Gut Microbiota and Complications of Type-2 Diabetes
Abstract
1. Introduction
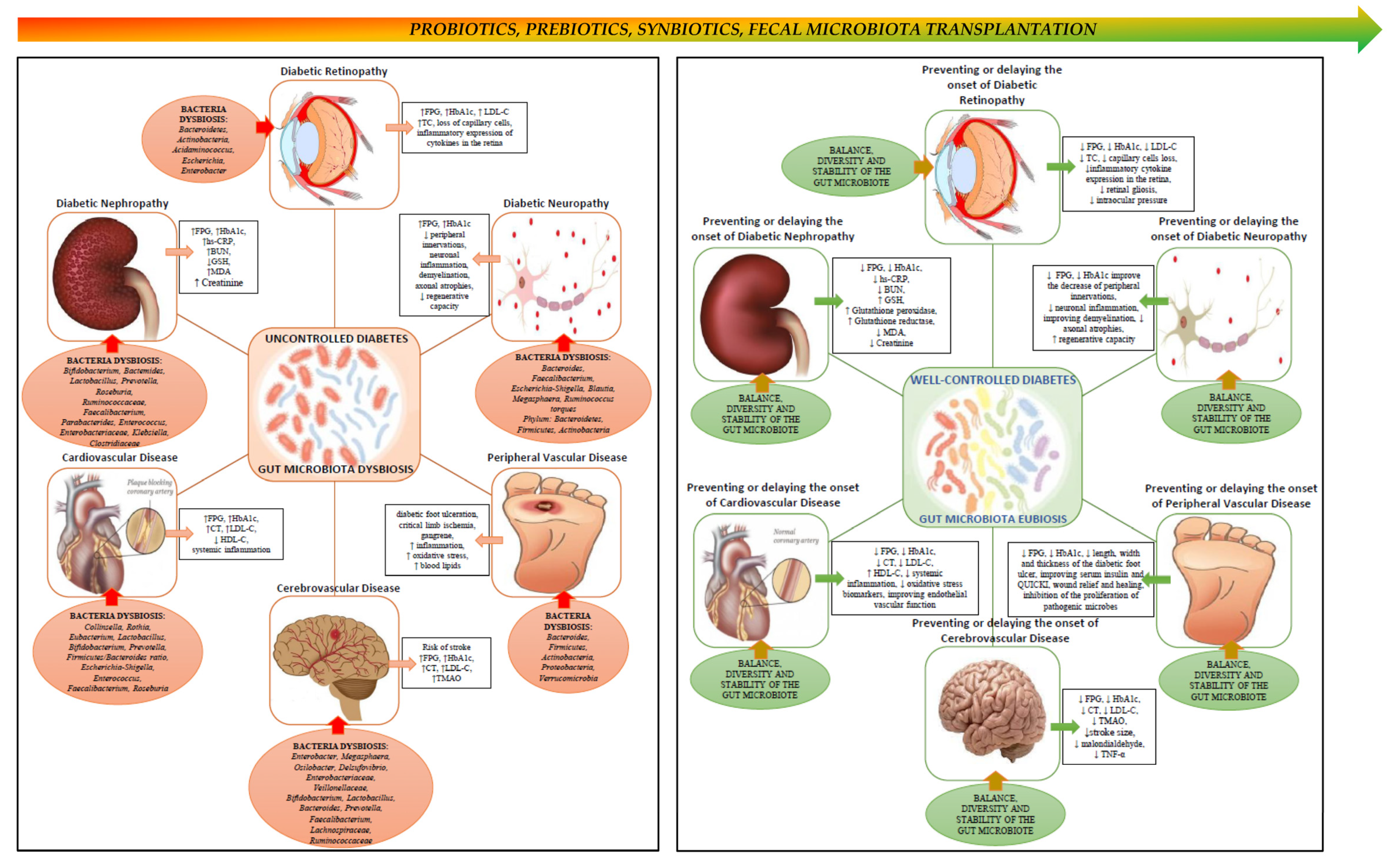
2. Gut Microbiota, Type 2 Diabetes and Its Complications
2.1. Gut Microbiota in Diabetic Nephropathy
2.2. Gut Microbiota in Diabetic Retinopathy
2.3. Gut Microbiota in Diabetic Neuropathy
2.4. Gut Microbiota in Cerebrovascular Disease
2.5. Gut Microbiota in Coronary Heart Disease
2.6. Gut Microbiota in Peripheral Vascular Disease
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Matijasic, M.; Mestrovic, T.; Paljetak, H.C.; Peric, M.; Baresic, A.; Verbanac, D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Jayasudha, R.; Das, T.; Kalyana Chakravarthy, S.; Sai Prashanthi, G.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Shivaji, S. Gut mycobiomes are altered in people with type 2 Diabetes Mellitus and Diabetic Retinopathy. PLoS ONE 2020, 15, e0243077. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Mar Rodriguez, M.; Perez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jove, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, B.; Zorena, K.; Szmigiero-Kawko, M.; Waz, P.; Mysliwiec, M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Prefer. Adherence 2016, 10, 591–599. [Google Scholar] [CrossRef]
- Pataky, Z.; Bobbioni-Harsch, E.; Hadengue, A.; Carpentier, A.; Golay, A. Gut microbiota, responsible for our body weight? Rev. Med. Suisse 2009, 5, 662–664. [Google Scholar]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naive T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Asghari Jafarabadi, M.; Hassanalilou, T.; Mesgari Abbasi, M.; Faraji, I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2019, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Current issues in the treatment of type 2 diabetes. Overview of newer agents: Where treatment is going. Am. J. Med. 2010, 123, S38–S48. [Google Scholar] [CrossRef]
- Bekyarova, G.Y.; Ivanova, D.G.; Madjova, V.H. Molecular mechanisms associating oxidative stress with endothelial dysfunction in the development of various vascular complications in diabetes mellitus. Folia Med. 2007, 49, 13–19. [Google Scholar]
- Baig, M.A.; Panchal, S.S. Streptozotocin-Induced Diabetes Mellitus in Neonatal Rats: An Insight into its Applications to Induce Diabetic Complications. Curr. Diabetes Rev. 2019, 16, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Gourgari, E.; Dabelea, D.; Rother, K. Modifiable Risk Factors for Cardiovascular Disease in Children with Type 1 Diabetes: Can Early Intervention Prevent Future Cardiovascular Events? Curr. Diabetes Rep. 2017, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Sun, D.; Ma, W.; Zheng, Y.; Champagne, C.M.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut-microbiome-related LCT genotype and 2-year changes in body composition and fat distribution: The POUNDS Lost Trial. Int. J. Obes. 2018, 42, 1565–1573. [Google Scholar] [CrossRef]
- Tajabadi-Ebrahimi, M.; Sharifi, N.; Farrokhian, A.; Raygan, F.; Karamali, F.; Razzaghi, R.; Taheri, S.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effect of Synbiotic Administration on Markers of Insulin Metabolism and Lipid Profiles in Overweight Type 2 Diabetic Patients with Coronary Heart Disease. Exp. Clin. Endocrinol. Diabetes 2017, 125, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Constantino, M.I.; Molyneaux, L.; Limacher-Gisler, F.; Al-Saeed, A.; Luo, C.; Wu, T.; Twigg, S.M.; Yue, D.K.; Wong, J. Long-term complications and mortality in young-onset diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013, 36, 3863–3869. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Boren, J.; Smith, U. Confounding Effects of Metformin on the Human Gut Microbiome in Type 2 Diabetes. Cell Metab. 2016, 23, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Manneras-Holm, L.; Stahlman, M.; Olsson, L.M.; Serino, M.; Planas-Felix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Guo, Y.; Wang, Z.; Liu, Q.; Yan, R.; Wang, Y.; Wu, Q.; Yuan, K.; Sun, W. The Profile and Function of Gut Microbiota in Diabetic Nephropathy. Diabetes Metab. Syndr. Obes. 2021, 14, 4283–4296. [Google Scholar] [CrossRef]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, P.; Biyani, M. Targeted therapies in diabetic nephropathy: An update. J. Nephrol. 2011, 24, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E. Nephropathy in type 2 diabetes. J. Intern. Med. 1999, 245, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr. Diabetes Rep. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef]
