The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets
Abstract
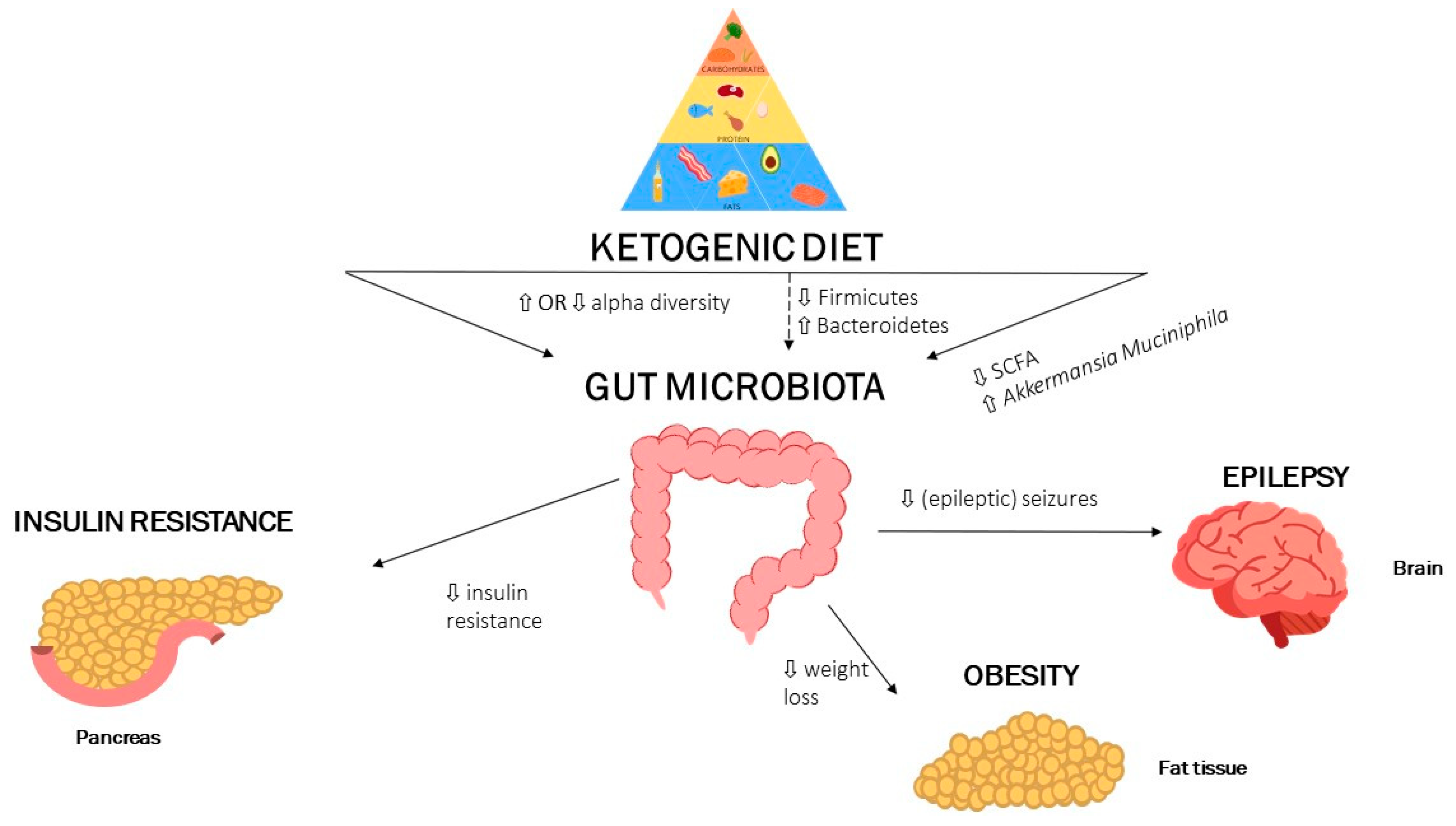
1. Introduction
2. The Gut Microbiota and Ketogenic Diet: Epilepsy
3. The Gut Microbiota and Ketogenic Diet: Obesity
4. The Gut Microbiota and Ketogenic Diet: Dyslipidaemia
5. The Gut Microbiota and Ketogenic Diet: Insulin Resistance
6. Summary and Future Perspectives
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CMD | Cardiometabolic diseases |
| En% | Energy percentage |
| GLUT1-DS | Glucose Transporter-1Deficiency syndrome |
| SCFA | Short-chain fatty acid |
| VLCKD | Very low-caloric ketogenic diet |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
References
- Wheless, J.W. History of the Ketogenic Diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic Diets for Drug-Resistant Epilepsy. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.H.; Lyczkowski, D.A.; Thiele, E.A. Low Glycemic Index Treatment: Implementation and New Insights into Efficacy. Epilepsia 2008, 49, 42–45. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO) European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Khadada, M.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I.; Al-Zaid, N.S. Beneficial Effects of Ketogenic Diet in Obese Diabetic Subjects. Mol. Cell. Biochem. 2007, 302, 249–256. [Google Scholar] [CrossRef]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: ADose-Response Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef]
- Chen, Z.; Franco, O.H.; Lamballais, S.; Ikram, M.A.; Schoufour, J.D.; Muka, T.; Voortman, T. Associations of Specific Dietary Protein with Longitudinal Insulin Resistance, Prediabetes and Type 2 Diabetes: The Rotterdam Study. Clin. Nutr. 2020, 39, 242–249. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Marette, A. Amino Acid and Insulin Signaling via the MTOR/P70 S6 Kinase Pathway. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [CrossRef]
- Rietman, A.; Schwarz, J.; Tomé, D.; Kok, F.J.; Mensink, M. High Dietary Protein Intake, Reducing or Eliciting Insulin Resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red Meat Consumption and Risk of Type 2 Diabetes: 3 Cohorts of US Adults and an Updated Meta-Analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Attaye, I.; Pinto-Sietsma, S.-J.; Herrema, H.; Nieuwdorp, M. A Crucial Role for Diet in the Relationship between Gut Microbiota and Cardiometabolic Disease. Annu. Rev. Med. 2020, 71, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Liu, X.; Zhang, J.; Liu, G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediat. Inflamm. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef] [PubMed]
- Vining, E.P.G. A Multicenter Study of the Efficacy of the Ketogenic Diet. Arch. Neurol. 1998, 55, 1433. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Burloiu, C.M.; Barca, D.G.; Magureanu, S.A.; Craiu, D.C.; Davila, C. Ketogenic Diet in Patients with GLUT1 Deficiency Syndrome. J. Clin. Med. 2019, 14, 93–97. [Google Scholar]
