Calcium Absorption from Food Products: Food Matrix Effects
Abstract
1. Introduction
2. The Importance of Dietary Calcium for Human Health
3. Absorption of Calcium: Physiological and Physicochemical Perspectives
3.1. Physiological Perspectives of Calcium Absorption
3.2. Physicochemical Aspects of Calcium Absorption
4. Calcium Absorption from Calcium Salts
5. Calcium Absorption from Food Products: Methodological Aspects
6. Calcium Absorption from Foods
6.1. Milk and Dairy Products
6.2. Non-Dairy Products
6.3. Calcium-Fortified Foods
7. Selection of Calcium Sources for Health and Sustainable Diets
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Word Health Organization. Human Vitamin and Mineral Requirements; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Barr, S.I. Associations of social and demographic variables with calcium intakes of high school students. J. Am. Diet. Assoc. 1994, 94, 260–266. [Google Scholar] [CrossRef]
- Albertson, A.M.; Tobelmann, R.C.; Marquart, L. Estimated dietary calcium intake and food sources for adolescent females: 1980–1992. J. Adolesc. Health 1997, 20, 20–26. [Google Scholar] [CrossRef]
- Fleming, K.H.; Heimbach, J.T. Consumption of calcium in the U.S.: Food sources and intake levels. J. Nutr. 1994, 124, 1426S–1430S. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Story, M.; Dixon, L.B.; Resnick, M.D.; Blum, R.W. Correlates of inadequate consumption of dairy products among adolescents. J. Nutr. Educ. Behav. 1997, 29, 12–20. [Google Scholar] [CrossRef]
- Subar, A.F.; Krebs-Smith, S.M.; Cook, A.; Kahle, L.L. Dietary sources of nutrients among US children, 1989–1991. Pediatrics 1998, 102, 913–992. [Google Scholar] [CrossRef]
- Johnson, R.K.; Panely, C.; Wang, M. The association between noon beverage consumption and the diet quality of school-age children. J. Child. Nutr. Manag. 1998, 22, 95–100. [Google Scholar]
- Weaver, C.M.; Heaney, R.P. (Eds.) Calcium in Human Health; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Cormick, G.; Belizan, J.M. Calcium intake and health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, Z.; Zhang, J.; Du, W.; Su, C.; Jiang, H.; Jia, X.; Ouyang, Y.; Wang, Y.; Li, L.; et al. Dietary calcium intake and food sources among Chinese adults in CNTCS. PLoS ONE 2018, 13, e0205045. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Fransen, H.; Jenab, M.; Boutron-Ruault, M.C.; Tumino, R.; Agnoli, C.; Ericson, U.; Johansson, I.; Ferrari, P.; Engeset, D.; et al. Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the european prospective investigation into cancer and nutrition study. Eur. J. Clin. Nutr. 2009, 63, S101–S121. [Google Scholar] [CrossRef] [PubMed]
- Poliquin, S.; Joseph, L.; Gray-Donald, K. Calcium and Vitamin d intakes in an adult Canadian population. Can. J. Diet. Pract. Res. 2009, 70, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Guéguen, L.; Pointillart, A. The bioavailability of dietary calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef] [PubMed]
- Rouf, A.S.; Sui, Z.; Rangan, A.; Grech, A.; Allman-Farinelli, M. Low calcium intakes among Australian adolescents and young adults are associated with higher consumption of discretionary foods and beverages. Nutrition 2018, 55–56, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Harinarayan, C.; Ramalakshmi, T. Patterns of dietary calcium intake in south Indian rural, urban and metropolitan city subjects. J. Clin. Sci. Res. 2015, 4, 143–148. [Google Scholar] [CrossRef]
- Visioli, F.; Strata, A. Milk, dairy products, and their functional effects in humans: A narrative review of recent evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef]
- Weaver, C.M.; Proulx, W.R.; Heaney, R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr. 1999, 70, 543S–548S. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Jarvis, J.K.; McBean, L.D. The importance of meeting calcium needs with foods. J. Am. Coll. Nutr. 2001, 20, 168S–185S. [Google Scholar] [CrossRef]
- Di Maio, G.; Pittia, P.; Mazzarino, L.; Maraschin, M.; Kuhnen, S. Cow milk enriched with nanoencapsulated phenolic extract of jaboticaba (Plinia peruviana). J. Food Sci. Technol. 2019, 56, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Theobald, H. Dietary Calcium and health. Nutr. Bull. 2005, 30, 237–277. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M. Le Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Camara-Martos, F.; Amaro-López, M.A. Influence of dietary factors on calcium bioavailability. Biol. Trace Elem. Res. 2002, 89, 43–52. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium intake and disease prevention. Arq. Bras. Endocrinol. Metabol. 2006, 50, 685–693. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in bone health revisited: A story beyond calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Sesso, H.D. Calcium intake and risk of cardiovascular disease. Am. J. Cardiovasc. Drugs 2012, 12, 105–116. [Google Scholar] [CrossRef]
- De Barboza, G.D.; Guizzardi, S.; de Talamoni, N.T. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W. Overview of bioactive components in milk and dairy products. In Bioactive Components in Milk and Dairy Products; Park, Y.W., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 3–12. [Google Scholar]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Francischetti, E.A.; Genelhu, V.; Sanjuliani, A.F. Effect of a high-calcium energy-reduced diet on abdominal obesity and cardiometabolic risk factors in obese Brazilian subjects. Int. J. Clin. Pract. 2010, 64, 1076–1083. [Google Scholar] [CrossRef]
- Torres, M.R.S.G.; Sanjuliani, A.F. Does calcium intake affect cardiovascular risk factors and/or events? Clinics 2012, 67, 839–844. [Google Scholar] [CrossRef]
- Vinarova, L.; Vinarov, Z.; Tcholakova, S.; Denkov, N.D.; Stoyanov, S.; Lips, A. The mechanism of lowering cholesterol absorption by calcium studied by using an in vitro digestion model. Food Funct. 2016, 7, 151–163. [Google Scholar] [CrossRef]
