Effects of Malted Rice Amazake on Constipation Symptoms and Gut Microbiota in Children and Adults with Severe Motor and Intellectual Disabilities: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Malted Rice Amazake
2.3. Investigation
2.4. Fecal Sample Collection
2.5. DNA Extraction
2.6. 16S rRNA Sequencing
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Patients Being Analyzed
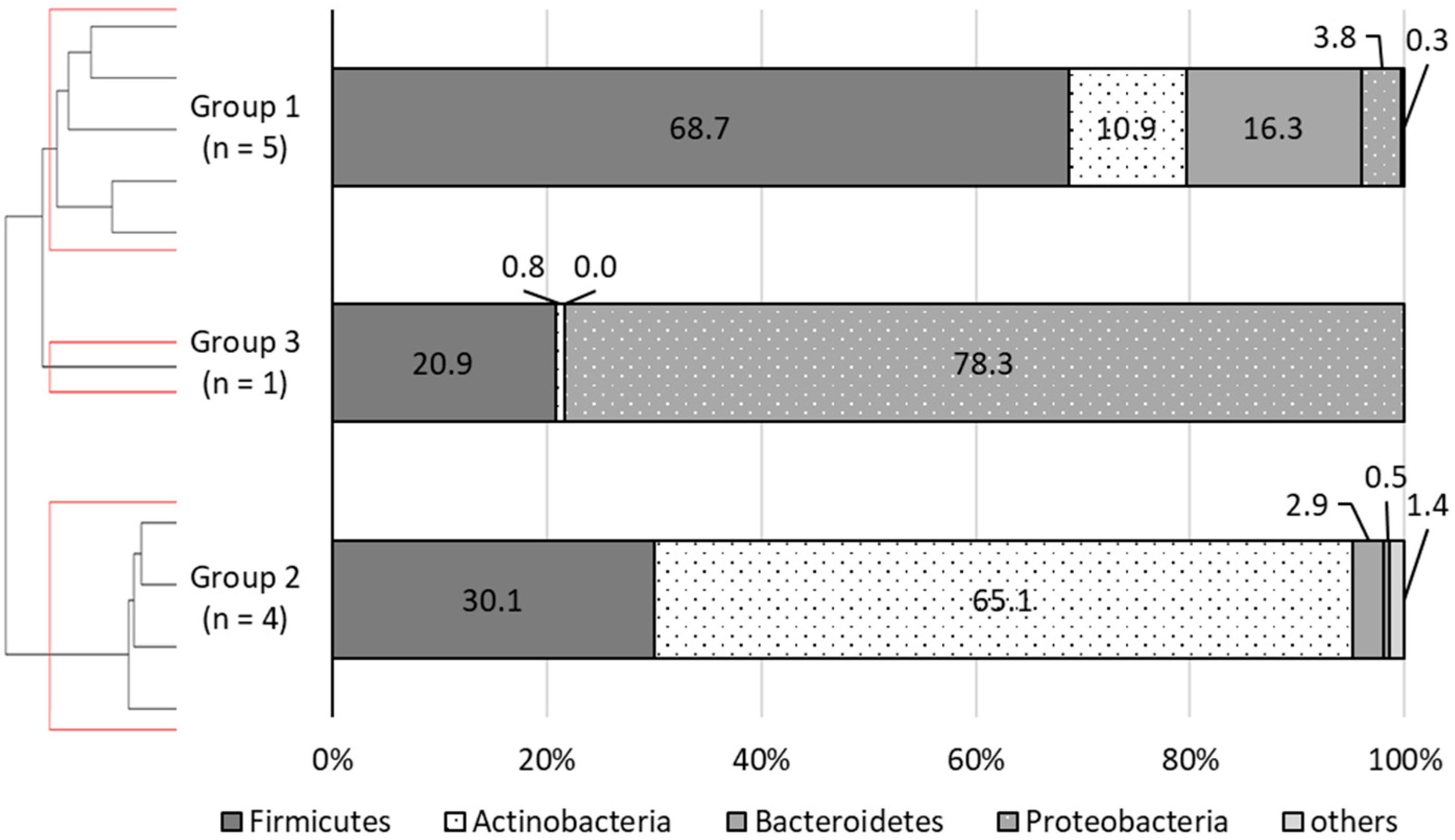
3.2. Grouping of the Patients Being Analyzed
3.3. Changes Due to the Intervention and Comparison between Groups
3.3.1. Weight, Nutrient Intake, and Prescription Drugs
3.3.2. CAS
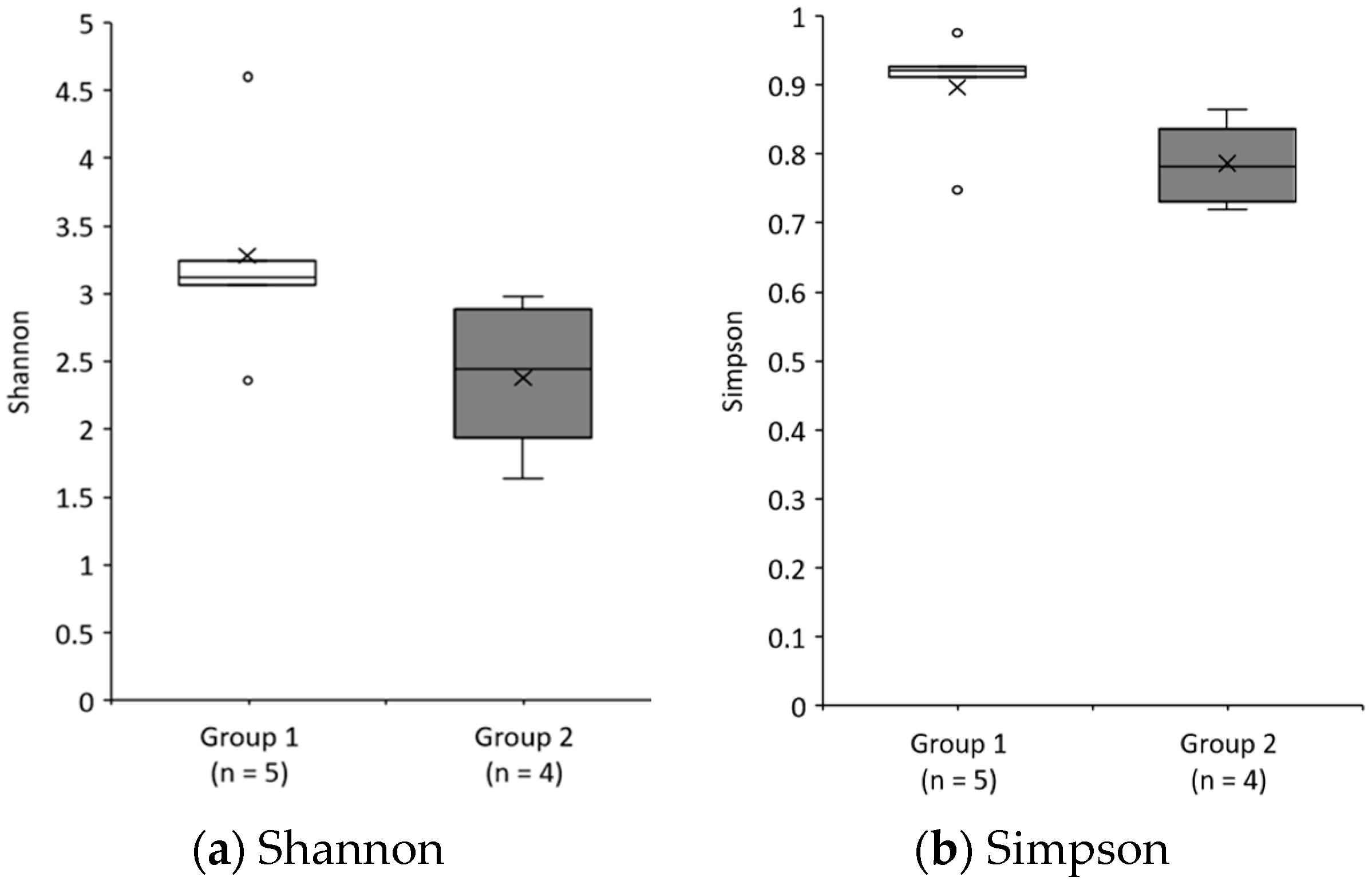
3.3.3. Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Timmeren, E.A.; van der Schans, C.P.; van der Putten, A.A.; Krijnen, W.P.; Steenbergen, H.A.; van Schrojenstein Lantman-de Valk, H.M.; Waninge, A. Physical health issues in adults with severe or profound intellectual and motor disabilities: A systematic review of cross-sectional studies. J. Intellect Disabil. Res. 2017, 61, 30–49. [Google Scholar] [CrossRef]
- Veugelers, R.; Benninga, M.A.; Calis, E.A.; Willemsen, S.P.; Evenhuis, H.; Tibboel, D.; Penning, C. Prevalence and clinical presentation of constipation in children with severe generalized cerebral palsy. Dev. Med. Child Neurol. 2010, 52, e216–e221. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, E.A.; van der Putten, A.A.; van Schrojenstein Lantman-de Valk, H.M.; van der Schans, C.P.; Waninge, A. Prevalence of reported physical health problems in people with severe or profound intellectual and motor disabilities: A cross-sectional study of medical records and care plans. J. Intellect Disabil. Res. 2016, 60, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Momma, E.; Koeda, M.; Tanabe, T.; Hoshikawa, Y.; Hoshino, S.; Kawami, N.; Kawagoe, T.; Tatsuguchi, A.; Kaise, M.; Iwakiri, K. Relationship between gastroesophageal reflux disease (GERD) and constipation: Laxative use is common in GERD patients. Esophagus 2021, 18, 152–155. [Google Scholar] [CrossRef]
- Marpole, R.; Blackmore, A.M.; Gibson, N.; Cooper, M.S.; Langdon, K.; Wilson, A.C. Evaluation and Management of Respiratory Illness in Children with Cerebral Palsy. Front Pediatr. 2020, 8, 333. [Google Scholar] [CrossRef]