- Medel-Matus, J.-S.; Shin, D.; Dorfman, E.; Sankar, R.; Mazarati, A. Facilitation of Kindling Epileptogenesis by Chronic Stress May Be Mediated by Intestinal Microbiome. Epilepsia Open 2018, 3, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Eor, J.Y.; Tan, P.L.; Son, Y.J.; Kwak, M.J.; Kim, S.H. Gut Microbiota Modulation by Both Lactobacillus Fermentum MSK 408 and Ketogenic Diet in a Murine Model of Pentylenetetrazole-Induced Acute Seizure. Epilepsy Res. 2021, 169, 106506. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut Microbiota and Metabolic Health among Overweight and Obese Individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia Muciniphila: Key Player in Metabolic and Gastrointestinal Disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Ferraris, C.; Meroni, E.; Casiraghi, M.C.; Tagliabue, A.; De Giorgis, V.; Erba, D. One Month of Classic Therapeutic Ketogenic Diet Decreases Short Chain Fatty Acids Production in Epileptic Patients. Front. Nutr. 2021, 8, 613100. [Google Scholar] [CrossRef]
- Gong, X.; Cai, Q.; Liu, X.; An, D.; Zhou, D.; Luo, R.; Peng, R.; Hong, Z. Gut Flora and Metabolism Are Altered in Epilepsy and Partially Restored after Ketogenic Diets. Microb. Pathog. 2021, 155, 104899. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The Ketogenic Diet Influences Taxonomic and Functional Composition of the Gut Microbiota in Children with Severe Epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Blackford, R. Gut Microbiota, the Ketogenic Diet and Epilepsy. Pediatr. Neurol. Briefs 2018, 32, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic Diet Poses a Significant Effect on Imbalanced Gut Microbiota in Infants with Refractory Epilepsy. WJG 2017, 23, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered Gut Microbiome Composition in Children with Refractory Epilepsy after Ketogenic Diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but Not Faecal Short-Chain Fatty Acids Are Related to Insulin Sensitivity, Lipolysis and GLP-1 Concentrations in Humans. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-Term Impact of a Classical Ketogenic Diet on Gut Microbiota in GLUT1 Deficiency Syndrome: A 3-Month Prospective Observational Study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.M. The Effects of Ketonemia on the Course of Epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.-F.; Kahn, B.B.; Libermann, T.A.; et al. A High-Fat, Ketogenic Diet Induces a Unique Metabolic State in Mice. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; van Dijck, L.; Töns, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.P.B.; de Koning, E.J.P. Long-Term Ketogenic Diet Causes Glucose Intolerance and Reduced β- and α-Cell Mass but No Weight Loss in Mice. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-Low-Carbohydrate Ketogenic Diet v. Low-Fat Diet for Long-Term Weight Loss: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, K.; Krzyżak, K.; Hajzler, W.; Drożdż, K.