- Schachter, D.; Dowdle, E.B.; Schenker, H. Active transport of calcium by the small intestine of the rat. Am. J. Physiol. Content 1960, 198, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Berner, L.A. Is solubility in vitro a reliable predictor of iron bioavailability? Biol. Trace Elem. Res. 1989, 19, 11–24. [Google Scholar] [CrossRef]
- Smith, R.H.; McAllan, A.B. Binding of magnesium and calcium in the contents of the small intestine of the calf. Br. J. Nutr. 1966, 20, 703–718. [Google Scholar] [CrossRef][Green Version]
- Pérez, A.V.; Picotto, G.; Carpentieri, A.R.; Rivoira, M.A.; López, M.E.P.; De Talamoni, N.G.T. Minireview on regulation of intestinal calcium absorption. Digestion 2008, 77, 22–34. [Google Scholar] [CrossRef]
- Bronner, F. Calcium absorption—A paradigm for mineral absorption. J. Nutr. 1998, 128, 917–920. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; van der Kemp, A.W.C.M.; Hartog, A.; van de Graaf, S.F.J.; van Os, C.H.; Willems, P.H.G.M.; Bindels, R.J.M. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 1999, 274, 8375–8378. [Google Scholar] [CrossRef]
- Peng, J.-B.; Chen, X.-Z.; Berger, U.V.; Vassilev, P.M.; Tsukaguchi, H.; Brown, E.M.; Hediger, M.A. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 1999, 274, 22739–22746. [Google Scholar] [CrossRef]
- Karbach, U.; Feldmeier, H. The cecum is the site with the highest calcium absorption in rat intestine. Dig. Dis. Sci. 1993, 38, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Hoenderop, J.G.J.; van der Kemp, A.W.C.M.; Bindels, R.J.M. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 2003, 14, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. Regulation of intestinal calcium transport. Annu. Rev. Nutr. 2008, 28, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Lameris, A.L.; Nevalainen, P.I.; Reijnen, D.; Simons, E.; Eygensteyn, J.; Monnens, L.; Bindels, R.J.M.; Hoenderop, J.G.J. Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am. J. Physiol. Liver Physiol. 2015, 308, G206–G216. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Intestinal calcium absorption: Mechanisms and applications. J. Nutr. 1987, 117, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Current concepts of calcium absorption: An overview. J. Nutr. 1992, 122, 641–643. [Google Scholar] [CrossRef]
- Bellaton, C.; Roche, C.; Rémy, C.; Pansu, D. Absorption du calcium. Données physiologiques récentes. Gastroentérol. Clin. Biol. 1992, 16, 239–247. [Google Scholar]
- Beggs, M.R.; Alexander, R.T. Intestinal absorption and renal reabsorption of calcium throughout postnatal development. Exp. Biol. Med. 2017, 242, 840–849. [Google Scholar] [CrossRef]
- Bronner, F. Recent developments in intestinal calcium absorption. Nutr. Rev. 2009, 67, 109–113. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Schiller, L.R.; Fordtran, J.S. In vivo intestinal absorption of calcium in humans. Miner. Electrolyte Metab. 1990, 16, 130–146. [Google Scholar]
- Alexander, R.T.; Rievaj, J.; Dimke, H. Paracellular calcium transport across renal and intestinal epithelia. Biochem. Cell Biol. 2014, 92, 467–480. [Google Scholar] [CrossRef]
- Anderson, J.M. Molecular structure of tight junctions and their role in epithelial transport. Physiology 2001, 16, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.S.; Lengemann, F.W. Absorption of Ca 45 and Sr 85 from solid and liquid food at various levels of the alimentary tract of the rat. J. Nutr. 1962, 77, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.H. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004, 134, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Barger-Lux, M.J.; Heaney, R.P.; Recker, R.R. Time course of calcium absorption in humans: Evidence for a colonic component. Calcif. Tissue Int. 1989, 44, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F.; Pansu, D. Nutritional aspects of calcium absorption. J. Nutr. 1999, 129, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; John Wiley & Sons: Sussex, UK, 2011. [Google Scholar]
- Smeets-Peeters, M.J.E.; Minekus, M.; Havenaar, R.; Schaafsma, G.; Verstegen, M.W.A. Description of a dynamic in vitro model of the dog gastrointestinal tract and an evaluation of various transit times for protein and calcium. Altern. Lab. Anim. 1999, 27, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Vergne, M.; Parker, T.F.; Pak, C.Y.C.; Hull, A.R.; De Luca, H.F.; Fordtran, J.S. Jejunal and ileal calcium absorption in patients with chronic renal disease. J. Clin. Investig. 1976, 57, 861–866. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Duflos, C.; Bellaton, C.; Pansu, D.; Bronner, F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J. Nutr. 1995, 125, 2348–2355. [Google Scholar] [CrossRef]
- Reid, I.R.; Bristow, S.M.; Bolland, M.J. Calcium supplements: Benefits and risks. J. Intern. Med. 2015, 278, 354–368. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.C.; Barrett-Connor, E.; Black, D.; Bonjour, J.P. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteoporos. Int. 1999, 10, 259. [Google Scholar] [CrossRef]
- Li, K.; Kaaks, R.; Linseisen, J.; Rohrmann, S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg). Heart 2012, 98, 920–925. [Google Scholar]
- Onakpoya, I.J.; Perry, R.; Zhang, J.; Ernst, E. Efficacy of calcium supplementation for management of overweight and obesity: Systematic review of randomized clinical trials. Nutr. Rev. 2011, 69, 335–343. [Google Scholar] [CrossRef]
- Kressel, G. Bioavailability and solubility of different calcium-salts as a basis for calcium enrichment of beverages. Food Nutr. Sci. 2010, 1, 53–58. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Santa Ana, C.A.; Nicar, M.J.; Schiller, L.R.; Fordtran, J.S. Gastrointestinal absorption of calcium from milk and calcium salts. N. Engl. J. Med. 1987, 317, 532–536. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.L.; Lanou, A.J.; D Barnard, N.E.A.L. The consumer cost of calcium from food and supplements. J. Am. Diet. Assoc. 2002, 102, 1669–1671. [Google Scholar] [CrossRef]
- De Vrese, M. Tricalcium Citrate (TCC) and Health. J. Nutr. Health Food Eng. 2017, 6, 130–146. [Google Scholar] [CrossRef][Green Version]
