Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Change in Circulating Fatty Acids with Supplementation
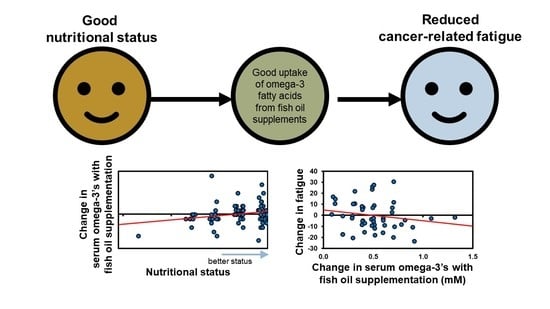
3.3. Associations between Nutritional Status, Omega-3 Uptake, and Cancer-Related Fatigue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015, Clinical Practice Guidelines in Oncology. Nat. Comp. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Al Maqbali, M.; Al Sinani, M.; Al Naamani, Z.; Al Badi, K.; Tanash, M.I. Prevalence of Fatigue in Patients With Cancer: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2021, 61, 167–189.e14. [Google Scholar] [CrossRef] [PubMed]
- Servaes, P.; Verhagen, C.; Bleijenberg, G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur. J. Cancer 2002, 38, 27–43. [Google Scholar] [CrossRef]
- Shi, Q.; Ma, T.G.S.; Ms, J.D.M.; Stein, K.D.; Kaw, C.K.; Cleeland, C.S. Symptom burden in cancer survivors 1 year after diagnosis. Cancer 2011, 117, 2779–2790. [Google Scholar] [CrossRef] [Green Version]
- Horneber, M.; Fischer, I.; Dimeo, F.; Rüffer, J.U.; Weis, J. Cancer-Related Fatigue. Dtsch. Aerzteblatt Online 2012, 109, 161–172. [Google Scholar] [CrossRef]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-Related Fatigue: The Scale of the Problem. Oncologist 2007, 12, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Saligan, L.N.; Multinational Association of Supportive Care in Cancer Fatigue Study Group–Biomarker Working Group; Olson, K.; Filler, K.; Larkin, D.; Cramp, F.; Sriram, Y.; Escalante, C.P.; Del Giglio, A.; Kober, K.; et al. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer 2015, 23, 2461–2478. [Google Scholar] [CrossRef]
- Zick, S.M.; Colacino, J.; Cornellier, M.L.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Wright, O. Nutrition therapy for the management of cancer-related fatigue and quality of life: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Schlemmer, M.; Suchner, U.; Schäpers, B.; Duerr, E.-M.; Alteheld, B.; Zwingers, T.; Stehle, P.; Zimmer, H.-G. Is glutamine deficiency the link between inflammation, malnutrition, and fatigue in cancer patients? Clin. Nutr. 2015, 34, 1258–1265. [Google Scholar] [CrossRef]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, Inflammation, and ω-3 and ω-6 Fatty Acid Intake Among Breast Cancer Survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the Association of Diet and Persistent Cancer-Related Fatigue: A Pilot Study. Oncol. Nurs. Forum 2013, 40, E41–E49. [Google Scholar] [CrossRef] [Green Version]