- McMullan, C.J.; Lambers Heerspink, H.J.; Parving, H.H.; Dwyer, J.P.; Forman, J.P.; de Zeeuw, D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: A post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am. J. Kidney Dis. 2014, 64, 714–722. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; Garcia-Perez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Singh, D.K.; Winocour, P.; Farrington, K. Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat. Rev. Endocrinol. 2011, 7, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharm. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Nazertehrani, S.; Ni, Z.; Liu, S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am. J. Nephrol. 2013, 38, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol.-Renal Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef]
- Fukuuchi, F. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin. Exp. Nephrol. 2002, 6, 99–104. [Google Scholar] [CrossRef]
- Xu, K.Y.; Xia, G.H.; Lu, J.Q.; Chen, M.X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J.; et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef]
- Kanbay, M.; Onal, E.M.; Afsar, B.; Dagel, T.; Yerlikaya, A.; Covic, A.; Vaziri, N.D. The crosstalk of gut microbiota and chronic kidney disease: Role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int. Urol. Nephrol. 2018, 50, 1453–1466. [Google Scholar] [CrossRef]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 2012, 17, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Taki, K.; Niwa, T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am. J. Kidney Dis. 2003, 41, S142–S145. [Google Scholar] [CrossRef]
- Cruz-Mora, J.; Martinez-Hernandez, N.E.; Martin del Campo-Lopez, F.; Viramontes-Horner, D.; Vizmanos-Lamotte, B.; Munoz-Valle, J.F.; Garcia-Garcia, G.; Parra-Rojas, I.; Castro-Alarcon, N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Renal Nutr. 2014, 24, 330–335. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Miranda Alatriste, P.V.; Urbina Arronte, R.; Gomez Espinosa, C.O.; Espinosa Cuevas Mde, L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. [Google Scholar] [CrossRef]
- Simenhoff, M.L.; Dunn, S.R.; Zollner, G.P.; Fitzpatrick, M.E.; Emery, S.M.; Sandine, W.E.; Ayres, J.W. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowith in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Min. Electrolyte Metab. 1996, 22, 92–96. [Google Scholar]
- Dunn, S.R.; Simenhoff, M.L.; Ahmed, K.E.; Gaughan, W.J.; Eltayeb, B.O.; Fitzpatrick, M.E.; Emery, S.M.; Ayres, J.W.; Holt, K.E. Effect of Oral Administration of Freeze-Dried Lactobacillus acidophilus on Small Bowel Bacterial Overgrowith in Patients with End Stage Kidney Disease: Reducing Uremic Toxins and Improving Nutrition. Int. Dairy J. 1998, 8, 545–553. [Google Scholar] [CrossRef]
- Mafi, A.; Namazi, G.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Asemi, Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Food Funct. 2018, 9, 4763–4770. [Google Scholar] [CrossRef]
- Abbasi, B.; Ghiasvand, R.; Mirlohi, M. Kidney Function Improvement by Soy Milk Containing Lactobacillus plantarum A7 in Type 2 Diabetic Patients with Nephropathy: A Double-Blinded Randomized Controlled Trial. Iran. J. Kidney Dis. 2017, 11, 36–43. [Google Scholar]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Mazruei Arani, N.; Emam-Djomeh, Z.; Tavakolipour, H.; Sharafati-Chaleshtori, R.; Soleimani, A.; Asemi, Z. The Effects of Probiotic Honey Consumption on Metabolic Status in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1195–1201. [Google Scholar] [CrossRef]
- Miraghajani, M.; Zaghian, N.; Dehkohneh, A.; Mirlohi, M.; Ghiasvand, R. Probiotic Soy Milk Consumption and Renal Function Among Type 2 Diabetic Patients with Nephropathy: A Randomized Controlled Clinical Trial. Probiotics Antimicrob. Proteins 2019, 11, 124–132. [Google Scholar] [CrossRef]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: A Randomized Controlled Clinical Trial. J. Renal Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Papanas, N.; Banach, M.; Papazoglou, D.; Edmonds, M. Complications of Diabetes 2016. J. Diabetes Res. 2016, 2016, 6989453. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharm. Toxicol. 2010, 50, 439–465. [Google Scholar] [CrossRef] [PubMed]
