Nutritional Therapy in Persons Suffering from Psoriasis
Abstract
1. Introduction
- physical factors (X-rays, subcutaneous and intradermal injections, surgical procedures, vaccinations, tattoos, insect bites, abrasions, burns (including sunburns), acupuncture, UV irradiation);
- chemical factors (chemical burns, topical treatments, others);
- skin diseases (rosacea, fungal infections, allergic contact dermatitis);
- infections (mainly streptococcal pharyngitis, viral infections);
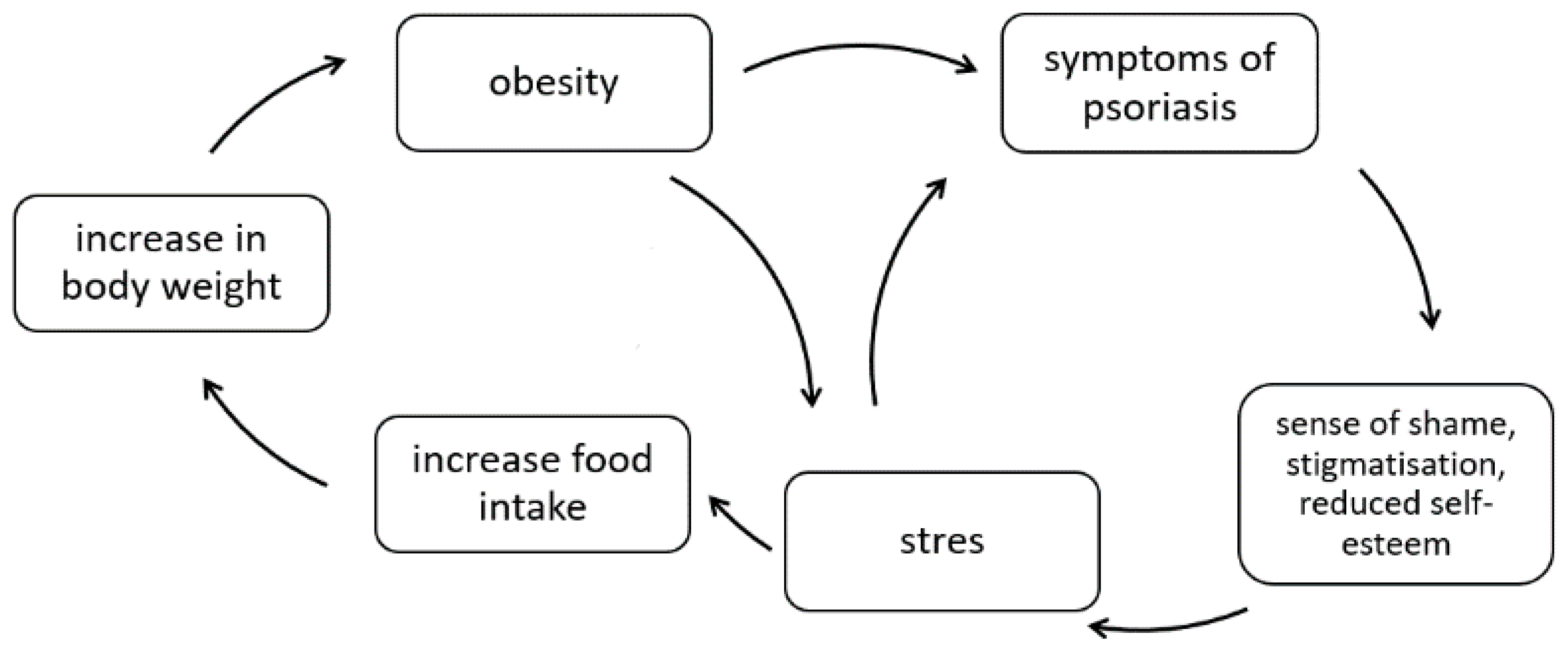
- stress;
- medications (β-adrenolytics, angiotensin-converting enzyme inhibitors, lithium, terbinafine, nonsteroidal anti-inflammatory drugs, anti-malarial drugs, tetracyclines, rapid withdrawal of systemic corticosteroids);
- diet;
- tobacco smoking;
- alcohol consumption.