- Heaney, R.P.; Recker, R.R.; Weaver, C.M. Absorbability of calcium sources: The limited role of solubility. Calcif. Tissue Int. 1990, 46, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, E.M.; McDonald, S.J.; De Wardener, H.E. The effect of a high intake of calcium carbonate in normal subjects and patients with chronic renal failure. Clin. Sci. 1966, 30, 425–438. [Google Scholar] [PubMed]
- Recker, R.R. Calcium absorption and achlorhydria. N. Engl. J. Med. 1985, 313, 70–73. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Stegman, M.R.; Moy, A.J. Calcium absorption in women: Relationships to calcium intake, estrogen status, and age. J. Bone Miner. Res. 1989, 4, 469–475. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.C.; Hsu, C.W.; Chang, W.H. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology 2009, 20, 375102. [Google Scholar] [CrossRef]
- Elble, A.E.; Hill, K.M.; Park, C.Y.; Martin, B.R.; Peacock, M.; Weaver, C.M. Effect of calcium carbonate particle size on calcium absorption and retention in adolescent girls. J. Am. Coll. Nutr. 2011, 30, 171–177. [Google Scholar] [CrossRef]
- Davidsson, L.; Tanumihardjo, S. New frontiers in science and technology: Nuclear techniques in nutrition. Am. J. Clin. Nutr. 2011, 94, 691S–695S. [Google Scholar] [CrossRef]
- Weaver, C.M.; Heaney, R.P.; Connor, L.; Martin, B.R.; Smith, D.L.; Nielsen, S. Bioavailability of calcium from tofu as compared with milk in premenopausal women. J. Food Sci. 2002, 67, 3144–3147. [Google Scholar] [CrossRef]
- Charoenkiatkul, S.; Kriengsinyos, W.; Tuntipopipat, S.; Suthutvoravut, U.; Weaver, C.M. Calcium absorption from commonly consumed vegetables in healthy Thai women. J. Food Sci. 2008, 73, H218–H221. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.B.; Bügel, S.; Stürup, S.; Jensen, M.; Mølgaard, C.; Hansen, M.; Krogsgaard, O.W.; Sandström, B. A novel dual radio-and stable-isotope method for measuring calcium absorption in humans: Comparison with the whole-body radioisotope retention method. Am. J. Clin. Nutr. 2003, 77, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Fairweather-Tait, S.; Hurrell, R.; Van Dokkum, W. Methods for studying mineral and trace element absorption in humans using stable isotopes. Nutr. Res. Rev. 1993, 6, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Chaney, R.L. Intrinsic mineral labeling of edible plants: Methods and uses. Crit. Rev. Food Sci. Nutr. 1985, 23, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.P.; Martin, B.R.; Smith, D.L.; Smith, J.B.; Miller, G.D.; Weaver, C.M. Calcium bioavailability from bovine milk and dairy products in premenopausal women using intrinsic and extrinsic labeling techniques. J. Nutr. 1996, 126, 1406–1411. [Google Scholar] [CrossRef]
- Weaver, C.M.; Heaney, R.P.; Martin, B.R.; Fitzsimmons, M.L. Human calcium absorption from whole-wheat products. J. Nutr. 1991, 121, 1769–1775. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Rafferty, K.; Bierman, J. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am. J. Clin. Nutr. 2000, 71, 1166–1169. [Google Scholar] [CrossRef]
- Zhao, Y.; Martin, B.R.; Weaver, C.M. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow’s milk in young women. J. Nutr. 2005, 135, 2379–2382. [Google Scholar] [CrossRef]
- Weaver, C.M.; Heaney, R.P. Isotopic exchange of ingested calcium between labeled sources. Evidence that ingested calcium does not form a common absorptive pool. Calcif. Tissue Int. 1991, 49, 244–247. [Google Scholar] [CrossRef]
- Neville, M.C. Calcium secretion into milk. J. Mammary Gland Biol. Neoplasia 2005, 10, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lenton, S.; Nylander, T.; Teixeira, S.C.M.; Holt, C. A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci. Technol. 2015, 95, 3–14. [Google Scholar] [CrossRef]
- Huppertz, T.; Heck, J.; Bijl, E.; Poulsen, N.A.; Larsen, L.B. Variation in casein distribution and mineralisation in the milk from Holstein-Friesian cows. Int. Dairy J. 2021, 119, 105064. [Google Scholar] [CrossRef]
- Akkerman, M.; Larsen, L.B.; Sørensen, J.; Poulsen, N.A. Natural variations of citrate and calcium in milk and their effects on milk processing properties. J. Dairy Sci. 2019, 102, 6830–6841. [Google Scholar] [CrossRef]
- Faulkner, A.; Peaker, M. Reviews of the progress of dairy science: Secretion of citrate into milk. J. Dairy Res. 1982, 49, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Bijl, E.; van Valenberg, H.J.F.; Huppertz, T.; van Hooijdonk, A.C.M. Protein, casein, and micellar salts in milk: Current content and historical perspectives. J. Dairy Sci. 2013, 96, 5455–5464. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein micelle structure, functions and interactions. In Advanced Dairy Chemistry—1 Proteins; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 233–276. [Google Scholar]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Law, A.J.R. pH-Induced dissociation of bovine casein micelles II. Mineral solubilization and its relation to casein release. J. Dairy Res. 1989, 56, 727–735. [Google Scholar] [CrossRef]
- Le Graët, Y.; Gaucheron, F. pH-induced solubilization of minerals from casein micelles: Influence of casein concentration and ionic strength. J. Dairy Res. 1999, 66, 215–224. [Google Scholar] [CrossRef]
- Huppertz, T.; Chia, L.W. Milk protein coagulation under gastric conditions: A review. Int. Dairy J. 2021, 113, 104882. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
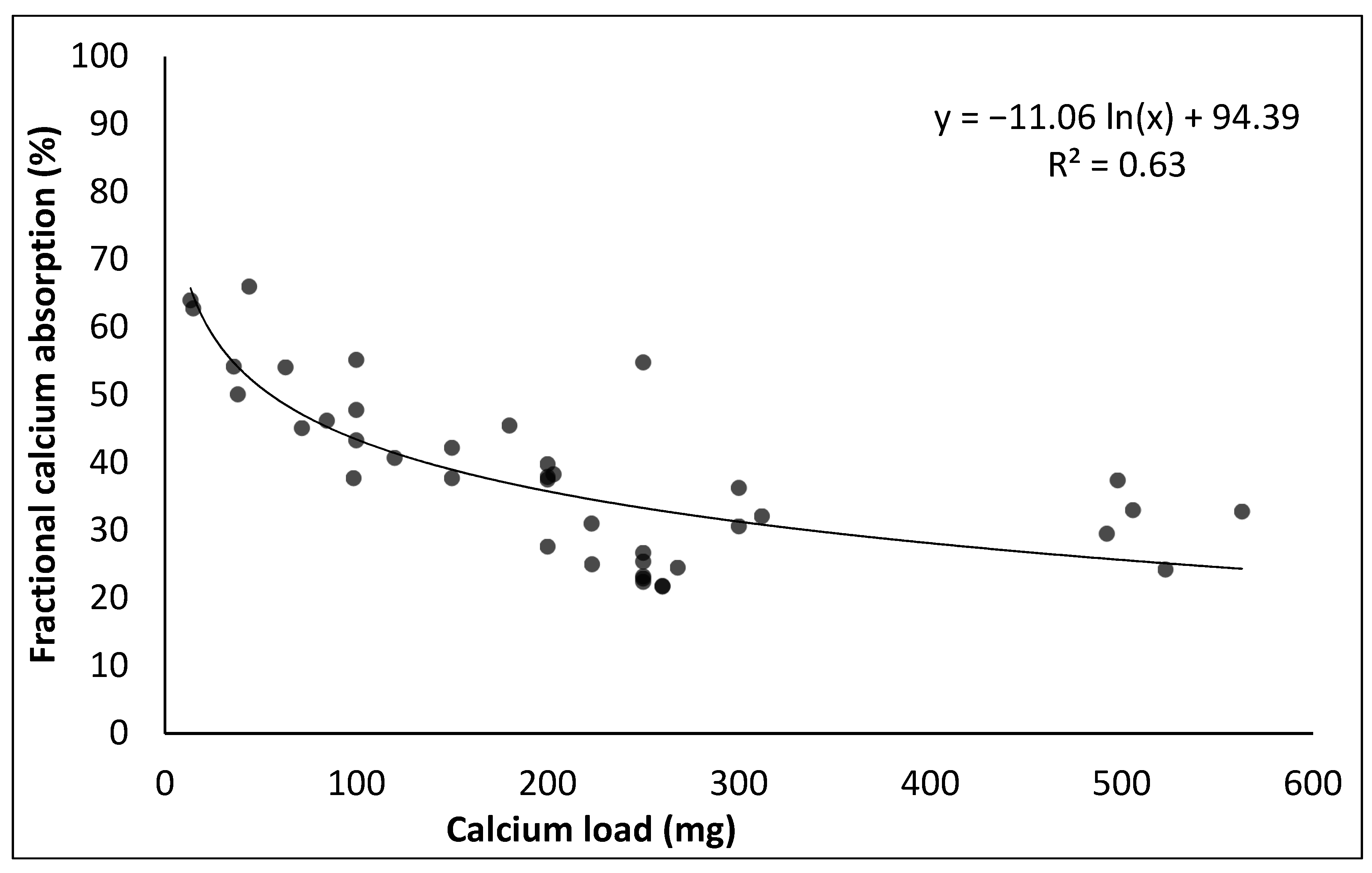
- Heaney, R.P.; Weaver, C.M.; Lee Fitzsimmons, M. Influence of calcium load on absorption fraction. J. Bone Miner. Res. 1990, 5, 1135–1138. [Google Scholar] [CrossRef]
- Weaver, C.M.; Heaney, R.P.; Proulx, W.R.; Hinders, S.; Packard, P.T. Absorbability of calcium from common beans. J. Food Sci. 1993, 58, 1401–1403. [Google Scholar] [CrossRef]
- Weaver, C.M.; Heaney, R.P.; Nickel, K.P.; Packard, P.I. Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes and rhubarb. J. Food Sci. 1997, 62, 524–525. [Google Scholar] [CrossRef]