- Das, A. Diabetic Retinopathy: Battling the Global Epidemic. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6669–6682. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.M., 2nd; Hu, P.; Caballero, S.; Moldovan, L.; Verma, A.; Oudit, G.Y.; Li, Q.; Grant, M.B. Adeno-Associated Virus Overexpression of Angiotensin-Converting Enzyme-2 Reverses Diabetic Retinopathy in Type 1 Diabetes in Mice. Am. J. Pathol. 2016, 186, 1688–1700. [Google Scholar] [CrossRef]
- Jeganathan, V.S. The therapeutic implications of renin-angiotensin system blockade in diabetic retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Perkins, B.A.; Aiello, L.P.; Krolewski, A.S. Diabetes complications and the renin-angiotensin system. N. Engl. J. Med. 2009, 361, 83–85. [Google Scholar] [CrossRef]
- Sjolie, A.K.; Dodson, P.; Hobbs, F.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int. J. Clin. Pract. 2011, 65, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shan, Z.; Lei, B.; Yuan, L.; Liu, X.; Nakagawa, T.; Grant, M.B.; Lewin, A.S.; Hauswirth, W.W.; Raizada, M.K.; et al. ACE2 and Ang-(1–7) confer protection against development of diabetic retinopathy. Mol. Ther. 2012, 20, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. In this issue: Immunology of the eye—Inside and out. Int. Rev. Immunol. 2013, 32, 1–3. [Google Scholar] [CrossRef]
- Tap, J.; Mondot, S.; Levenez, F.; Pelletier, E.; Caron, C.; Furet, J.P.; Ugarte, E.; Munoz-Tamayo, R.; Paslier, D.L.; Nalin, R.; et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009, 11, 2574–2584. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, 643.e7–643.e12. [Google Scholar] [CrossRef]
- Ozkan, J.; Willcox, M.; Wemheuer, B.; Wilcsek, G.; Coroneo, M.; Thomas, T. Biogeography of the human ocular microbiota. Ocul. Surf. 2019, 17, 111–118. [Google Scholar] [CrossRef]
- Lu, L.J.; Liu, J. Human Microbiota and Ophthalmic Disease. Yale J. Biol. Med. 2016, 89, 325–330. [Google Scholar]
- Das, T.; Jayasudha, R.; Chakravarthy, S.; Prashanthi, G.S.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Sharma, S.; Shivaji, S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci. Rep. 2021, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, C.; Xia, Y.; Xia, W.; Liu, G.; Ren, C.; Gu, Y.; Li, X.; Lu, P. Elevated plasma trimethylamine-N-oxide levels are associated with diabetic retinopathy. Acta Diabetol. 2021, 58, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Ma, H.; Ji, S.; Chen, Z.; Cui, Z.; Chen, J.; Tang, S. Dysbiosis and Implication of the Gut Microbiota in Diabetic Retinopathy. Front. Cell Infect. Microbiol. 2021, 11, 646348. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Xu, K.; Du, T.; Zhu, P.; Liang, Z.; Liao, S.; Zhang, J.; Raizada, M.K.; Grant, M.B.; Li, Q. Expression of Human ACE2 in Lactobacillus and Beneficial Effects in Diabetic Retinopathy in Mice. Mol. Methods Clin. Dev. 2019, 14, 161–170. [Google Scholar] [CrossRef]
- LI, Q.; XU, K.; DU, T.; ZHU, P.; VERMA, A. Recombinant Probiotics Expressing Angiotensin-(1-7) Improves Glucose Metabolism and Diabetes-Induced Renal and Retinal Injury. Diabetes 2018, 67, 33-LB. [Google Scholar] [CrossRef]
- Petit Homme, R.; George, A.K.; Stanisic, D.N.; Malonee, C.; Molnar, J.; Smolenkova, I.; Sandhu, H.A.S.; Tyagi, S.C.; Singh, M. Effects of Probiotic on the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4961. [Google Scholar]
- Vinik, A.I.; Nevoret, M.L.; Casellini, C.; Parson, H. Diabetic neuropathy. Endocrinol. Metab. Clin. Neurol. Am. 2013, 42, 747–787. [Google Scholar] [CrossRef]
- Grasset, E.; Burcelin, R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef]
- Rolim, L.C.; da Silva, E.M.; Flumignan, R.L.; Abreu, M.M.; Dib, S.A. Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2019, 6, CD011265. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Ding, D.; Lu, Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520936806. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural. Transm. 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Chiu, I.M. Bacterial Signaling to the Nervous System through Toxins and Metabolites. J. Mol. Biol. 2017, 429, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.J.; Baral, P.; Voisin, T.; Lubkin, A.; Pinho-Ribeiro, F.A.; Adams, K.L.; Roberson, D.P.; Ma, Y.C.; Otto, M.; Woolf, C.J.; et al. Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat. Commun. 2018, 9, 37. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Stevens, R.J.; Coleman, R.L.; Adler, A.I.; Stratton, I.M.; Matthews, D.R.; Holman, R.R. Risk Factors for Myocardial Infarction Case Fatality and Stroke Case Fatality in Type 2 Diabetes. UKPDS 2004, 27, 201–207. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An insight into intestinal mucosal microbiota disruption after stroke. Sci. Rep. 2018, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fak, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Backhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, X.; Peng, Y.; Wu, Q.; Zhu, J.; Tan, C.; Xia, G.; You, C.; Xu, R.; Pan, S.; et al. Higher Risk of Stroke Is Correlated with Increased Opportunistic Pathogen Load and Reduced Levels of Butyrate-Producing Bacteria in the Gut. Front. Cell. Infect. Microbiol. 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Krankel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients with Stroke and Is Related to Proinflammatory Monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.T.; Zuo, H.; Ueland, P.M.; Seifert, R.; Loland, K.H.; Pedersen, E.R.; Schuster, P.M.; Karlsson, T.; Tell, G.S.; Schartum-Hansen, H.; et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018, 267, 100–106. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Xie, L.; Zhao, B.X.; Li, Y.; Qiu, B.; Zhu, F.; Li, G.F.; He, M.; Wang, Y.; Wang, B.; et al. Serum Trimethylamine N-Oxide Concentration Is Positively Associated with First Stroke in Hypertensive Patients. Stroke 2018, 49, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota with Reduced Trimethylamine-N-Oxide Level in Patients with Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699. [Google Scholar] [CrossRef]
- Akhoundzadeh, K.; Vakili, A.; Shadnoush, M.; Sadeghzadeh, J. Effects of the Oral Ingestion of Probiotics on Brain Damage in a Transient Model of Focal Cerebral Ischemia in Mice. Iran. J. Med. Sci. 2018, 43, 32–40. [Google Scholar]
- Aronson, D.; Edelman, E.R. Coronary artery disease and diabetes mellitus. Cardiol. Clin. 2014, 32, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef]
- Lee, S.H. Update on Familial Hypercholesterolemia: Diagnosis, Cardiovascular Risk, and Novel Therapeutics. Endocrinol. Metab. 2017, 32, 36–40. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Kobayashi, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: Gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessel. 2017, 32, 39–46. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, T.; Hu, H.; Zhang, W.; Hua, X. Association Study of Gut Flora in Coronary Heart Disease through High-Throughput Sequencing. Biomed. Res. Int. 2017, 2017, 3796359. [Google Scholar] [CrossRef]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840. [Google Scholar] [CrossRef]
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012, 26, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.; Fialho, A.; Kochhar, G.; Schenone, A.L.; Thota, P.; McCullough, A.J.; Shen, B. Association Between Small Intestinal Bacterial Overgrowith by Glucose Breath Test and Coronary Artery Disease. Dig. Dis. Sci. 2018, 63, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018, 16, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, C.C.K.; Ueland, T.; Broch, K.; Vincent, R.P.; Cross, G.F.; Dahl, C.P.; Aukrust, P.; Gullestad, L.; Hov, J.R.; Troseid, M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017, 23, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, F.; Homayouni, A. Dairy Probiotic Foods and Coronary Heart Disease: A Review on Mechanism of Action. In Probiotics; Rigobelo, E., Ed.; InTech: London, UK, 2012; pp. 121–128. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men with Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51. [Google Scholar] [CrossRef]
- Sawacha, Z.; Guarneri, G.; Avogaro, A.; Cobelli, C. A New Classification of Diabetic Gait Pattern Based on Cluster Analysis of Biomechanical Data. J. Diabetes Sci. Technol. 2010, 4, 1127–1138. [Google Scholar] [CrossRef]
- Karadurmus, N.; Sahin, M.; Tasci, C.; Naharci, I.; Ozturk, C.; Ilbasmis, S.; Dulkadir, Z.; Sen, A.; Saglam, K. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol. Pol. 2010, 61, 275–279. [Google Scholar] [PubMed]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lepantalo, M.; Apelqvist, J.; Setacci, C.; Ricco, J.B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.H.; De Rango, P.; et al. Chapter V: Diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. 2), S60–S74. [Google Scholar] [CrossRef]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.J.; Seelig, A.D.; Ahroni, J.H. Limb- and Person-Level Risk Factors for Lower-Limb Amputation in the Prospective Seattle Diabetic Foot Study. Diabetes Care 2018, 41, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Nativel, M.; Potier, L.; Alexandre, L.; Baillet-Blanco, L.; Ducasse, E.; Velho, G.; Marre, M.; Roussel, R.; Rigalleau, V.; Mohammedi, K. Lower extremity arterial disease in patients with diabetes: A contemporary narrative review. Cardiovasc. Diabetol. 2018, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723. [Google Scholar] [CrossRef]