- Peppone, L.J.; Inglis, J.E.; Mustian, K.M.; Heckler, C.E.; Padula, G.D.A.; Mohile, S.G.; Kamen, C.S.; Culakova, E.; Lin, P.-J.; Kerns, S.L.; et al. Multicenter Randomized Controlled Trial of Omega-3 Fatty Acids Versus Omega-6 Fatty Acids for the Control of Cancer-Related Fatigue Among Breast Cancer Survivors. JNCI Cancer Spectr. 2019, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, U.J.; Gonzalez, M.A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguze, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hos-pital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sugano, K.; Ikeya, T.; Muguruma, K.; Tanaka, H.; Toyokawa, T.; et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLoS ONE 2015, 10, e0132488. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Sakamoto, K.; Shinden, S.; Ogawa, K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin. Nutr. 2017, 36, 1681–1685. [Google Scholar] [CrossRef]
- Kuroda, D.; Sawayama, H.; Kurashige, J.; Iwatsuki, M.; Eto, T.; Tokunaga, R.; Kitano, Y.; Yamamura, K.; Ouchi, M.; Nakamura, K.; et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018, 21, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bistrian, B.R.; Blackburn, G.L.; Hallowell, E.; Heddle, R. Protein status of general surgical patients. JAMA 1974, 230, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shronts, E.P. Basic Concepts of Immunology and Its Application to Clinical Nutrition. Nutr. Clin. Pr. 1993, 8, 177–183. [Google Scholar] [CrossRef]
- Nozoe, T.; Ninomiya, M.; Maeda, T.; Matsukuma, A.; Nakashima, H.; Ezaki, T. Prognostic nutritional Index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg. Today 2010, 40, 440–443. [Google Scholar] [CrossRef]
- Madroño, A.G. The use of biochemical and immunological parameters in nutritional screening and assessment. Nutr. Hosp 2011, 26, 184–191. [Google Scholar]
- Stein, K.D.; Jacobsen, P.B.; Blanchard, C.M.; Thors, C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manag. 2004, 27, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.D.; Ma, S.C.M.; Hann, D.M.; Jacobsen, P.B. A Multidimensional Measure of Fatigue for Use with Cancer Patients. Cancer Pr. 1998, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.J. Robust Statistics, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Liposits, G.; Orrevall, Y.; Kaasa, S.; Österlund, P.; Cederholm, T. Nutrition in Cancer Care: A Brief, Practical Guide With a Focus on Clinical Practice. JCO Oncol. Pr. 2021, 17, e992–e998. [Google Scholar] [CrossRef]
- Presley, C.J.; Dotan, E.; Soto-Perez-De-Celis, E.; Jatoi, A.; Mohile, S.G.; Won, E.; Alibhai, S.; Kilari, D.; Harrison, R.; Klepin, H.D.; et al. Gaps in nutritional research among older adults with cancer. J. Geriatr. Oncol. 