- Heaney, R.P.; Weaver, C.M.; Fitzsimmons, M.L. Soybean phytate content: Effect on calcium absorption. Am. J. Clin. Nutr. 1991, 53, 745–747. [Google Scholar] [CrossRef]
- Heaney, R.P.; Weaver, C.M. Calcium absorption from kale. Am. J. Clin. Nutr. 1990, 51, 656–657. [Google Scholar] [CrossRef]
- Heaney, R.P.; Weaver, C.M.; Recker, R.R. Calcium absorbability from spinach. Am. J. Clin. Nutr. 1988, 47, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, K.M.; Morris, J.; Hotze, T.; Hirschi, K.D.; Abrams, S.A. Biotechnologically-modified carrots: Calcium absorption relative to milk. J. Bioequivalence Bioavailab. 2009, 1, 34–38. [Google Scholar]
- Heaney, R.P.; Dowell, M.S. Absorbability of the calcium in a high-calcium mineral water. Osteoporos. Int. 1994, 4, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Couzy, F.; Kastenmayer, P.; Vigo, M.; Clough, J.; Munoz-Box, R.; Barclay, D. V Calcium bioavailability from a calcium-and sulfate-rich mineral water, compared with milk, in young adult women. Am. J. Clin. Nutr. 1995, 62, 1239–1244. [Google Scholar] [CrossRef]
- Martin, B.R.; Weaver, C.M.; Heaney, R.P.; Packard, P.T.; Smith, D.L. Calcium absorption from three salts and CaSO4-fortified bread in premenopausal women. J. Agric. Food Chem. 2002, 50, 3874–3876. [Google Scholar] [CrossRef]
- Hansen, M.; Thilsted, S.H.; Sandström, B.; Kongsbak, K.; Larsen, T.; Jensen, M.; Sørensen, S.S. Calcium absorption from small soft-boned fish. J. Trace Elem. Med. Biol. 1998, 12, 148–154. [Google Scholar] [CrossRef]
- Sirichakwal, P.P.; Kamchansuppasin, A.; Akoh, C.C.; Kriengsinyos, W.; Charoenkiatkul, S.; O’Brien, K.O. Vitamin D status is positively associated with calcium absorption among postmenopausal Thai women with low calcium intakes. J. Nutr. 2015, 145, 990–995. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Johnson, A.; Eagles, J.; Ganatra, S.; Kennedy, H.; Gurr, M.I. Studies on calcium absorption from milk using a double-label stable isotope technique. Br. J. Nutr. 1989, 62, 379–388. [Google Scholar] [CrossRef]
- Tang, A.L.; Walker, K.Z.; Wilcox, G.; Strauss, B.J.; John, F. Ashton, J.F.; Stojanovska, L. Calcium absorption in Australian osteopenic postmenopausal women: An acute comparative study of fortified soymilk to cows’ milk. Asia Pac. J. Clin. Nutr. 2010, 19, 243–249. [Google Scholar]
- Recker, R.R.; Bammi, A.; Barger-Lux, M.J.; Heaney, R.P. Calcium absorbability from milk products, an imitation milk, and calcium carbonate. Am. J. Clin. Nutr. 1988, 47, 93–95. [Google Scholar] [CrossRef]
- Van Dokkum, W.; De La Gueronniere, V.; Schaafsma, G.; Bouley, C.; Luten, J.; Latge, C. Bioavailability of calcium of fresh cheeses, enteral food and mineral water. A study with stable calcium isotopes in young adult women. Br. J. Nutr. 1996, 75, 893–903. [Google Scholar] [CrossRef]
- van der Hee, R.M.; Miret, S.; Slettenaar, M.; Duchateau, G.S.M.J.E.; Rietveld, A.G.; Wilkinson, J.E.; Quail, P.J.; Berry, M.J.; Dainty, J.R.; Teucher, B.; et al. Calcium absorption from fortified ice cream formulations compared with calcium absorption from milk. J. Am. Diet. Assoc. 2009, 109, 830–835. [Google Scholar] [CrossRef]
- López-Huertas, E.; Teucher, B.; Boza, J.J.; Martínez-Férez, A.; Majsak-Newman, G.; Baró, L.; Carrero, J.J.; González-Santiago, M.; Fonollá, J.; Fairweather-Tait, S. Absorption of calcium from milks enriched with fructooligosaccharides, caseinophosphopeptides, tricalcium phosphate, and milk solids. Am. J. Clin. Nutr. 2006, 83, 310–316. [Google Scholar] [CrossRef]
- Gao, K.P.; Mitsui, T.; Fujiki, K.; Ishiguro, H.; Kondo, T. Effect of lactase preparations in asymptomatic individuals with lactase deficiency-gastric digestion of lactose and breath hydrogen analysis. Nagoya J. Med. Sci. 2002, 65, 21–28. [Google Scholar] [PubMed]
- Huppertz, T.; Lambers, T.T. Influence of micellar calcium phosphate on in vitro gastric coagulation and digestion of milk proteins in infant formula model systems. Int. Dairy J. 2020, 107, 104717. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Mackie, A.R.; Brodkorb, A.; Wilde, P.J. Dairy structures and physiological responses: A matter of gastric digestion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3737–3752. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, T.; van Dijk, H.J.M.; Zoon, P.; Walstra, P. Relation between syneresis and rheological properties of particle gels. Colloid Polym. Sci. 1991, 269, 620–627. [Google Scholar] [CrossRef]
- Walstra, P. The syneresis of curd. In Cheese: Chemistry, Physics and Microbiology; Springer: Boston, MA, USA, 1993. [Google Scholar]
- Hindmarsh, J.P.; Smith, J.R.; Carr, A.J.; Watkinson, P.J. Expanding solid-state phosphorus nuclear magnetic resonance insights into Mozzarella cheese ageing. J. Food Eng. 2019, 242, 31–46. [Google Scholar] [CrossRef]
- Floury, J.; Jeanson, S.; Aly, S.; Lortal, S. Determination of the diffusion coefficients of small solutes in cheese: A review. Dairy Sci. Technol. 2010, 90, 477–508. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Liu, W.; Jin, Y.; Wilde, P.J.; Hou, Y.; Wang, Y.; Han, J. Mechanisms, physiology, and recent research progress of gastric emptying. Crit. Rev. Food Sci. Nutr. 2021, 61, 2742–2755. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef]
- Buchheim, W. Influences of Different Technological Treatments of Milk on the Digestion in the Stomach. IV. Electron Microscopical Characterization of the Coagulum and of Lipolytic Processes in the Stomach (German) . Milchwissenschaft 1984, 39, 271–275. [Google Scholar]
- Pfeil, R. Influence of Different Technological Treatments of Milk on the Digestion in the Stomach. III. Proteolysis in the Stomach (German). Milchwissenschaft 1984, 39, 267–270. [Google Scholar]
- Horstman, A.M.H.; Ganzevles, R.A.; Kudla, U.; Kardinaal, A.F.M.; van den Borne, J.J.G.C.; Huppertz, T. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. Int. Dairy J. 2021, 113, 104890. [Google Scholar] [CrossRef]