- Mohseni, S.; Bayani, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Bayani, M.A.; Jafari, P.; Asemi, Z. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. Res. Rev. 2018, 34, e2970. [Google Scholar] [CrossRef]
- Sonal Sekhar, M.; Unnikrishnan, M.K.; Vijayanarayana, K.; Rodrigues, G.S.; Mukhopadhyay, C. Topical application/formulation of probiotics: Will it be a novel treatment approach for diabetic foot ulcer? Med. Hypotheses 2014, 82, 86–88. [Google Scholar] [CrossRef]
- Covasa, M.; Stephens, R.W.; Toderean, R.; Cobuz, C. Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides. Front. Endocrinol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Azmal Ali, S.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Johansson, M.L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255. [Google Scholar] [CrossRef]
- Simons, L.A.; Amansec, S.G.; Conway, P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 531–535. [Google Scholar] [CrossRef]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2009, 101, 1679–1687. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Moller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Di Pietro, E.; Simon, R.R.; Prakash, S. Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: A randomized control trial. Regul. Toxicol. Pharm. 2012, 63, 313–320. [Google Scholar] [CrossRef]
- Gobel, R.J.; Larsen, N.; Jakobsen, M.; Molgaard, C.; Michaelsen, K.F. Probiotics to adolescents with obesity: Effects on inflammation and metabolic syndrome. J. Pediatric Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shavakhi, A.; Minakari, M.; Firouzian, H.; Assali, R.; Hekmatdoost, A.; Ferns, G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int. J. Prev. Med. 2013, 4, 531–537. [Google Scholar]
- Jung, S.P.; Lee, K.M.; Kang, J.H.; Yun, S.I.; Park, H.O.; Moon, Y.; Kim, J.Y. Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial. Korean J. Fam. Med. 2013, 34, 80–89. [Google Scholar] [CrossRef]
- Sharafedtinov, K.K.; Plotnikova, O.A.; Alexeeva, R.I.; Sentsova, T.B.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—A randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi Rad, M.; Rahimi Foroushani, A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Shahbazian, H.; Kaydani, G.A.; Mohammadi, F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014, 4, 83–88. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Kennelly, M.; Culliton, M.; Smith, T.; Maguire, O.C.; Shanahan, F.; Brennan, L.; McAuliffe, F.M. Probiotics in obese pregnancy do not reduce maternal fasting glucose: A double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am. J. Clin. Nutr. 2014, 99, 1432–1439. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 447–452. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef]
- Ogawa, A.; Kadooka, Y.; Kato, K.; Shirouchi, B.; Sato, M. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis. 2014, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: A randomised, double-blind, placebo-controlled pilot study. Br. J. Nutr. 2014, 112, 438–445. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: A randomized controlled clinical trial. Hormones 2014, 13, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Simon, M.C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc. Pharm. 2015, 20, 289–298. [Google Scholar] [CrossRef]
- Bjerg, A.T.; Kristensen, M.; Ritz, C.; Stark, K.D.; Holst, J.J.; Leser, T.D.; Wellejus, A.; Astrup, A. Four weeks supplementation with Lactobacillus paracasei subsp. paracasei L. casei W8(R) shows modest effect on triacylglycerol in young healthy adults. Benef. Microbes. 2015, 6, 29–39. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Madjd, A.; Taylor, M.A.; Mousavi, N.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Zilmer, K.; Salum, T.; Rehema, A.; Zilmer, M. The use of probiotic L. fermentum ME-3 containing Reg’Activ Cholesterol supplement for 4 weeks has a positive influence on blood lipoprotein profiles and inflammatory cytokines: An open-label preliminary study. Nutr. J. 2016, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Berteus Forslund, H.; Perkins, R.; Backhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: A double-blind randomized clinical trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. 2018, 12, 617–624. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Alipour, B.; Asghari Jafar-Abadi, M.; Faraji, I.; Hassanalilou, T.; Mesgari Abbasi, M.; Vaghef-Mehrabany, E.; Alizadeh Sani, M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran. Biomed. J. 2019, 23, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr. 2019, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: A double blind, randomized, placebo controlled study. PLoS ONE 2019, 14, e0225168. [Google Scholar] [CrossRef] [PubMed]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef]
