin, A.; Czysz, A.H.; Coughlin, L.A.; Hussain, S.K.; Koh, A.Y.; Trivedi, M.H. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.; Thompson, D.; Fowler, J.C. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 2020, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; He, S.; Fang, L.; Wang, B.; Bai, S.-J.; Xie, J.; Zhou, C.-J.; Wang, W.; Xie, P. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging 2020, 12, 2764–2776. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.; Zhao, J. Similarly in depression, nuances of gut microbiota_ Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Vinberg, M.; Ottesen, N.M.; Meluken, I.; Sørensen, N.; Pedersen, O.; Kessing, L.V.; Miskowiak, K.W. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta Psychiatr. Scand. 2019, 139, 174–184. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.; Zhang, X.; Zhang, Z.; Ruan, B. The Microbiome in Bipolar Depression: A Longitudinal Study of One Pair of Monozygotic Twins. Bipolar Disord. 2018, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; Fournier, C.; Durdevic, M.; Knoblich, L.; Keip, B.; Dejaco, C.; Trauner, M.; Moser, G. A Microbial Signature of Psychological Distress in Irritable Bowel Syndrome. Psychosom. Med. 2018, 80, 698–709. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, P.; Liu, Y.; Zhong, X.; Wang, H.; Guo, Y.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiman, S.C.; Bulik-Sullivan, E.C.; Glenny, E.M.; Zerwas, S.C.; Huh, E.Y.; Tsilimigras, M.C.B.; Fodor, A.A.; Bulik, C.M.; Carroll, I.M. The Gut-Brain Axis in Healthy Females: Lack of Significant Association between Microbial Composition and Diversity with Psychiatric Measures. PLoS ONE 2017, 12, e0170208. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Coello, K.; Hansen, T.H.; Sørensen, N.; Munkholm, K.; Kessing, L.V.; Pedersen, O.; Vinberg, M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav. Immun. 2019, 75, 112–118. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Subramaniapillai, M.; Shekotikhina, M.; Carmona, N.E.; Lee, Y.; Mansur, R.B.; Brietzke, E.; Fus, D.; Coles, A.S.; Iacobucci, M.; et al. Characterizing the gut microbiota in adults with bipolar disorder: A pilot study. Nutr. Neurosci. 2019, 24, 173–180. [Google Scholar] [CrossRef]
- Hu, S.; Li, A.; Huang, T.; Lai, J.; Li, J.; Sublette, M.E.; Lu, H.; Lu, Q.; Du, Y.; Hu, Z.; et al. Gut Microbiota Changes in Patients with Bipolar Depression. Adv. Sci. 2019, 6, 1900752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. NeuroReport 2018, 29, 417–425. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Taylor, A.M.; Thompson, S.V.; Edwards, C.G.; Musaad, S.M.A.; Khan, N.A.; Holscher, H.D. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2020, 23, 983–992. [Google Scholar] [CrossRef]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhi, F. Lower Level of Bacteroides in the Gut Microbiota Is Associated with Inflammatory Bowel Disease: A Meta-Analysis. BioMed Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef] [Green Version]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Cheng, S.; Han, B.; Ding, M.; Wen, Y.; Ma, M.; Zhang, L.; Qi, X.; Cheng, B.; Li, P.; Kafle, O.P.; et al. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Brief. Bioinform. 2020, 21, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lai, J.; Lu, H.; Ng, C.; Huang, T.; Zhang, H.; Ding, K.; Wang, Z.; Jiang, J.; Hu, J.; et al. Gut Microbiota in Bipolar Depression and Its Relationship to Brain Function: An Advanced Exploration. Front. Psychiatry 2019, 10, 784. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Koga, N.; Hattori, K.; Ota, M.; Kunugi, H. Bifidobacterium and Lactobacillus Counts in the Gut Microbiota of Patients With Bipolar Disorder and Healthy Controls. Front. Psychiatry 2019, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Heym, N.; Heasman, B.C.; Hunter, K. The role of microbiota and inflammation in self-judgement and empathy: Implications for understanding the brain-gut-microbiome axis in depression. Psychopharmacology 2019, 12, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Baj, J.; Sitarz, E.; Forma, A.; Wróblewska, K.; Karakuła-Juchnowicz, H. Alterations in the Nervous System and Gut Microbiota after β-Hemolytic Streptococcus Group A Infection—Characteristics and Diagnostic Criteria of PANDAS Recognition. Int. J. Mol. Sci. 2020, 21, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, C.; Subasic, K. PANDAS Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection. AJN Am. J. Nurs. 2020, 120, 32–37. [Google Scholar] [CrossRef] [PubMed]