- Van Timmeren, E.A.; Waninge, A.; van Schrojenstein Lantman-de, H.M.J.; van der Putten, A.A.J.; van der Schans, C.P. Patterns of multimorbidity in people with severe or profound intellectual and motor disabilities. Res. Dev. Disabil. 2017, 67, 28–33. [Google Scholar] [CrossRef]
- Ohmori, H.; Kodama, H.; Yamasaki, M.; Murata, Y.; Fukuba, H.; Matsumoto, N.; Ichikawa, M.; Takemoto, M.; Ikeda, M.; Harada, A.; et al. Fecal Microbiota and Fecal Characteristics of Patients with Severe Motor and Intellectual Disabilities Undergoing Long-Term Tube Feeding. J. Intest. Microbiol. 2013, 27, 1–6. [Google Scholar] [CrossRef]
- Ino, M.; Matsukawa, Y.; Yamaoka, Y.; Hanada, K.; Fujii, C. Prophylactic Effects of Kefir-Fermented Milk on Constipation among Mentally and Physically Handicapped Persons. J. Probiotics Health 2014, 3, 100126. [Google Scholar] [CrossRef]
- Takano, S. Diet, Exercise and Physical Therapies for Chronic Constipation. J. Japan Soc. Coloproctol. 2019, 72, 621–627. [Google Scholar] [CrossRef]
- Maki, R.; Matsukawa, M.; Matsuduka, A.; Hashinaga, M.; Anai, H.; Yamaoka, Y.; Hanada, K.; Fujii, C. Therapeutic effect of lyophilized, Kefir-fermented milk on constipation among persons with mental and physical disabilities. Jpn. J. Nurs. Sci. 2018, 15, 218–225. [Google Scholar] [CrossRef]
- Hashimoto, H.; Fujiwara, T.; Takahashi, H.; Fukui, H. Urinary tract infection in mentally and physically handicapped patients. Iryo 1991, 45, 294–297. [Google Scholar] [CrossRef]
- Kawai, M.; Setoyama, H.; Takeda, T.; Shimizu, K.; Satoh, M.; Manabe, K.; Makino, T.; Watanabe, O.; Yoshioka, M.; Nanaka, C.; et al. Effect of Fermented Milk Containing Bifidobacterium on Bowel Habits of Healthy Volunteers with Mild Constipation. J. Intest. Microbiol. 2011, 25, 181–187. [Google Scholar] [CrossRef]
- Kurahashi, A. Ingredients, Functionality, and Safety of the Japanese Traditional Sweet Drink Amazake. J. Fungi 2021, 7, 469. [Google Scholar] [CrossRef]
- Watanabe, T. lngredients in “Sake Cake” Contribute to Health and Beauty. J. Brew. Soc. Jpn. 2012, 107, 282–291. [Google Scholar] [CrossRef][Green Version]
- Yamada, H.; Hirata, H.; Ooya, M.; Matsuda, M.; Mizumoto, K.; Tsukamoto, K. Effects of millet amazake on constipation in residents of long-term care health facility. J. Hum. Nurs. Stud. 2018, 16, 35–40. [Google Scholar] [CrossRef]