- De La Fuente, M.A.; Montes, F.; Guerrero, G.; Juárez, M. Total and soluble contents of calcium, magnesium, phosphorus and zinc in yoghurts. Food Chem. 2003, 80, 573–578. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in plant-based foods: A review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Heaney, R.P.; Weaver, C.M.; Hinders, S.M.; Martin, B.; Packard, P.T. Absorbability of calcium from brassica vegetables: Broccoli, bok choy, and kale. J. Food Sci. 1993, 58, 1378–1380. [Google Scholar] [CrossRef]
- Hansen, M.; Sandström, B.; Jensen, M.; Sørensen, S.S. Casein phosphopeptides improve zinc and calcium absorption from rice-based but not from whole-grain infant cereal. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 56–62. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.L.; Sian, L.; Miller, L.V.; Peterson, K.L.; Raboy, V. Absorption of calcium from tortilla meals prepared from low-phytate maize. Am. J. Clin. Nutr. 2005, 82, 84–87. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Ebner, J.S.; Krueger, C.A. Oxalic acid decreases calcium absorption in rats. J. Nutr. 1987, 117, 1903–1906. [Google Scholar] [CrossRef]
- Hodgman, C. Handbook of Chemistry and Physics; CRC Press, Inc.: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hanes, D.A.; Weaver, C.M.; Heaney, R.P.; Wastney, M. Absorption of calcium oxalate does not require dissociation in rats. J. Nutr. 1999, 129, 170–173. [Google Scholar] [CrossRef]
- Hughes, J.; Norman, R.W. Diet and calcium stones. Can. Med. Assoc. J. 1992, 146, 137. [Google Scholar]
- Prenen, J.A.; Boer, P.; Dorhout Mees, E.J. Absorption kinetics of oxalate from oxalate-rich food in man. Am. J. Clin. Nutr. 1984, 40, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.; Wang, H.I.; Wu, W.J.; Yang, R.Y. Anti-nutrient components and their concentrations in edible parts of vegetable families. CAB Rev. 2018, 13, 1–30. [Google Scholar] [CrossRef]
- Noonan, S.C.; Savage, G.P. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [PubMed]
- Chai, W.; Liebman, M. Effect of different cooking methods on vegetable oxalate content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Plawecki, K.L. Dietary calcium: Adequacy of a vegetarian diet. Am. J. Clin. Nutr. 1994, 59, 1238S–1241S. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.C.; Weaver, C.M.; Kimmel, S.; Brown, R.K. Effect of soybean phytate content on calcium bioavailability in mature and immature rats. J. Agric. Food Chem. 1993, 41, 246–249. [Google Scholar] [CrossRef]
- Iqbal, T.H.; Lewis, K.O.; Cooper, B.T. Phytase activity in the human and rat small intestine. Gut 1994, 35, 1233–1236. [Google Scholar] [CrossRef]
- Grynspan, F.; Cheryan, M. Calcium phytate: Effect of pH and molar ratio on in vitro solubility. J. Am. Oil Chem. Soc. 1983, 60, 1761–1764. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Walker, A.R.P.; Fox, F.W.; Irving, J.T. Studies in human mineral metabolism: 1. The effect of bread rich in phytate phosphorus on the metabolism of certain mineral salts with special reference to calcium. Biochem. J. 1948, 42, 452. [Google Scholar] [CrossRef] [PubMed]
- Bellostas, N.; Kachlicki, P.; Sørensen, J.C.; Sørensen, H. Glucosinolate profiling of seeds and sprouts of B. oleracea varieties used for food. Sci. Hortic. 2007, 114, 234–242. [Google Scholar] [CrossRef]
- Pak, C.Y.; Stewart, A.; Haynes, S.D. Effect of added citrate or malate on calcium absorption from calcium-fortified orange juice. J. Am. Coll. Nutr. 1994, 13, 575–577. [Google Scholar] [CrossRef]
- Jones, A.D.; Hoey, L.; Blesh, J.; Miller, L.; Green, A.; Shapiro, L.F. A systematic review of the measurement of sustainable diets. Adv. Nutr. 2016, 7, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Fanzo, J.C.; Cogill, B. Understanding sustainable diets: A descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv. Nutr. 2014, 5, 418–429. [Google Scholar] [CrossRef]
- RIVM. Database-Milieubelasting-Voedingsmiddelen. Available online: https://www.rivm.nl/voedsel-en-voeding/duurzaam-voedsel/database-milieubelasting-voedingsmiddelen (accessed on 4 October 2021).
- RIVM. Dutch Food Composition Database (NEVO). Available online: https://nevo-online.rivm.nl (accessed on 4 October 2021).
- van Rossum, C.T.M.; Buurma-Rethans, E.J.M.; Dinnissen, C.S.; Beukers, M.H.; Brants, H.A.M.; Dekkers, A.L.M.; Ocké, M.C. The Diet of the Dutch: Results of the Dutch National Food Consumption Survey 2012–2016; National Institute for Public Health and the Environment RIVM: Bilthoven, The Netherlands, 2020. [Google Scholar]
| Country | Calcium Intake (mg/day) | Ages (Years) | Main Food Sources of Calcium | References |
|---|---|---|---|---|
| Greece | 1039 | 35–74 | Dairy products, cereals and products, vegetables | [11] |
| The Netherlands | 1033 | 35–74 | Dairy products, cereals and products, beverages (non-alcoholic) | [11] |
| Denmark | 1011 | 35–74 | Dairy products, cereals and products, beverages (non-alcoholic) | [11] |
| Canada | 973 | ≥25 | Milk and dairy products | [12] |
| Spain | 972 | 35–74 | Dairy products, cereals and products, beverages (non-alcoholic) | [11] |
| United Kingdom | 969 | 35–74 | Dairy products, cereals and products, cakes | [11] |
| Sweden | 955 | 35–74 | Dairy products, cereals and products, beverages (non-alcoholic) | [11] |
| Germany | 942 | 35–74 | Dairy products, cereals and products, beverages (non-alcoholic) | [11] |
| USA | 934 | ≥19 | Dairy products, vegetables, fruits | [13,14] |
| France | 918 | 35–74 | Dairy products, beverages (non-alcoholic), vegetables | [11] |
| Italy | 808 | 35–74 | Dairy products, cereals and products, vegetables | [11] |
| Australia | 672 | 14–25 | Regular milk, cheese, bread, low-fat milk | [15] |
| India (metropolitan) | 526 | 43 | Cereals and products, dairy products, vegetables | [16] |
| China | 369 | 18–64 | Vegetables, legumes, cereals and products | [10] |
| India (urban) | 308 | 47 | Cereals and products, dairy products, vegetables | [16] |
| India (rural) | 269 | 40 | Cereals and products, vegetables and dairy products | [16] |
| Humans 1 | Rats 2 | |
|---|---|---|
| Transit Time (min) | Transit Time (min) | |
| Duodenum | 15.6 | 3 |
| Jejunum | 102 | 43 |
| Ileum | 78 | 141 |