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Fat, Sugar or Gut Microbiota in Reducing Cardiometabolic Risk: Does Diet Type Really Matter? Nutrients 2021, 13, 639. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Nieuwenhuizen, A.; Tomé, D.; Soenen, S.; Westerterp, K.R. Dietary Protein, Weight Loss, and Weight Maintenance. Annu. Rev. Nutr. 2009, 29, 21–41. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Gibson, G.R.; Beatty, E.; Cummings, J.H. Estimation of Short-Chain Fatty Acid Production from Protein by Human Intestinal Bacteria Based on Branched-Chain Fatty Acid Measurements. FEMS Microbiol. Ecol. 1992, 10, 81–88. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Dissimilatory Amino Acid Metabolism in Human Colonic Bacteria. Anaerobe 1997, 3, 327–337. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [PubMed]
- Kübeck, R.; Bonet-Ripoll, C.; Hoffmann, C.; Walker, A.; Müller, V.M.; Schüppel, V.L.; Lagkouvardos, I.; Scholz, B.; Engel, K.-H.; Daniel, H.; et al. Dietary Fat and Gut Microbiota Interactions Determine Diet-Induced Obesity in Mice. Mol. Metab. 2016, 5, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, 1900167. [Google Scholar] [CrossRef] [PubMed]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Bird, A.R. Comparative Effects of Very Low-Carbohydrate, High-Fat and High-Carbohydrate, Low-Fat Weight-Loss Diets on Bowel Habit and Faecal Short-Chain Fatty Acids and Bacterial Populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81. [Google Scholar] [CrossRef]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and Risk Factors for Dyslipidemia among Adults in Rural and Urban China: Findings from the China National Stroke Screening and Prevention Project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.; Cabezas, M. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of Weight Reduction on Blood Lipids and Lipoproteins: A Meta-Analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, J.; Jiang, T.; Zhang, Z.; Wang, Z.; Gong, M.; Zhao, L.; Zhang, C. Ketogenic Diets Induced Glucose Intolerance and Lipid Accumulation in Mice with Alterations in Gut Microbiota and Metabolites. Am. Soc. Microbiol. mBio 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Warmbrunn, M.V.; Herrema, H.; Aron-Wisnewsky, J.; Soeters, M.R.; Van Raalte, D.H.; Nieuwdorp, M. Gut Microbiota: A Promising Target against Cardiometabolic Diseases. Expert Rev. Endocrinol. Metab. 2020, 15, 13–27. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin Resistance Is a Cardiovascular Risk Factor in Humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O. Nutritional Modulation of Insulin Resistance. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef][Green Version]
- Shen, J.; Obin, M.S.; Zhao, L. The Gut Microbiota, Obesity and Insulin Resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef]