- Sumiyoshi, K.; Nakao, M. Effect of Amazake Ingestion on Constipation. Jpn. J. Nurs. Art. Sci. 2017, 16, 36–40. [Google Scholar] [CrossRef]
- Inoue, R.; Ayabe, M.; Hiramatsu, S.; Sato, Y.; Ogawa, A.; Doi, M.; Syauki, A.Y.; Kageyama, S.; Seto, C.; Sumida, M.; et al. Malted rice amazake ingestion changes constipation and microbiota in independently living older adults. J. Jpn. Soc. Clin. Nutr. 2020, 42, 54–65. [Google Scholar]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Fukuda, S. Shedding Light on the Function of Gut Microbiota by Metabologenomics. J. Intest. Microbiol. 2015, 29, 145–155. [Google Scholar] [CrossRef]
- Kaneko, T.; Kohmoto, T.; Kikuchi, H.; Shiota, M.; Yatake, T.; Iino, H.; Tsuji, K. Effects of Isomaltooligosaccharides Intake on Defecation and Intestinal Environment in Healthy Volunteers. J. Home Econ. Jpn. 1993, 44, 245–254. [Google Scholar] [CrossRef]
- Kohmoto, T.; Fukui, F.; Takaku, H.; Machida, Y.; Arai, M.; Mitsuoka, T. Effect of Isomalto-oligosaccharides on Human Fecal Flora. Bifidobactria Microflora 1988, 7, 61–69. [Google Scholar] [CrossRef]
- Murata, S.; Inoue, K.; Aomatsu, T.; Yoden, A.; Tamai, H. Supplementation with carnitine reduces the severity of constipation: A retrospective study of patients with severe motor and intellectual disabilities. J. Clin. Biochem. Nutr. 2017, 60, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K. Basic issue on severe motor and intellectual disabilities. Nippon. Koshu Eisei Zasshi 1971, 35, 648–655. [Google Scholar]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.C.; Williams, F.A. Validity and reliability of the Constipation Assessment Scale. Cancer Nurs. 1989, 12, 183–188. [Google Scholar] [CrossRef]
- Inoue, R.; Kageyama, S.; Suka, T.; Kurohashi, Y.; Teramoto, K.; Ayabe, M.; Doi, M.; Syauki, A.Y.; Irie, Y. Changes in constipation symptoms associated with ingestion of malted-rice ‘amazake’ for 6 weeks in home-care patients with severe motor and intellectual disabilities. J. Child Health 2022, 81. in press. [Google Scholar]