| Total | 195.6 | 187 |
| Calcium Salts | Calcium Content (%) | pH | Solubility (g/L) | References |
|---|---|---|---|---|
| Calcium lactate | 13 | not reported (water) | 66 | [72] |
| Calcium gluconate | 9.0 | not reported (water 21 °C) | 30 | [67] |
| Calcium carbonate | 40.0 | 4.5 | 46 | [67,68] |
| 6.0 | 3.8 | [68] | ||
| 7.5 | 0.13 | [68] | ||
| 8.5 (water) | <0.005 a | [68] | ||
| not reported (water 21 °C) | 0.014 | [67] | ||
| Calcium citrate | 21 | 2.0 | 17 | [68,72] |
| 4.5 | 0.33 | [68] | ||
| 5.6 (water) | 0.23 | [68] | ||
| 6.0 | 0.20 | [68] | ||
| 7.5 | 0.23 | [68] | ||
| Calcium phosphate | 17–36 | 2.0 | 21 | [68,72] |
| 4.5 | 0.63 | [68] | ||
| 6.0 | 0.079 | [68] | ||
| 6.3 (water) | 0.037 | [68] | ||
| 7.5 | 0.019 | [68] | ||
| not reported (water) | slightly soluble | [72] | ||
| Calcium lactate citrate | 16.2 | not reported (water 21 °C) | 98 | [67] |
| Calcium lactate malate | 18.1 | not reported (water 21 °C) | 115 | [67] |
| Food | Serving Size (g or mL) | Calcium Load (mg) | Fractional Calcium Absorption (%) a | Meal Composition | Isotopic Method b | References |
|---|---|---|---|---|---|---|
| Milk | DNS | 71.6 | 45.1 (1.8) | Milk was ingested with one or two slices of low-calcium Italian bread toasted and served with butter, as well as coffee or tea, with artificial sweetener if desired | DNS | [104] |
| Milk | DNS | 200 | 37.9 (2.6) | Milk was ingested as a part of a neutral breakfast consisting of two pieces of white bread toast with butter or margarine and coffee after an overnight fast | R/E | [105] |
| Milk | 63 | 54.1 (4.5) | ||||
| Milk | 120 | 40.7 (2.1) | ||||
| Milk 2% fat | DNS | 98.5 | 37.7 (1.4) | Milk was ingested in the middle of a neutral meal consisting of two pieces of white bread toast with butter or margarine as well as coffee or tea | R/E | [106] |
| Milk | DNS | 312 | 32.1 (2.7) | Milk was ingested in the middle of a neutral meal consisting of two pieces of white-bread toast with butter or margarine and coffee, tea or diet cola (without sugar) | R/E | [107] |
| Milk | DNS | 200 | 27.6 | Milk was ingested as a part of a standard breakfast consisting of two pieces of toasted white bread with butter or margarine and a cup of coffee or tea (with artificial sweetener) | DR/E | [108] |
| Milk low fat | 30 mL | 38 | 50.1 (3) | Milk was ingested with 170 g calcium fortified orange juice in order to provide approximately 300 mg calcium | SSI/E | [109] |
| Milk | 90.3 g | 100 | 43.3 | Water was consumed midway through a light breakfast consisting of low-calcium white bread, toasted, with butter and tea or coffee (with artificial sweetener, if desired) | R/E | [110] |
| Milk (2.8 g fat/L) | 200 mL | 223.2 | 25.0 (2.2) | 200 mL of milk was ingested with 300 mL distilled water after an overnight fast | DSI/E | [111] |
| Milk | A cup of milk | 300 | 36.3 (2.2) | Milk was ingested as a part of a standard test breakfast consisting of two pieces of toasted white bread with butter or margarine and a cup of coffee or tea (with artificial sweetener, if desired). One of the two pieces of bread was calcium fortified | R/E | [112] |
| Milk 2% fat | 240 mL | 260 | 21.7 (0.9) | Milk was ingested with a slice of calcium-free toast | DSI/E | [88] |
| Milk | 6.9 g | 13.3 | 64 (2.9) | Milk was ingested without meal | R/E | [86] |
| Milk | 140.1 g | 200 | 37.5 (1.7) | |||
| Skimmed milk | 208 g | 260 | 21.8 (1.4) | 208 g of milk was ingested together with 120 g white wheat bread, 27 g unsalted butter and 100 g ultrapure water | R/E | [113] |
| Milk 2% fat | 67.3 g | 250 | 54.8 (4) | Milk was ingested with breakfast (not specified) | SSI/E | [80] |
| Skim milk | DNS | 200 | 39.8 (3) | Milk was ingested with two slices of low-calcium Italian bread toasted and served with butter as well as coffee or tea (with artificial sweetener, if desired) | R/E | [80] |
| Milk | 92.4 g | 100 | 55.2 (2.7) | Milk was served as part of breakfast after an overnight fast with 100 g cooked rice | SSI/E | [81] |
| Whole milk | DNS | 100 | 47.8 (2.9) | Milk was ingested with 90 g of cooked white rice | TSI/E | [114] |
| Skimmed milk | 136 mL | 180 | 45.5 (1.9) | Milk was ingested without meal | DSI/E | [115] |
| Milk 2% fat | DNS | 300 | 30.6 (1.5) | Milk was ingested as part of a breakfast after an overnight fast with three pieces of low-calcium Italian-style white bread toasted with butter a cup of coffee (with artificial sweetener if desired) | R/E | [87] |
| Milk | 20 mL | 44 | 66 (0.1) | Milk was ingested without meal, but the ingestion was followed immediately by 200 mL of distilled water | R/E | [116] |
| Milk 2% fat | 240 mL | 14.7 | 62.8 | Milk was taken in the middle of a light breakfast consisting of two pieces of toasted Italian bread with butter, together with coffee (decaffeinated or regular) or tea | DSI/E | [103] |
| Milk 2% fat | 240 mL | 35.9 | 54.2 | |||
| Milk 2% fat | 240 mL | 84.6 | 46.2 | |||
| Milk 2% fat | 240 mL | 203.1 | 38.3 | |||
| Milk 2% fat | 240 mL | 492.4 | 29.5 | |||
| Milk | 488 g | 563 | 32.8 (4.0) | Test foods were ingested with breakfast (not specified) after an overnight fast | SSI/I | [85] |
| Cheddar cheese | 108 g | 498 | 37.4 (9.2) | |||
| Processed cheese | 145 g | 506 | 33.0 (4.3) | |||
| Yoghurt | 481 g | 523 | 24.2 (3.4) | |||
| Whole milk | DNS | 250 | 26.7 (2.5) | DNS | DR/E | [117] |
| Chocolate milk | 250 | 23.2 (1.8) | ||||
| Yoghurt | 250 | 25.4 (2.9) | ||||
| Imitation milk | 250 | 22.4 (1.8) | ||||
| Cheese | 250 | 22.9 (1.7) | ||||
| Fresh cheese | 208 g | 150 | 42.2 (3.3) | Test meals were ingested without meal | DSI/E | [118] |
| New fresh cheese | 94 g | 150 | 37.7 (2.9) | |||
| Milk 1.7% fat | 200 g | 223 | 31 (2.3) | Milk was ingested within five minutes after the standardized breakfast which consisted of two slices of white bread with margarine and strawberry jam and 125 mL mineral water | DSI/E | [119] |
| Milk 1.9% fat | 165–224 mL | 268 | 24.5 (1.9) | Test foods were ingested in the middle of a light breakfast consisting of 2 slices of toasted white bread with butter and jam | DSI/E | [120] |