2016, 7, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Ljungblad, L.; Gleissman, H.; Hedberg, G.; Wickström, M.; Eissler, N.; Pickova, J.; Johnsen, J.; Tedroff, K.; Strandvik, B.; Kogner, P. Body surface area-based omega-3 fatty acids supplementation strongly correlates to blood concentrations in children. Prostaglandins, Leukot. Essent. Fat. Acids 2021, 169, 102285. [Google Scholar] [CrossRef]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P. Determinants of Erythrocyte Omega-3 Fatty Acid Content in Response to Fish Oil Supplementation: A Dose–Response Randomized Controlled Trial. J. Am. Hear. Assoc. 2013, 2, e000513. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Schwichtenberg, K.A.; Hanson, N.Q.; Tsai, M.Y. Incorporation and Clearance of Omega-3 Fatty Acids in Erythrocyte Membranes and Plasma Phospholipids. Clin. Chem. 2006, 52, 2265–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, A.H.; Pedersen, T.L.; Fillaus, K.; Larson, M.K.; Shearer, G.C.; Newman, J.W. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J. Lipid Res. 2012, 53, 1662–1669. [Google Scholar] [CrossRef] [Green Version]
- Pollack, M.N.; Huynh, H.T.; Pratt, L. Tamoxifen reduces serum insulin-like growth factor I (IGF-I). Breast Cancer Res. Treat. 1992, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharour, L.A. Cancer-Related Fatigue, Laboratory Markers as Indicators for Nutritional Status among Patients with Colorectal Cancer. Nutr. Cancer 2020, 72, 903–908. [Google Scholar] [CrossRef]
- Vergara, N.; Montoya, J.E.; Luna, H.G.; Amparo, J.R.; Cristal-Luna, G. Quality of Life and Nutritional Status Among Cancer Patients on Chemotherapy. Oman Med. J. 2013, 28, 270–274. [Google Scholar] [CrossRef]
- Lim, H.-S.; Cho, G.-S.; Park, Y.-H.; Kim, S.-K. Comparison of Quality of Life and Nutritional Status in Gastric Cancer Patients Undergoing Gastrectomies. Clin. Nutr. Res. 2015, 4, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urquiaga, I.; Guasch, V.; Marshall, G.; Martín, A.S.; Castillo, O.; Rozowski, J.; Leighton, F. Effect of Mediterranean and Occidental diets, and red wine, on plasma fatty acids in humans. An intervention study. Biol. Res. 2004, 37, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Toobert, D.J.; Glasgow, R.E.; Strycker, L.A.; Barrera, M.; Radcliffe, J.L.; Wander, R.C.; Bagdade, J.D. Biologic and Quality-of-Life Outcomes From the Mediterranean Lifestyle Program: A randomized clinical trial. Diabetes Care 2003, 26, 2288–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, Å.; Taft, C.; Lundgren-Nilsson, Å.; Dencker, A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med. Res. Methodol. 2016, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kleckner, A.S.; Magnuson, A. The nutritional needs of older cancer survivors. J. Geriatr. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Kleckner, A.S.; Lin, P.-J.; Gilmore, N.J.; Culakova, E.; VanderWoude, A.C.; Mustian, K.M.; Fernandez, I.D.; Dunne, R.F.; Deutsch, J.; et al. Excess Body Weight and Cancer-Related Fatigue, Systemic Inflammation, and Serum Lipids in Breast Cancer Survivors. Nutr. Cancer 2021, 73, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thapa, S.; Zhou, T.; Liu, H.; Li, L.; Peng, G.; Yu, S. Cancer-related fatigue and biochemical parameters among cancer patients with different stages of sarcopenia. Support. Care Cancer 2020, 28, 581–588. [Google Scholar] [CrossRef]