- Hosomi, K.; Murakami, H.; Natsume-Kitatani, Y.; Tanisawa, K.; Hirata, S.; Suzuki, H.; Nagatake, T.; Nishino, T.; Mizuguchi, K.; Miyachi, M.; et al. Method for preparing DNA from feces in guanidine thiocyanate solution affects 16S rRNA-based profiling of human microbiota diversity. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Sheweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Mohsen, A.; Park, J.; Kawashima, H.; Chen, Y.A.; Natsume-Kitatani, Y.; Mizuguchi, K. Auto-q Qiime analysis automating script. Zenodo 2018, 10, 1. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Bezek, K.; Petelin, A.; Praznikar, J.; Nova, E.; Redondo, N.; Marcos, A.; Praznikar, Z.J. Obesity Measures and Dietary Parameters as Predictors of Gut Microbiota Phyla in Healthy Individuals. Nutrients 2020, 12, 2695. [Google Scholar] [CrossRef]
- Mori, S.; Tanaka, Y.; Watabe, K.; Yamada, M.; Morita, M.; Matsuo, T. Amazake Using the Lees and Rice Koji Promotes Regular Bowel Movements—A Randomized, Placebo-controlled Parallel-group Comparison Study. Jpn. Pharmacol. Ther. 2019, 47, 759–765. [Google Scholar]
- Johanson, J.F.; Sonnenberg, A.; Koch, T.R.; Mccarty, D.J. Association of constipation with neurologic diseases. Dig. Dis. Sci. 1992, 37, 179–186. [Google Scholar] [CrossRef]
- Romano, C.; van Wynckel, M.; Hulst, J.; Broekaert, I.; Bronsky, J.; Dall’Oglio, L.; Mis, N.F.; Hojsak, I.; Orel, R.; Papadopoulou, A.; et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children with Neurological Impairment. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 242–264. [Google Scholar] [CrossRef]
- Yen, C.H.; Tseng, Y.H.; Kuo, Y.W.; Lee, M.C.; Chen, H.L. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people-A placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 445–450. [Google Scholar] [CrossRef]
- Mitsuoka, T. Prebiotics and Intestinal Flora. Biosci. Microflora 2002, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Kamath, P.S.; Phillips, S.F.; Zinsmeister, A.R. Short-Chain Fatty Acids Stimulate Ileal Motility in Humans. Gastroenterology 1988, 95, 1496–1502. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dsfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Pindo, M.; Renzi, D.; et al. Altered gut microbiota in Rett syndrome. Microbiome 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Bentley, R.; Meganathan, R. Biosynthesis of Vitamin K (Menaquinone) in Bacteria. Microbiol. Rev. 1982, 46, 241–280. [Google Scholar] [CrossRef] [PubMed]