- Brussow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Koh, A.; Backhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef]
| Reference | Design | Probiotic Source | Probiotic Dose, CFU | Study Period (wk/d) | Effects |
|---|---|---|---|---|---|
| [50] | RD, DB, CT | tablet | L. acidophilus strain ZT-L1, B. bifidum strain ZT-B1, L. reuteri strain ZT-Lre, L. fermentum strain ZT-L3 8 × 109 CFU/d | 12 wk | S↓ FG, I, HOMA-IR, TG, VLDL, TC/HDL-C ratio, hs-CRP, MDA, AGEs, BUN, creatinine, urine protein S↑ QUICKI, HDL-C, GSH, CG = HbA1c, LDL-C, NO, TAC |
| [51] | RD, DB, CT | soy milk | L. plantarum A7 | 8 wk | S↓ albuminuria, serum creatinine, serum interleukin-18, serum sialic acid S improvment in estimated GFR |
| [52] | RD, DB, CT | capsule | L. acidophilus L. casei B. bifium | 12 wk | S↓ FG, I, HOMA-IR, HbA1c, hs-CRP, MDA, SGA score, TIBC S↑ QUICKI =HOMA-B, TG, VLDL, CT, LDL-C, HDL-C, NO, TAC, GSH, GFR, creatinine, BUN, albumin, Na, K |
| [53] | RD, DB, CT | honey | Bacillus coagulans T4 (IBRC-N10791) 108 CFU/g | 12 wk | S↓ I, HOMA-IR, CT/HDL-C ratio, hs-CRP hs-CRP, MDA, creatinine S↑ QUICKI =FG, TG, VLDL, CT, LDL-C, HDL-C, NO, TAC, GSH, BUN |
| [54] | RD, DB, CT | soy milk | L. plantarum A7 2 × 107 CFU/mL | 8 wk | S↓ Cys-C, PGRN, NGAL =sTNFR1 |
| [55] | RD, DB, CT | soy milk | L plantarum A7 (KC 355240, LA7) 2 × 107 CFUmL | 8 wk | S↑ Glutathione, Glutathione peroxidase, Glutathione reductase S↓ Oxidized glutathione =MDA, 8-iso-PGF2a, TAC |
| Reference | Year | Location | Design | Participants, Age, Nr. Treated/ Nr. Controls | Probiotic Source | Probiotic Dose, CFU | Study Period (wk/d) | Glycemia | Insulin | Lipid Metabolism | Incretins |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [135] | 2002 | Poland | RD, DB, CT | Healthy participants 35–45 y 18/18 | rose-hip drink | L. plantarum 299v, 5 × 107 CFU/mL | 6 wk | =FG | =I | =TC, LDL-C, HDL-C, TG, lipoprotein(a) | S↓ leptin |
| [136] | 2006 | Australia | DB, PC, parallel design trial, single centre | Healthy volunteers 30–75 y 23/21 | capsule | L. fermentum, 2 × 109 CFU | 10 wk | =FG | - | =LDL-C, TC, HDL-C, TGL | - |
| [137] | 2009 | Finland | RD, prospective, parallel-group | Pregnant women 29.7/30.1/30.2 y 85/86/85 | capsule | L. rhamnosus GG, ATCC 53 103, B. lactis Bb12, 1010 CFU/d each | 4 wk | S↓ FG, =HbA1c | S↓ I, HOMA, S↑ QUICKI | - | - |
| [138] | 2010 | Denmark | RD, PC, DB | T2DM/non-diabetic 48–66 y 24/24 | capsule | L. acidophilus NCFM, 1 g; about 1010 CFU | 4 wk | - | =QUICKI | - | - |
| [139] | 2012 | Iran | DB, RD, CT | T2DM 30–60 y 32/32 | yogurt | L. acidophilus La5, 7.23 × 106–1.85 × 106 CFU/g B. lactis Bb12, 6.04 × 106 CFU/g–1.79 × 106 CFU/g | 6 wk | S↓ FG, HbA1c | =I | - | - |
| [140] | 2012 | Brazil | DB, PC, RD | Healthy participants 50–65 y 10/10 | shake | L. acidophillus, 4 × 108 CFU/100 mL B. bifidum 4 ×108 CFU/100 mL 1 g/100 mL FOS | 30 d | S↓ FG | - | S↑ HDL-C =TC, TG | - |
| [141] | 2012 | Canada | DB, PC, multi-center study | Healthy hypercholester-olemic human subjects 20–75 y 67/64 | capsule | L. reuteri NCIMB 30242, 2.9 × 10⁹ CFU | 9 wk | =FG | - | - | - |
| [142] | 2012 | Denmark | DB, PC, RD | Ob adolescents 12–15 y 27/23 | capsule | L. salivarius Ls-33 ATCC SD5208, 1010 CFU | 12 wk | =FG | =I, HOMA-IR | =TC, HDL-C, LDL-C, TG | - |
| [143] | 2013 | Iran | RD, DB, PC, CT | T2DM 35–70 y 27/27 | capsule | L. acidophilus, 2 × 109 CFU L. casei, 7 × 109 CFU L. rhamnosus, 1.5 × 109 CFU L. bulgaricus, 2 × 108 CFU B. breve, 2 × 1010 CFU B. longum, 7 × 109 CFU S. thermophiles, 1.5 ×109 CFU 100 mg FOS | 8 wk | S↓ FG | S↑ I, HOMA-IR | S↑ LDL-C | - |
| [144] | 2013 | Iran | RD, DB, CT | Patients with NASH 18–75 y 34/36 | tablet | L. acidophilus, 1 × 108 CFU L. casei, 5 × 108 CFU L. rhamnosus, 7.5 × 107 CFU L. bulgaricus, 1.5 × 108 CFU B. breve, 5 × 107 CFU B. longum, 2.5 × 107 CFU S. thermophilus, 5 × 107 CFU 350 mg FOS | 24 wk | S↓ FG | - | S↓ TC, TG | - |