- Braeckman, R.A.; Manku, M.S.; Bays, H.E.; Stirtan, W.G.; Soni, P.N. Icosapent ethyl, a pure EPA omega-3 fatty acid: Effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 195–201. [Google Scholar] [CrossRef]
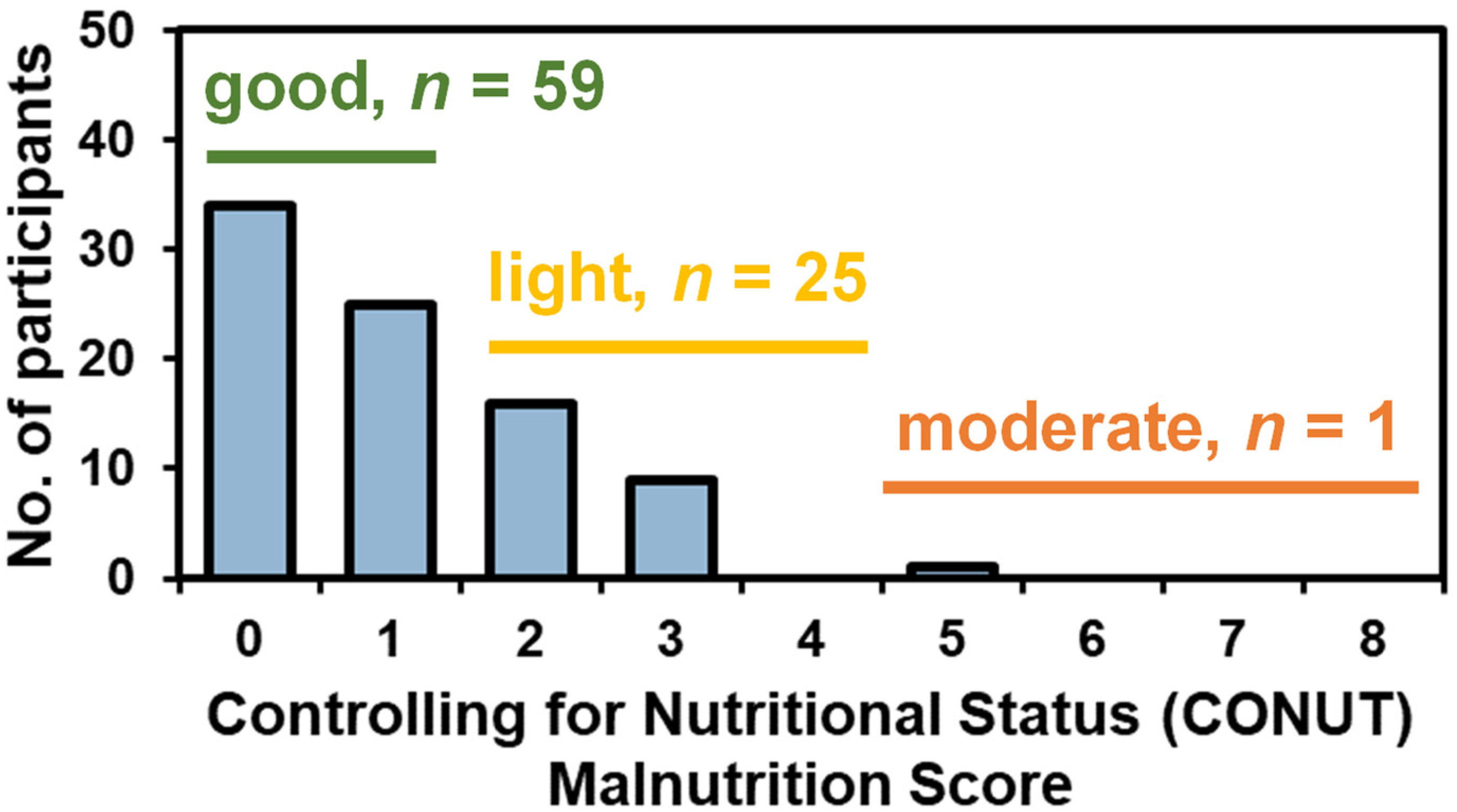

| Good Nutritional Status (n = 59) | Light-Moderate Malnutrition (n = 26) | p-Value * | |
|---|---|---|---|
| Mean ± SD or n (Percent) | Mean ± SD or n (Percent) | ||
| Age (years) | 61.3 ± 9.4 | 61.2 ± 10.7 | 0.97 |
| Body mass index (kg/m2) | 31.5 ± 6.0 | 32.8 ± 8.3 | 0.47 |
| Race (self-identified) | 0.28 | ||
| White | 56 (94.9%) | 23 (88.5%) | |
| Other | 3 (5.1%) | 3 (11.5%) | |
| Menopausal status | 0.88 | ||
| Pre-menopausal | 4 (6.8%) | 2 (7.7%) | |
| Peri- or post-menopausal or medically induced | 55 (93.2%) | 24 (92.3%) | |
| Marital status | 0.29 | ||
| Married or long-term relationship | 41 (69.5%) | 15 (57.7%) | |
| Divorced, separated, single, or widowed | 16 (27.1%) | 10 (38.5%) | |
| Education | 0.44 | ||
| Up to a high school degree | 20 (33.9%) | 11 (42.3%) | |
| At least some college | 37 (62.7%) | 14 (53.8%) | |
| Cancer stage | 0.27 | ||
| 0 | 4 (6.8%) | 0 (0%) | |
| 1 | 23 (39.0%) | 13 (50.0%) | |
| 2 | 26 (44.1%) | 8 (30.8%) | |
| 3 | 5 (8.5%) | 4 (15.4%) | |
| Cancer treatment | |||
| Surgery (yes) | 56 (94.9%) | 26 (100%) | 0.24 |
| Months since treatment | 17.7 (9.7%) | 19.0 (9.4%) | 0.57 |
| Chemotherapy (yes) | 30 (50.8%) | 11 (42.3%) | 0.47 |
| Months since treatment | 17.0 (8.3%) | 17.8 (9.2%) | 0.79 |
| Radiation therapy (yes) | 41 (69.5%) | 22 (84.6%) | 0.14 |
| Months since treatment | 19.4 (28.8%) | 17.4 (9.1%) | 0.67 |
| Current hormonal therapy | 0.045 | ||
| No | 19 (32.2%) | 3 (11.5%) | |
| Yes | 40 (67.8%) | 23 (88.5%) | |
| Fatty acid concentration | |||
| Total omega-3 fatty acids (mM) | 0.33 ± 0.10 | 0.27 ± 0.12 | 0.013 |
| Docosahexaenoic acid (DHA, μM) | 135.24 ± 47.70 | 125.77 ± 56.08 | 0.458 |
| Eicosapentaenoic acid (EPA, μM) | 70.68 ± 34.08 | 48.12 ± 35.23 | 0.009 |
| α-Linolenic acid (ALA, μM) | 87.61 ± 37.70 | 70.08 ± 28.77 | 0.022 |
| Total omega-6 fatty acids (mM) | 4.65 ± 0.79 | 3.92 ± 0.77 | <0.001 |
| Linoleic acid (μM) | 3497.3 ± 562.4 | 2958.1 ± 546.0 | <0.001 |
| Arachidonic acid (μM) | 844.95 ± 243.42 | 721.38 ± 240.89 | 0.035 |
| Omega-6:omega-3 ratio | 14.88 ± 4.06 | 16.56 ± 6.12 | 0.207 |
| 6 g Fish Oil (n = 30) | 3 g Fish Oil + 3 g Soybean Oil (n = 23) | 6 g Soybean Oil (n = 30) | p-Value | |