- Hudault, S.; Guignot, J.; Servin, A.L. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 2001, 49, 47–55. [Google Scholar] [CrossRef]
- Reid, G.; Howard, J.; Gan, B.S. Can bacterial interference prevent infection? Trends Microbiol. 2001, 9, 424–428. [Google Scholar] [CrossRef]
- Watanabe, K. Contribution of gut microbiota to the etiology of human diseases outside of the gut. Mod. Media 2014, 60, 356–368. [Google Scholar]
- Ge, X.; Zhao, W.; Ding, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Furuta, A.; Suzuki, Y.; Takahashi, R.; Jakobsen, B.P.; Kimura, T.; Egawa, S.; Yoshimura, N. Clinical Medicine Effects of Transanal Irrigation on Gut Microbiota in Pediatric Patients with Spina Bifida. J. Clin. Med. 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.; Yao, X.; Xu, L.; Zhou, Y.; Zhang, L.; Liu, Y.; Pei, S.; Zhou, C. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb. Cell Fact. 2021, 20, 98. [Google Scholar] [CrossRef]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, e1875796. [Google Scholar] [CrossRef] [PubMed]
- Morello, W.; D’ Amico, F.; Serafinelli, J.; Turroni, S.; Abati, I.; Fiori, J.; Baskin, E.; Yalcinkaya, F.; Jankauskiene, A.; Pennesi, M.; et al. Low-Dose Antibiotic Prophylaxis Induces Rapid Modifications of the Gut Microbiota in Infants with Vesicoureteral Reflux. Front. Pediatr. 2021, 9, 674716. [Google Scholar] [CrossRef]


| Component | Amount per 35 g/Day |
|---|---|
| Energy | 76.7 kcal |
| Protein | 1.2 g |
| Fat | 0.1 g |
| Carbohydrate | 18.0 g |
| Sugars | 17.4 g |
| Isomaltose | 0.83 g |
| Panose | 0.07 g |
| Isomaltotriose | 0.06 g |
| Soluble dietary fiber | 0.1 g |
| Insoluble dietary fiber | 0.5 g |
| Sodium | 10.1 mg |
| Water | 15.7 g |
| Ash | 0.035 g |
| 1 Sex | Age (yr.) | 2 Ht (m) | 2 Wt (kg) | 3 H/A (<18yr.) | 3 W/H (<18yr.) | 4 BMI (kg/m2) | 5 Motor Function | 6 Intake Method | Energy Intake (kcal/d.) | Fiber Intake (g/d.) | Weaning Experience | Diagnosis | 7 Group | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 11 | 1.19 | 19.2 | 82.9 | 85.3 | 13.5 | Gait disturbance | Oral | 1676 | 8.9 | Yes | 21st monosomy | 1 |
| 2 | F | 11 | 1.12 | 15.1 | 77.8 | 79.5 | 12.0 | Bedridden | Nasal, oral | 2099 | 18.6 | Yes | Leuko- dystrophy | 2 |
| 3 | M | 13 | 1.27 | 21.0 | 80.9 | 80.8 | 13.0 | Bedridden | Oral | 642 | 5.8 | Yes | Cerebral palsy | 1 |