- The Prokaryotes; Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. (Eds.) Springer: Berlin, Germany, 2014; ISBN 978-3-642-30119-3. [Google Scholar]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Morotomi, M.; Nagai, F.; Watanabe, Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 144–149. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1880221. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wong, C.C.; Tong, L. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Altemani, F.; Barrett, H.L.; Gomez-Arango, L.; Josh, P.; David McIntyre, H.; Callaway, L.K.; Morrison, M.; Tyson, G.W.; Dekker Nitert, M. Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy Hypertens. 2021, 23, 211–219. [Google Scholar] [CrossRef]
- Ai, D.; Pan, H.; Li, X.; Gao, Y.; Liu, G.; Xia, L.C. Identifying Gut Microbiota Associated With Colorectal Cancer Using a Zero-Inflated Lognormal Model. Front. Microbiol. 2019, 10, 826. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Dong, L.; Chang, P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv. Nutr. 2019, 10, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-Y.; Huang, S.-Y.; Su, K.-P. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, J.; Yuan, W.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses: Roseburia intestinalis reduces colitis. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Schwiertz, A.; Aminov, R.I.; Blaut, M.; Flint, H.J. Oligonucleotide Probes That Detect Quantitatively Significant Groups of Butyrate-Producing Bacteria in Human Feces. Appl. Environ. Microbiol. 2003, 69, 4320–4324. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Ogita, T.; Yamamoto, Y.; Mikami, A.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Strongly Suppresses Th2 Immune Responses in Mice. Front. Immunol. 2020, 11, 379. [Google Scholar] [CrossRef] [Green Version]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii, a Bacteria Increased With Green Tea Consumption, Promotes Recovery From Acute Colitis in Mice via Suppression of IL-17. Front. Nutr. 2021, 7, 610946. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Dai, L.; Chen, H.; Yi, B.; Niu, J.; Wang, L.; Zhang, F.; Luo, J.; Wang, K.; et al. Ecological and network analyses identify four microbial species with potential significance for the diagnosis/treatment of ulcerative colitis (UC). BMC Microbiol. 2021, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Straub, T.J.; Chou, W.-C.; Manson, A.L.; Schreiber, H.L.; Walker, B.J.; Desjardins, C.A.; Chapman, S.B.; Kaspar, K.L.; Kahsai, O.J.; Traylor, E.; et al. Limited effects of long-term daily cranberry consumption on the gut microbiome in a placebo-controlled study of women with recurrent urinary tract infections. BMC Microbiol. 2021, 21, 53. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, 45. [Google Scholar] [CrossRef]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut Microbiota Composition Is Correlated to Grid Floor Induced Stress and Behavior in the BALB/c Mouse. PLoS ONE 2012, 7, e46231. [Google Scholar] [CrossRef] [Green Version]
- Frost, F.; Storck, L.J.; Kacprowski, T.; Gärtner, S.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Aghdassi, A.A.; Steveling, A.; et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS ONE 2019, 14, e0219489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.D.; Chen, C.; Knox, N.C.; Marrie, R.-A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases—does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y. Health benefits of lactobacillus gasseri cp2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
- Sawada, D.; Kawai, T.; Nishida, K.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct. Foods 2017, 31, 188–197. [Google Scholar] [CrossRef]