|---|---|---|---|---|
| Fatty Acid | Mean ± SE | Mean ± SE | Mean ± SE | |
| Total omega-3 fatty acids (mM) | 0.59 ± 0.33 | 0.38 ± 0.19 | −0.01 ± 0.09 | <0.001 |
| Docosahexaenoic acid (DHA, µM) | 207.13 ± 101.79 | 164.83 ± 80.48 | −8.30 ± 44.82 | <0.001 |
| Eicosapentaenoic acid (EPA, µM) | 358.00 ± 223.02 | 187.78 ± 107.86 | −5.04 ± 31.44 | <0.001 |
| Total omega-6 fatty acids (mM) | −0.56 ± 0.67 | −0.11 ± 0.73 | 0.05 ± 0.83 | 0.008 |
| Linoleic acid (µM) | −257.43 ± 105.77 | 54.39 ± 120.80 | 85.26 ± 111.49 | 0.054 |
| Arachidonic acid (µM) | −208.20 ± 172.49 | −105.48 ± 169.55 | −31.78 ± 180.74 | 0.001 |
| Omega-6:omega-3 ratio | −11.19 ± 6.94 | −9.13 ± 3.80 | 0.57 ± 2.94 | <0.001 |
| Dependent Variable | Effect Estimate | Standard Error | p-Value |
|---|---|---|---|
| Total omega-3 fatty acids (mM) | −0.0492 | 0.017 | 0.005 |
| Docosahexaenoic acid (DHA, μM) | −20.233 | 6.764 | 0.003 |
| Eicosapentaenoic acid (EPA, μM) | −19.400 | 9.055 | 0.032 |
| Total omega-6 fatty acids (mM) | 0.026 | 0.069 | 0.705 |
| Linoleic acid (µM) | 6.160 | 60.782 | 0.919 |
| Arachidonic acid (µM) | 15.309 | 15.489 | 0.323 |
| Omega-6:omega-3 ratio | 0.596 | 0.470 | 0.205 |
| Dependent Variable | Estimate (β) *,† | Std Error | p-Value |
|---|---|---|---|
| Multidimensional Fatigue Symptom Inventory-Short Form (MFSI) total score | −11.731 | 6.500 | 0.071 |
| General fatigue | −2.745 | 2.347 | 0.242 |
| Physical fatigue | −4.532 | 1.983 | 0.022 |
| Emotional fatigue | 0.257 | 1.484 | 0.863 |
| Mental fatigue | −1.051 | 1.321 | 0.426 |
| Vigor | 4.852 | 2.041 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleckner, A.S.; Culakova, E.; Kleckner, I.R.; Belcher, E.K.; Demark-Wahnefried, W.; Parker, E.A.; Padula, G.D.A.; Ontko, M.; Janelsins, M.C.; Mustian, K.M.; et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients 2022, 14, 184. https://doi.org/10.3390/nu14010184
Kleckner AS, Culakova E, Kleckner IR, Belcher EK, Demark-Wahnefried W, Parker EA, Padula GDA, Ontko M, Janelsins MC, Mustian KM, et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients. 2022; 14(1):184. https://doi.org/10.3390/nu14010184
Chicago/Turabian StyleKleckner, Amber S., Eva Culakova, Ian R. Kleckner, Elizabeth K. Belcher, Wendy Demark-Wahnefried, Elizabeth A. Parker, Gilbert D. A. Padula, Mary Ontko, Michelle C. Janelsins, Karen M. Mustian, and et al. 2022. "Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study" Nutrients 14, no. 1: 184. https://doi.org/10.3390/nu14010184
APA StyleKleckner, A. S., Culakova, E., Kleckner, I. R., Belcher, E. K., Demark-Wahnefried, W., Parker, E. A., Padula, G. D. A., Ontko, M., Janelsins, M. C., Mustian, K. M., & Peppone, L. J. (2022). Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients, 14(1), 184. https://doi.org/10.3390/nu14010184