- Gadgil, M.D.; Appel, L.J.; Yeung, E.; Anderson, C.A.M.; Sacks, F.M.; Miller, E.R. The Effects of Carbohydrate, Unsaturated Fat, and Protein Intake on Measures of Insulin Sensitivity: Results from the OmniHeart Trial. Diabetes Care 2013, 36, 1132–1137. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Bielohuby, M.; Sisley, S.; Sandoval, D.; Herbach, N.; Zengin, A.; Fischereder, M.; Menhofer, D.; Stoehr, B.J.M.; Stemmer, K.; Wanke, R.; et al. Impaired Glucose Tolerance in Rats Fed Low-Carbohydrate, High-Fat Diets. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1059–E1070. [Google Scholar] [CrossRef]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Short-Term Low Carbohydrate/High-Fat Diet Intake Increases Postprandial Plasma Glucose and Glucagon-like Peptide-1 Levels during an Oral Glucose Tolerance Test in Healthy Men. Eur. J. Clin. Nutr. 2012, 66, 926–931. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut Microbiota–Bile Acid–Interleukin-22 Axis Orchestrates Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Koopen, A.M.; Almeida, E.L.; Attaye, I.; Witjes, J.J.; Rampanelli, E.; Majait, S.; Kemper, M.; Levels, J.H.M.; Schimmel, A.W.M.; Herrema, H.; et al. Effect of Fecal Microbiota Transplantation Combined With Mediterranean Diet on Insulin Sensitivity in Subjects With Metabolic Syndrome. Front. Microbiol. 2021, 12, 662159. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Iñiguez, A.J.; Yang, G.E.; Fang, P.; Pronovost, G.N.; Jameson, K.G.; Rendon, T.K.; Paramo, J.; Barlow, J.T.; Ismagilov, R.F.; et al. Alterations in the Gut Microbiota Contribute to Cognitive Impairment Induced by the Ketogenic Diet and Hypoxia. Cell Host Microbe 2021, 29, 1378–1392.e6. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut Microbiome and Serum Metabolome Alterations in Obesity and after Weight-Loss Intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Frost, F.; Storck, L.J.; Kacprowski, T.; Gärtner, S.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Aghdassi, A.A.; Steveling, A.; et al. A Structured Weight Loss Program Increases Gut Microbiota Phylogenetic Diversity and Reduces Levels of Collinsella in Obese Type 2 Diabetics: A Pilot Study. PLoS ONE 2019, 14, e0219489. [Google Scholar] [CrossRef]
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2022, 14, 191. https://doi.org/10.3390/nu14010191
Attaye I, van Oppenraaij S, Warmbrunn MV, Nieuwdorp M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients. 2022; 14(1):191. https://doi.org/10.3390/nu14010191
Chicago/Turabian StyleAttaye, Ilias, Sophie van Oppenraaij, Moritz V. Warmbrunn, and Max Nieuwdorp. 2022. "The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets" Nutrients 14, no. 1: 191. https://doi.org/10.3390/nu14010191
APA StyleAttaye, I., van Oppenraaij, S., Warmbrunn, M. V., & Nieuwdorp, M. (2022). The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients, 14(1), 191. https://doi.org/10.3390/nu14010191