- Haghighat, N.; Rajabi, S.; Mohammadshahi, M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: A randomized, double-blinded, clinical trial. Nutr. Neurosci. 2019, 24, 490–499. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Katz, E.G.; Blacketer, C. Microbiome-Gut-Brain Axis: Probiotics and Their Association With Depression. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 39–44. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Eslami Shahrbabaki, M.; Sabouri, S.; Sabahi, A.; Barfeh, D.; Divsalar, P.; Divsalar, P.; Esmailzadeh, M.; Ahmadi, A. The Efficacy of Probiotics for Treatment of Bipolar Disorder- Type 1: A Randomized, Double-Blind, Placebo-Controlled Trial. Iran. J. Psychiatry 2020, 15, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R. A case report looking at the effects of faecal microbiota transplantation in a patient with bipolar disorder. Aust. N. Z. J. Psychiatry 2020, 54, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Roesch, L.; Thiago, P.; Russell, J.T.; Pepine, C.J.; Holbert, R.C.; Raizada, M.K.; Triplett, E.W. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol. Psychiatry 2020, 26, 4277–4287. [Google Scholar] [CrossRef]
| Source | Genus Bacteroides | |
|---|---|---|
| [42] | aMDD ↓ | rMDD ↑ |
| [45] | negative mood ↑ | |
| [46] | BD ↑ (1 OTU) | |
| MDD ↑/↓ (OTUs) | ||
| [47] | anhedonia ↑ | |
| anxiety ↓ | ||
| [50] | MDD ↓, BD ↓ | |
| [55] | anxiety ↑ | |
| [57] | MDD ↑ (m) | |
| [60] | D ↑; IBS ↑; D + IBS ↑ | |
| [66] | BD ↑ | |
| [74] | MDD ↑ | |
| [75] | BD ↑ (B-P group) | |
| Source | Genus Roseburia |
|---|---|
| [42] | aMDD ↑ |
| [45] | ↓ positive mood |
| [46] | BD ↓ (1 OTU) |
| [47] | anhedonia ↓ |
| [48] | P ↓ remission (R. inuliniforans) |
| [52] | BD ↓ * |
| [55] | D ↓ (unclassified species) |
| [57] | MDD ↑ (f) |
| [60] | D ↓ |
| [63] | MDD ↓ (OTUs) |
| [66] | BD ↓ |
| Source | Family Ruminococcaceae | Genus Ruminococcus | Genus Faecalibacterium |
|---|---|---|---|
| [42] | MDD ↓ | aMDD ↓ | MDD ↓ |
| [44] | MDD ↓ | MDD ↓ (Ruminococcus 1) | MDD ↓ |
| [45] | negative mood ↑ | ||
| [46] | MDD ↓ (OTUs) | ||
| BD ↑/↓ (OTUs) | |||
| [47] | anhedonia ↓ | ||
| [48] | P ↓ remission | P ↑ (Ruminococcus 1) | P ↓ remission (F. prausnitzii) |
| [49] | MDD ↑/↓ (OTUs) | ||
| [51] | MDD ↑ | ||
| [52] | BD ↓ | BD ↓ | |
| [54] | BD ↓ | BD ↓ | |
| [55] | D ↑ (unclassified species) | ||
| [57] | MDD ↑/↓ (OTUs) | MDD ↑ (f) | |
| [58] | MDD ↓ | ||
| [60] | D ↓ | ||
| [63] | MDD ↑ (OTUs) | MDD ↓ (OTUs) | |
| [66] | BD ↓ | BD ↓ | |
| [67] | MDD ↑ | MDD ↓ | |
| [75] | BD ↑ (F. prausnitzii) | ||
| [77] | lower QoL ↓ | ||
| [99] | DASS ↑ (R. gnavus) | ||
| [100] | BD ↓ (1 OTU) | BD ↓ |
| Source | Family Bifidobacteriaceae | Genus Bifidobacterium | |
|---|---|---|---|
| [43] | MDD ↑ | ||
| [45] | negative mood ↑ | ||
| [46] | MDD ↑ | BD = | |
| [47] | anhedonia ↓ | anhedonia ↓ | |
| [50] | MDD ↑, BD ↑ | ||
| [51] | MDD ↑ | MDD ↑ | |
| [56] | HDRS ↑ (B. longum) | ||
| [57] | MDD ↑ (f) | ||
| [67] | MDD ↑ | ||
| [79] | BD = | ||
| [113] | MDD ↓ | ||
| More Abundant in Depressive Subjects | Less Abundant in Depressive Subjects |
|---|---|
| Actinobacteria (phylum) | Christensenellaceae and Christensenella (family and genus) |
| Alistipes (genus) | Coprococcus (genus) |
| Bacteroides (genus) | Eubacterium and E. rectale (genus and species) |
| Bifidobacteriaceae and Bifidobacterium (family and genus) | Faecalibacterium and F. prausnitzii (genus and species) |
| Flavonifractor (genus) | Roseburia (genus) |
| Parabacteroides (genus) | Ruminococcaceae (family) |
| Streptococcus (genus) | Sutterellaceae and Sutterella (family and genus) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knuesel, T.; Mohajeri, M.H. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients 2022, 14, 37. https://doi.org/10.3390/nu14010037
Knuesel T, Mohajeri MH. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients. 2022; 14(1):37. https://doi.org/10.3390/nu14010037
Chicago/Turabian StyleKnuesel, Tom, and M. Hasan Mohajeri. 2022. "The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder" Nutrients 14, no. 1: 37. https://doi.org/10.3390/nu14010037
APA StyleKnuesel, T., & Mohajeri, M. H. (2022). The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients, 14(1), 37. https://doi.org/10.3390/nu14010037