| 4 | F | 13 | 1.18 | 14.6 | 76.6 | 66.4 | 10.5 | Bedridden | Gastro | 1724 | 0 | No | Cerebral palsy | 2 |
| 5 | M | 15 | 1.28 | 15.6 | 76.6 | 58.9 | 9.5 | Bedridden | Gastro | 600 | 0 | Yes | Sequelae of encephalitis | 3 |
| 6 | M | 16 | 1.50 | 38.0 | 88.5 | 91.6 | 16.9 | Bedridden | Nasal | 750 | 0 | Yes | Tuberous sclerosis | 1 |
| 7 | F | 18 | 1.29 | 26.0 | - | - | 15.6 | Bedridden | Gastro | 800 | 0 | No | Sequelae of encephalitis | 2 |
| 8 | M | 19 | 1.58 | 43.0 | - | - | 17.2 | Gait disturbance | Oral | 1136 | 11.9 | Yes | Cerebral palsy | 1 |
| 9 | M | 20 | 1.30 | 25.0 | - | - | 14.8 | Bedridden | Gastro | 900 | 0 | Yes | Cerebral palsy | 2 |
| 10 | F | 28 | 1.37 | 35.0 | - | - | 18.6 | Sit with support | Nasal | 900 | 10.5 | Yes | Rett syndrome | 1 |
| Sex (Male) | Age (yr.) | 1 Ht (m) | 1 Wt (kg) | 2 BMI (kg/m2) | Motor Function (n) | 3 Intake Method (n) | Weaning Experience (n) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedridden | Sit with Support & Gait Disturbance | Oral | Nasal | Gastro | Yes | No | ||||||
| Group 1 (n = 5) | 3 | 17.4 ± 3.0 | 1.38 ± 0.07 | 31.2 ± 4.7 | 15.8 ± 1.1 | 2 | 3 | 3 | 2 | 0 | 5 | 0 |
| Group 2 (n = 4) | 1 | 15.5 ± 2.1 | 1.22 ± 0.04 | 20.2 ± 3.1 | 13.2 ± 1.2 | 4 | 0 | 0 | 1 | 3 | 2 | 2 |
| p value | a 0.294 | b 0.905 | b 0.190 | b 0.190 | b 0.190 | a 0.058 | a 0.043 * | a 0.073 | ||||
| Types of Prescription Drugs (Number of Types) | Overall (n = 10) | Group 1 (n = 5) | Group 2 (n = 4) | Group 1 vs. 2 b p Value | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| Laxative | 0.6 ± 0.3 | 0.7 ± 0.3 | 1.2 ± 0.6 | 1.4 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.190 |
| a p = 0.317 | a p = 0.317 | a p = 1.000 | |||||
| Intestinal peristalsis promoter | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.2 ± 0.2 | 0.2 ± 0.2 | 1.5 ± 0.9 | 1.5 ± 0.9 | 0.190 |
| a p = 1.000 | a p = 1.000 | a p = 1.000 | |||||
| Intestinal flora balance improving drug | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.413 |
| a p = 1.000 | a p = 1.000 | a p = 1.000 | |||||
| Anticonvulsant, Antiepileptic drug | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.2 ± 1.3 | 2.2 ± 1.3 | 2.8 ± 0.6 | 2.8 ± 0.6 | 0.413 |
| a p = 1.000 | a p = 1.000 | a p = 1.000 | |||||
| Muscle relaxant | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.8 ± 0.8 | 1.8 ± 0.8 | 0.016 * |