| [145] | 2013 | Korea | single center, RD, DB, PC, CT | Ob volunteers 19–60 y 31/31 | capsule | L. gasseri BNR17, 1010 CFU 25% FOS | 12 wk | =FG, HbA1c | =I | =TC, TG, LDL-C, HDL-C, | - |
| [146] | 2013 | Russian Federation | RD, DB, PC, parallel pilot study | Patients with metabolic syndrome 30–69 y 25/15 | cheese | L. plantarum TENSIA, 1.5 × 1011 CFU/g | 3 wk | =FG | - | =TC, LDL-C, HDL-C, TG | - |
| [147] | 2013 | Iran | RD, SB, CT | Pregnant women 37/33 18–30 y | yogurt | L. acidophilus LA5, B. animalis BB12, 1 × 10⁷ CFU | 9 wk | =FG | S↓ I, HOMA | - | - |
| [148] | 2014 | Iran | RD, DB, cross-over CT | T2DM 35–70 y 62/62 | package | L. sporogenes, 27 × 107 CFU 1.08 g inulin | 6 wk | =FG | S↓ I =HOMA-IR | =CT, LDL-C, TG, HDL-C | - |
| [149] | 2014 | Iran | RD, DB, CT | T2DM ov/ob obese 53.00 ± 5.9/ 49.00 ± 7.08 y 22/22 | yogurt | B. lactis Bb12, L. acidophilus strain La5, 3.7 × 106 CFU/g | 8 wk | S↓ HbA1c =FG | - | - | - |
| [150] | 2014 | Ireland | PC, DB, RD | Ob pregnant women, 31.4 ± 5.0/31.0 ± 5.2 y 63/75 | capsule | L. salivarius UCC118, 109 CFU | 4 wk | =FG | =I, HOMA-IR | =TC, HDL-C, LDL-C, TG | - |
| [151] | 2014 | Australia | RD, DB, parallel study | Ov >55 y 40/37/39/40 | Yogurt/ capsule | L. acidophilus La5, B. lactis Bb12, 3 × 109 CFU/d | 6 wk | S↑ FG =HbA1c | S↑ HOMA-IR =I | - | - |
| [152] | 2014 | India | RD, CT, DB | Ov/ob healthy adults 40–60 y 15/15/15/15 | capsule | B. longum, B. infantis, B. breve, L. acidophilus, L. paracasei, L. bulgaricus, L. plantarum, S. thermophilus. 112.5 × 109 CFU/capsule | 6 wk | S↓ FG | S↓ I, HOMA-IR | S↓ TC, TG, LDL-C, VLDL-C S↑ HDL-C | - |
| [153] | 2014 | Japan | SB, PC, within-subject, repeated-measure intervention trial | Adults with hypertriacylglycerolemia, 51.1 ± 6.6 y 10/10 | fermented mil | L. gasseri SBT2055 (LG2055), 5 × 1010 CFU/100 g | 4 wk | S↑ HbA1c =FG | =I | S↓ NEFA =TG, Apo B-48, TC, LDL-C, HDL-C | - |
| [154] | 2014 | Iran | RD, DB, PC, CT | NAFLD >18 y 26/26 | capsule | L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus 2 × 108 CFU 250 mg FOS | 28 wk | S↓ FG | S↓ I, HOMA-IR | - | - |
| [155] | 2014 | Iran | RD, DB, PC pilot study | Patients with MS >18 y 19/19 | capsule | L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus 2 × 108 CFU 250 mg FOS | 28 wk | S↓ FG | S↓ I, HOMA-IR S↑ QUICKI | =LDL-C S↓ TG, CT S↑ HDL | - |
| [156] | 2014 | Iran | RD, PC, CT | Pregnant women 18–35 y 26/26 | food | L. sporogenes, 1 × 107 CFU 0.04 g inulin | 9 wk | =FG | S↓ I, HOMA-IR, HOMA-B S↑ QUICKI | - | - |
| [157] | 2014 | Iran | RD, DB, CT | T2DM 35–70 y 26/26/26 | bread | L. sporogenes, 1 × 108 CFU 0.07 g inulin | 8 wk | =FG | - | S↓ TG, VLDL-C, TC/HDL-C S↑ HDL-C = TC, LDL-C, HDL-C | - |
| [158] | 2015 | Germany | DB, RD, prospective, longitudinal pilot | Lean/ob participants 40–65 y 11/10 | capsule | L. reuteri, 2 × 1010 CFU | 8 wk | =blood glucose levels during OGTT | S↑ QUICKI in lean participants compared with obese | - | S↑ GLP-1, GLP-2 |
| [159] | 2015 | Iran | RD, DB, PC, CT | T2DM 35–65 y 30/30 | fermented milk (kefir) | L. acidophilus, 3 × 106–25 × 106 L. casei, 2 × 106–15 × 106 B. lactis, 0.5 × 106–8 × 106 | 8 wk | S↓ HbA1c, FG | - | =TG, TC, LDL-C, HDL-C | - |
| [160] | 2015 | India | RD, CP, SB, pilot study | Healthy participants 20–25 y 15/15/15 | capsule | L. salivarius UBL S22, 2 × 109 CFU 10 g/d FOS | 6 wk | S↓ FG | S↓ I, HOMA-IR | S↓ TG, CT, LDL-C S↑ HDL-C | - |
| [161] | 2015 | Denmark | CT, DB, RD, PC, two-arm parallel | Young healthy adults 20–45 y 32/32 | capsule | L. casei W8, 1010 CFU | 4 wk | =FG | =I | S↓ TG =CT, HDL-C, LDL-C | =GLP1 |
| [162] | 2016 | Iran | RD, DB, PC, CT | GDM, 18–40 y 30/30 | capsule | L. acidophilus, 2 × 109 CFU/g L. casei, 2 × 109 CFU/g B. bifidum, 2 × 109 CFU/g | 6 wk | S↓ FG | S↓ I, HOMA-IR, HOMA-B S↑ QUICKI | S↓ TG, VLD-C, =TC, HDL-C | - |
| [163] | 2016 | Iran | RD, SB, CT | Ob/ov subjects 18–50 y 44/45 | yogurt | L. acidophilus LA5, B. lactis BB12 1 × 107 CFU | 12 wk | S↓ 2-h postprandial glucose, HbA1c =FG | S↓ HOMA-IR, I | S↓ TC, LDL-C =HDL-C, TG | - |
| [164] | 2016 | Estonia | preliminary, open label study | Clinically healthy volunteers 50–75 y | capsule | L. fermentum ME-3 (LFME-3), 6 × 109 CFU | 4 wk | S↓ HbA1c | S↓ HOMA-IR | S↓ LDL-C, oxLDL, TC, TG, TG/HDL-C ratio S↑ HDL-C | - |
| [165] | 2017 | Sweden | RD, PC | T2DM 50–75 y 15/15/16 | stick pack | L. reuteri DSM 17938, 108 CFU/day L. reuteri DSM 17938, 1010 CFU/day | 12 wk | =FG =HbA1c | S↑ QUICKI | =CT, HDL, LDL, TGL | - |