| Foods | Serving Size (g) | Calcium Load (mg) | Fractional Calcium Absorption (%) a | Meal Composition | Isotopic Method b | References |
|---|---|---|---|---|---|---|
| Broccoli | DNS | 82.4 | 47.8 (3.0) | Cooked and pureed vegetables were fed as a part of breakfast consisting of two slices of low-calcium Italian bread, toasted and served with butter as well as coffee or tea, with artificial sweetener, if desired | R/I | [136] |
| Bok choy stems | 83.0 | 51.9 (3.6) | ||||
| Bok choy leaves | 83.0 | 52.0 (1.9) | ||||
| Kale | 83.0 | 52.7 (2.4) | ||||
| White beans | DNS | 71.6 | 22.5 (1.5) | Cooked and pureed beans were ingested with one or two slices of low-calcium Italian bread, toasted and served with butter as well as coffee or tea, with artificial sweetener, if desired | R/I | [104] |
| Red beans | 71.6 | 19.3 (1.3) | ||||
| Pinto beans untreated | 71.6 | 23.1 (1.4) | ||||
| Pinto beans phytase treated | 71.6 | 31.8 (2.2) | ||||
| Kai Choy (Chinese mustard greens) | 144 | 200 | 39.9 (2.1) | Cooked and pureed vegetables were fed as a part of a neutral breakfast consisting of two pieces of white bread toast with butter or margarine and coffee after an overnight fast | R/I | [105] |
| Choy Sum (Chinese cabbage flower leaves) | 127 | 200 | 40.2 (1.7) | |||
| Chinese spinach | 119 | 200 | 9.3 (0.7) | |||
| Sweet potatoes | 115 | 63 | 22.8 (2.1) | |||
| Rhubarb | 58 | 120 | 9.2 (0.8) | |||
| Soybean high phytate Soybean low phytate | 88 88 |
97.8
99.2 | 31.0 (1.8) 41.4 (1.9) | Cooked soybeans were ingested as breakfast after an overnight fast and accompanied by coffee or tea (with artificial sweetener if desired) | R/I | [106] |
| Kale | 150 | 288 | 40.9 (3) | Cooked and pureed kale were ingested in the middle of a neutral meal consisting of two pieces of white-bread toast with butter or margarine and coffee, tea or diet cola (without sugar) | R/I | [107] |
| Spinach | DNS | 200 | 5.1 | Cooked and pureed spinach were administered as a part of a standard breakfast consisting of two pieces of white bread toast with butter or margarine and coffee or tea (with artificial sweetener if desired) after an overnight fast | DR/I | [108] |
| Wheat bread | 78.3 | 13.3 | 81.7 (3.7) | Bread was served with butter Wheat bran cereal was served with milk | R/I | [86] |
| Wheat Bran cereal | 44.5 | 200 | 22.3 (1.1) | |||
| Wheat bread (crust removed) | 61.0 | 10.4 | 70.3 (2.8) | |||
| Light cookies | 111.0 | 18.8 | 64.8 (2.2) | |||
| Dark cookies | 101.4 | 20.4 | 65.4 (2.7) | |||
| Winged beans | 400 | 110 | 39.1 (2.9) | The test foods were served as part of breakfast with 100 g cooked rice after an overnight fast | SSI/E | [81] |
| Ivy gourd | 150 | 114 | 47.6 (2.5) | |||
| Cassia | 142 | 100 | 42.6 (2.8) | Cooked cassia was ingested with 90 g of cooked white rice | TSI/E | [114] |
| Rice based cereal | DNS | 481 | 16.0 (1.2) | Test foods were ingested with together with 250 mL water | R/E | [137] |
| Whole grain cereal | 541 | 17.0 (1.0) | ||||
| Tortillas, maize with typical-phytate content | 140 | 140 | 35 (3.1) | Tortilla meals were ingested without meal | DSI/E | [138] |
| Tortillas, maize with low-phytate content | 140 | 140 | 50 (1.3) | |||
| Sangemini | 294.4 | 100 | 47.5 | Water was consumed midway through a light breakfast consisting of low-calcium white bread, toasted, with butter, and tea or coffee (with artificial sweetener, if desired) | R/E | [110] |
| Ferrarele | 340 | 150 | 37.0 (2.8) | Water was ingested without meal | DSI/E | [118] |
| Freeze-dried small Bengali fish | 6.28 | 280 | 23.8 (1.3) | 6.28 g of fish was ingested together with 120 g white wheat bread, 27 g unsalted butter and 308 g ultrapure water | R/E | [113] |
| pH | Calcium Oxalate Solubility (g/L) | References |
|---|---|---|
| 2.0 | 0.011 | [68] |
| 4.5 | <0.005 a | [68] |
| 6.0 | <0.005 a | [68] |
| 7.5 | <0.005 a | [68] |
| 8.5 (water) | <0.005 a | [68] |
| 8.5 (water) | 0.0061 | [140] |
| Total Oxalate (mg/100 g of Wet Weight) a | ||||
|---|---|---|---|---|
| Raw | Steamed | Boiled | Cooking Time (min) | |
| Rhubarb stalks | 532 ± 8 | 505 ± 2 | 309 ± 7 | 15 |
| Spinach | 1145 ± 33 | 797 ± 12 | 460 ± 9 | 12 |
| Foods | Serving Size (g or mL) | Calcium Load (mg) | Fractional Calcium Absorption (%) a | Meal Composition | Isotopic Method b | References |
|---|---|---|---|---|---|---|
| Orange juice with calcium citrate | 180 mL | 300 | 40.1 (2.1) | Orange juices were ingested without meal | R/E | [154] |
| Orange juice with calcium malate | 180 mL | 300 | 40.6 (2.2) | |||
| Milk 2% fat | 240 mL | 260 | 21.7 (0.9) | Foods were ingested with a slice of calcium-free toast | DSI/E | [88] |
| Soy drink with calcium carbonate | 240 mL | 260 | 21.1 (1.3) | |||
| Soy drink with tricalcium phosphate | 240 mL | 260 | 18.1 (0.9) | |||
| Milk | A cup of milk | 300 | 36.27 (2.2) | Foods were ingested as a part of a standard breakfast consisting of two pieces of toasted white bread with butter or margarine and a cup of coffee or tea (with artificial sweetener, if desired). One of the two pieces of bread was calcium fortified | R/E | [112] |
| Bread with calcium sulphate | 16.8 g | 300 | 43.02 (2.5) | |||
| Medium fat milk (2.8 g fat/L) | 200 mL | 223.2 | 25.0 (2.2) | Milk was ingested with 300 mL distilled water after an overnight fast | DSI/E | [111] |
| Mineral water with calcium sulfate | 500 mL | 224 | 23.8 (1.6) | Water was ingested without meal | ||
| Milk 2% fat | 67.3 g | 250 | 54.8 (4) | Test foods were ingested with breakfast (not specified) | SSI/E | [80] |
| Tofu with calcium chloride | DNS | 250 | 49.3 (5.6) | SSI/E | ||
| Skim milk | DNS | 200 | 39.8 (3) | Test meals were ingested at breakfast consisting in two slices of low-calcium Italian bread, toasted and served with butter as well as coffee or tea (with artificial sweetener, if desired) | R/E | [80] |
| Tofu with calcium sulfate | DNS | 200 | 39.0 (3.5) | R/E | ||
| Milk 2% fat | DNS | 300 | 30.6 (1.5) | Foods were ingested as part of a breakfast after an overnight fast with three pieces of low-calcium Italian-style white bread toasted with butter a cup of coffee (with artificial sweetener if desired) | R/E | [87] |