| a p = 0.317 | a p = 1.000 | a p = 1.000 | |||||
| Gastric acid secretion inhibitor | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.3 ± 0.3 | 1.000 |
| a p = 0.317 | a p = 0.317 | a p = 1.000 | |||||
| Antibiotics | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.000 |
| a p = 1.000 | a p = 1.000 | a p = 1.000 | |||||
| [Side effect] Constipation | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.2 ± 0.2 | 0.4 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.413 |
| a p = 1.000 | a p = 0.317 | a p = 1.000 | |||||
| [Side effect] Diarrhea | 0.7±0.2 | 0.7±0.2 | 0.2 ± 0.2 | 0.4 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.190 |
| a p = 1.000 | a p = 0.317 | a p = 1.000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, S.; Inoue, R.; Hosomi, K.; Park, J.; Yumioka, H.; Suka, T.; Kurohashi, Y.; Teramoto, K.; Syauki, A.Y.; Doi, M.; et al. Effects of Malted Rice Amazake on Constipation Symptoms and Gut Microbiota in Children and Adults with Severe Motor and Intellectual Disabilities: A Pilot Study. Nutrients 2021, 13, 4466. https://doi.org/10.3390/nu13124466
Kageyama S, Inoue R, Hosomi K, Park J, Yumioka H, Suka T, Kurohashi Y, Teramoto K, Syauki AY, Doi M, et al. Effects of Malted Rice Amazake on Constipation Symptoms and Gut Microbiota in Children and Adults with Severe Motor and Intellectual Disabilities: A Pilot Study. Nutrients. 2021; 13(12):4466. https://doi.org/10.3390/nu13124466
Chicago/Turabian StyleKageyama, Suzumi, Rikako Inoue, Koji Hosomi, Jonguk Park, Hitomi Yumioka, Tomo Suka, Yoshihiro Kurohashi, Kazuaki Teramoto, A. Yasmin Syauki, Miki Doi, and et al. 2021. "Effects of Malted Rice Amazake on Constipation Symptoms and Gut Microbiota in Children and Adults with Severe Motor and Intellectual Disabilities: A Pilot Study" Nutrients 13, no. 12: 4466. https://doi.org/10.3390/nu13124466
APA StyleKageyama, S., Inoue, R., Hosomi, K., Park, J., Yumioka, H., Suka, T., Kurohashi, Y., Teramoto, K., Syauki, A. Y., Doi, M., Sakaue, H., Mizuguchi, K., Kunisawa, J., & Irie, Y. (2021). Effects of Malted Rice Amazake on Constipation Symptoms and Gut Microbiota in Children and Adults with Severe Motor and Intellectual Disabilities: A Pilot Study. Nutrients, 13(12), 4466. https://doi.org/10.3390/nu13124466