| [17] | 2017 | Iran | RD, CT | T2DM, ov, CHD patients 40–85 y 30/30 | capsule | L. acidophilus, 2 × 109 L. casei, 2 × 109, B. bifidum, 2 × 109 CFU/g 800 mg inulin | 12 wk | S↓ FG | S↓ I, HOMA-B S↑ QUICKI =HOMA-IR | S↑ HDL-C =TG, TC, LDL-C, VLDL-C, TC/HDL-C ratio | - |
| [166] | 2017 | Malaysia | RD, DB, parallel-group, CT | T2DM, 30–70 y 68/68 | sachet | L. acidophilus, L. casei, L. lactis, B. bifidum, B. longum, B. infantis, 1010 CFU/d each | 12 wk | S↓ HbA1c =FG | S↓ I =HOMA-IR, QUICKI | =TC, TG, LDL-C, HDL-C | - |
| [167] | 2017 | Brazil | DB, RD, PC, CT | T2DM 35–60 y 25/25 | fermented goat milk | L. acidophilus La-5, B. lactis BB-12, 109 CFU/d each | 6 wk | S↓ FS =HbA1c, FG | = I, HOMA-IR | S↓ TC, LDL-C =HDL, VLDL, TG. CT/HLD-C ratio | - |
| [9] | 2017 | Saudi Arabia | DB, RD, CT | T2DM 30–60 y 48/46 | sachet | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19, Lactococcus lactis W58, 2.5 × 109 CFU/g | 12 wk | =FG | S↓ HOMA-IR = I | =TG, TC, HDL-C, LDL-C, TC/HDL ratio | - |
| [168] | 2017 | Iran | RD, DB, PC, CT | NAFLD patients with normal or low BMI >18 y 25/25 | capsule | L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus 2 × 108 CFU | 28 wk | S↓ FG | =HOMA-IR, I, QUICKI | =LDL-C, HDL-C, TC S↓ TG | - |
| [169] | 2018 | Taiwan | DB, RD, PC | T2DM 25–70 y 25/25/24 | capsule | ADR-1 (live L. reuteri), 4 × 109 CFU cells of ADR-3 (heat-killed L. reuteri), 2 × 1010 CFU | 24 wk | =fasting blood glucose S↓ HbA1c in liver ADR-1 =HbA1c in heat-killed ADR03, | =I, HOMA-IR | =LDL-C, free fatty acids S↓ TC in ADR-1 | - |
| [170] | 2018 | Iran | DB, RD, PC, parallel-group, CT | Prediabetes 40/40/40 35–75 y | powder | L. acidophilus, B. lactis, B. bifidum, B. longum 1 × 109 CFU/each inulin | 24 wk | S↓ FG, HbA1c | S↓ I, HOMA-IR S↑ QUICKI =HOMA-B | - | - |
| [171] | 2018 | Ukraine | DB, single center RD, CT | T2DM, ov 18–75 y 31/22 | sachet | 14 alive probiotic strains of L.+ Lactococcus, 6 × 1010 CFU/g B., 1 × 1010 CFU/g, Propionibacterium, 3 × 1010 CFU/g, Acetobacter, 1 × 106 CFU/g | 8 wk | S↓ HbA1c =FG | S↓ HOMA-IR =I | - | - |
| [172] | 2018 | Iran | RD, DB, PC, CT | T2DM, CHD 45–85 y 30/30 | capsule | L. acidophilus, B. bifidum, L. reuteri, L. fermentum 8 × 109 CFU/g | 12 wk | =FG | S↓ I, HOMA-IR S↑ QUICKI | S↑ HDL-C =LDL, TC, TG, VLDL-C | - |
| [82] | 2018 | Saudi Arabia | DB, RD, CT | T2DM, 30–60 y 30/31 | sachet | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19, Lactococcus lactis W58 2.5 × 109 CFU/g | 24 wk | S↓ FG, | S↓ I, HOMA-IR, | S↓ TC, TG, total/HDL-cholesterol ratio | - |
| [173] | 2019 | Iran | parallel-group, RD, CT | T2DM, 20/20 30–50 y | capsule | L. casei, 108 CFU/d | 8 wk | S↓ FG =HbA1c | S↓ I, HOMA-IR | - | - |
| [174] | 2019 | Iran | RD, DB, CT | T2DM 30–75 y 34/34 | capsule | L. acidophilus, 2 × 109 CFU L. casei, 7 × 109 CFU L. rhamnosus, 1.5 × 109 CFU L. bulgaricus, 2 × 108 CFU B. breve, 3 × 1010 CFU B. longum, 7 × 109 CFU S. thermophilus, 1.5 × 109 CFU 100 mg FOS | 6 wk | S↓ FG | =I, HOMA-IR | S↑ HDL-C =TG, TC | - |
| [175] | 2019 | India | RD, DB, CT | T2DM, Ob 18–65 y 39/40 | capsule | L. salivarius, L. casei, L. plantarum, L. acidophilus, B. breve, B. coagulans, 30 billion CFU 100 mg FOS | 12 wk | S↓ HbA1c =FG | =I, HOMA-IR | = TC, TG, HDL-C, LDL-C | - |
| [133] | 2019 | Belgium | RD, DB, PC, pilot study | Ob/ov insulin-resistant volunteers 18–70 y 14/13/13 | sachet | Live/pasteurized Akkermansia municiphila 1010 bacteria/day | 12 wk | =FG, HbA1c | S↑ insulin sensitivity S↓ I | S↓ TC =LDL-C, TG | =GLP-1 |
| [176] | 2020 | Australia | RD, DB, CT | T2DM BMI ≥ 25 kg/m2 ≥ 18 y 30/30 | capsule | L. plantarum, 6 × 109 CFU, L. bulgaricus, 3 × 109 CFU L. gasseri, 18 × 109 CFU B. breve, 7.5 × 109 CFU B. animalis sbsp. lactis, 8 × 109 CFU B. bifidum, 7 × 109 CFU S. thermophiles, 450 × 106 CFU Saccharomyces boulardii, 45 × 106 CFU | 12 wk | S↓ FG, HbA1c (in patients taking probiotics and metformin) | S↓ HOMA-IR (in patients taking probiotics and metformin) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. https://doi.org/10.3390/nu14010166
Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2022; 14(1):166. https://doi.org/10.3390/nu14010166
Chicago/Turabian StyleIatcu, Camelia Oana, Aimee Steen, and Mihai Covasa. 2022. "Gut Microbiota and Complications of Type-2 Diabetes" Nutrients 14, no. 1: 166. https://doi.org/10.3390/nu14010166
APA StyleIatcu, C. O., Steen, A., & Covasa, M. (2022). Gut Microbiota and Complications of Type-2 Diabetes. Nutrients, 14(1), 166. https://doi.org/10.3390/nu14010166