| Soy drink with tricalcium phosphate 2% fat | DNS | 300 | 35.8 (1.67) | R/E | ||
| Milk low fat | 30 mL | 38 | 50.1 (3) | Milk and carrots were ingested with 170 g calcium fortified orange juice in order to provide approximately 300 mg calcium | SSI/E | [109] |
| Ca-enriched carrots | 65 g | 40 | 42.6 (2.8) | SSI/I | ||
| Milk 1.9% fat | 165–224 mL | 268 | 24.5 (1.9) | Test foods were ingested in the middle of a light breakfast consisting of 2 slices of toasted white bread with butter and jam | DSI/E | [120] |
| Milk with tricalcium phosphate | 165–224 mL | 268 | 27.5 (2.0) | |||
| Skimmed milk | 136 mL | 180 | 45.5 (1.9) | Ingested without meal | DSI/E | [115] |
| Skimmed milk with calcium gluconate | 83 mL | 184 | 35.7 (4.7) | |||
| Milk 1.7% fat | 200 g | 223 | 31 (2.3) | Test foods were ingested within five minutes after the standardized breakfast which consisted of two slices of white bread with margarine and strawberry jam and 125 mL of mineral water | DSI/E | [119] |
| Ice cream 3% butterfat | 60 g | 227 | 26 (2.0) | |||
| Ice cream 9% coconut oil | 60 g | 224 | 28 (1.3) | |||
| Milk | 20 mL | 44 | 66 (0.1) | Test foods were ingested without meal, but the ingestion was followed | R/E | [116] |
| Soy drink calcium-fortified | 20 mL | 44 | 65 (0.1) | immediately by 200 mL of distilled water |
| Food Item | Food Category | Ca (mg/100 g) a | GHG (kg CO2-eq/100 g) b | Ca (mg/2250 kcal) a | Product (g/200 mg Ca) c | kg CO2-eq per 200 mg Ca c |
|---|---|---|---|---|---|---|
| Seaweed kelp, raw | Miscellaneous | 168 | 0.09 | 8043 | 119.0 | 0.10 |
| Flax seed | Nuts and seeds | 255 | 0.17 | 1203 | 78.4 | 0.13 |
| Kale | Vegetable | 231 | 0.16 | 15750 | 86.6 | 0.14 |
| Vegetarian schnitzel | Meat/dairy substitutes | 800 | 0.59 | 9574 | 25.0 | 0.15 |
| Cheese, 20+ | Dairy | 1059 | 1.04 | 9686 | 18.9 | 0.20 |
| Cheese Edam, 40+ | Dairy | 896 | 1.10 | 6222 | 22.3 | 0.25 |
| Lettuce, medium | Vegetable | 53 | 0.07 | 7950 | 377.4 | 0.26 |
| Herring in tomato sauce | Fish | 150 | 0.20 | 2021 | 133.3 | 0.26 |
| Spinach, frozen | Vegetable | 162 | 0.22 | 15188 | 123.5 | 0.28 |
| Milk, butter | Dairy | 109 | 0.15 | 8175 | 183.5 | 0.28 |
| Lettuce, crop | Vegetable | 49 | 0.07 | 8481 | 408.2 | 0.28 |
| Yogurt, low-fat | Dairy | 152 | 0.22 | 9243 | 131.6 | 0.29 |
| Root | Vegetable | 27 | 0.04 | 1841 | 740.7 | 0.30 |
| Onion | Vegetable | 29 | 0.04 | 1764 | 689.7 | 0.30 |
| Milk, skim | Dairy | 126 | 0.20 | 8100 | 158.7 | 0.31 |
| Cheese Gouda,48+ | Dairy | 816 | 1.31 | 4976 | 24.5 | 0.32 |
| Yogurt, whole | Dairy | 143 | 0.24 | 5547 | 139.9 | 0.33 |
| Milk, semi-skimmed | Dairy | 123 | 0.20 | 6150 | 162.6 | 0.33 |
| Yogurt, semi-skimmed | Dairy | 139 | 0.23 | 6255 | 143.9 | 0.33 |
| Yogurt, low-fat with fruits | Dairy | 129 | 0.22 | 3976 | 155.0 | 0.34 |
| Milk, whole | Dairy | 124 | 0.21 | 4574 | 161.3 | 0.34 |
| Old cheese, 48+ | Dairy | 740 | 1.31 | 4359 | 27.0 | 0.35 |
| Cheese spread, 48+ | Dairy | 467 | 0.88 | 3995 | 42.8 | 0.38 |
| Green beans (plastic) | Vegetable | 54 | 0.11 | 4860 | 370.4 | 0.39 |
| Figs | Fruit | 54 | 0.11 | 1446 | 370.4 | 0.40 |
| Figs, dried | Fruit | 162 | 0.34 | 1407 | 123.5 | 0.42 |
| Yogurt drink, sweetener | Dairy | 103 | 0.22 | 7725 | 194.2 | 0.43 |
| Tofu | Meat/dairy substitutes | 188 | 0.43 | 3743 | 106.4 | 0.46 |
| Chicory | Vegetable | 22 | 0.05 | 2605 | 909.1 | 0.46 |
| Green beans frozen (plastic) | Vegetable | 69 | 0.16 | 4566 | 289.9 | 0.47 |
| Custard, vanilla | Dairy | 86 | 0.20 | 2081 | 232.6 | 0.47 |
| Chocolate milk, semi-skimmed | Dairy | 102 | 0.24 | 2981 | 196.1 | 0.47 |
| Kiwi | Fruit | 30 | 0.07 | 1089 | 666.7 | 0.48 |
| Chocolate milk, whole | Dairy | 102 | 0.25 | 2579 | 196.1 | 0.49 |
| Custard, whole, various flavors | Dairy | 64 | 0.20 | 1516 | 312.5 | 0.64 |
| Orange | Fruit | 23 | 0.08 | 1078 | 869.6 | 0.68 |
| Tangerine | Fruit | 25 | 0.09 | 1223 | 800.0 | 0.69 |
| Cottage cheese, whole | Dairy | 125 | 0.47 | 2180 | 160.0 | 0.76 |
| Green beans (can) | Vegetable | 49 | 0.19 | 4240 | 408.2 | 0.78 |
| Canned salmon, wild caught | Fish | 91 | 0.37 | 1402 | 219.8 | 0.82 |
| Green beans (glass) | Vegetable | 49 | 0.20 | 4240 | 408.2 | 0.82 |
| Cream/vanilla ice cream | Dairy | 103 | 0.46 | 1130 | 194.2 | 0.90 |
| Mozzarella | Dairy | 160 | 0.85 | 1423 | 125.0 | 1.06 |
| Cauliflower | Vegetable | 25 | 0.14 | 2557 | 800.0 | 1.09 |
| Broccoli | Vegetable | 33 | 0.18 | 2750 | 606.1 | 1.11 |
| Canned salmon, farmed fish | Fish | 91 | 0.59 | 1402 | 219.8 | 1.29 |
| Chicken egg | Eggs | 64 | 0.43 | 1125 | 312.5 | 1.35 |
| Garden peas with carrots (can) | Vegetable | 29 | 0.20 | 1165 | 689.7 | 1.38 |
| Plaice | Fish | 101 | 0.73 | 1171 | 198.0 | 1.44 |
| Garden peas with carrots (glass) | Vegetable | 29 | 0.21 | 1165 | 689.7 | 1.44 |
| Apricots | Fruit | 20 | 0.15 | 1023 | 1000.0 | 1.47 |
| Coffee | Non-alcoholic drinks | 4 | 0.03 | 9000 | 5000.0 | 1.48 |
| Gourmet | Fish | 101 | 0.82 | 1077 | 198.0 | 1.62 |
| Goat cheese, fresh | Dairy | 101 | 0.85 | 1098 | 198.0 | 1.68 |
| Cucumber with peel | Vegetable | 20 | 0.19 | 3462 | 1000.0 | 1.88 |
| Dutch shrimps | Fish | 134 | 1.54 | 3207 | 149.3 | 2.30 |
| Bean sprouts | Vegetable | 16 | 0.20 | 1440 | 1250.0 | 2.48 |
| Soda, sugar and caffeine | Non-alcoholic drinks | 5 | 0.06 | 1023 | 4000.0 | 2.50 |
| Zucchini | Vegetable | 20 | 0.26 | 2368 | 1000.0 | 2.63 |
| Strawberries | Fruit | 15 | 0.64 | 1034 | 1333.3 | 8.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2022, 14, 180. https://doi.org/10.3390/nu14010180
Shkembi B, Huppertz T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients. 2022; 14(1):180. https://doi.org/10.3390/nu14010180
Chicago/Turabian StyleShkembi, Blerina, and Thom Huppertz. 2022. "Calcium Absorption from Food Products: Food Matrix Effects" Nutrients 14, no. 1: 180. https://doi.org/10.3390/nu14010180
APA StyleShkembi, B., & Huppertz, T. (2022). Calcium Absorption from Food Products: Food Matrix Effects. Nutrients, 14(1), 180. https://doi.org/10.3390/nu14010180