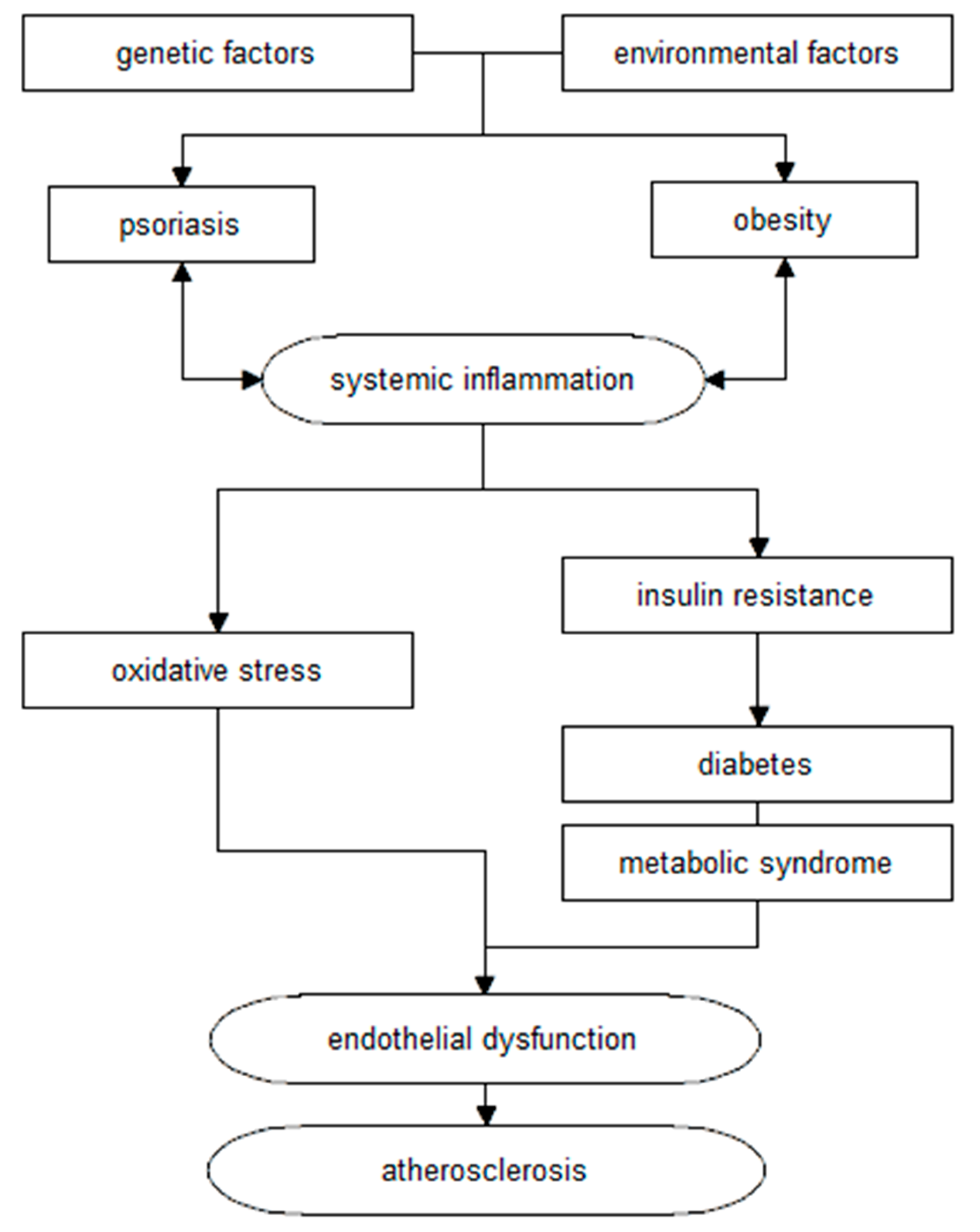
2. Metabolic Syndrome
3. Obesity
- body weight assessment;
- BMI assessment;
- assessment of waist/hip ratio (WHR);
- fasting blood glucose determination at least once a year;
- more frequent testing for hypertension;
- determination of serum lipids;
- determination of serum uric acid and liver enzymes;
- in patients with other cardiovascular risk factors (e.g., hypertension, obesity), performing an Oral Glucose Tolerance Test (OGTT).
4. Low-Energy Diet in the Treatment of Psoriasis
5. Selection of Fatty Acids
6. Selection of Carbohydrate Products
7. The Importance of Antioxidants
8. The Importance of Vitamin D3
9. Use of Seaweed in Psoriasis
10. Coffee and Psoriasis
11. Alternative Diets in the Treatment of Psoriasis
11.1. Gluten-Free Diet
11.2. Vegetarian Diet
11.3. Mediterranean Diet
- products with antioxidant and anti-inflammatory effects (fruit, vegetables, red wine, natural herbs);
- unsaturated fatty acids (fish, olive oil, nuts);
- products that are a source of dietary fibre;
- products that are a source of vitamins and minerals;
- and low consumption of:
- products that are sources of saturated fat;
- simple carbohydrates;
- highly processed products.
11.4. Ketogenic Diet
12. Diet Therapy and the Use of Medicines
13. Alcohol and Intensification of Psoriatic Lesions
14. Conclusions
15. Limitations of This Manuscript
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tupikowska, M.; Zdrojowy-Welna, A.; Maj, J. Psoriasis as metabolic and cardiovascular risk factor. Pol. Merkur Lek. 2014, 37, 124–127. [Google Scholar]
- WHO. Global Report on Psoriasis. Available online: https://apps.who.int/iris/handle/10665/204417 (accessed on 2 October 2021).
- Zuccotti, E.; Oliveri, M.; Girometta, C.; Ratto, D.; Di Iorio, C.; Occhinegro, A.; Rossi, P. Nutritional strategies for psoriasis: Current scientific evidence in clinical trials. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8537–8551. [Google Scholar] [CrossRef] [PubMed]
- Placek, W.; Mieszczak-Woszczyna, D. Dieta w schorzeniach dermatologicznych (II). Znaczenie kwasów omega-3 w leczeniu łuszczycy. Dermatol. Estet. 2011, 13, 125–131. [Google Scholar]
- Szczerkowska-Dobosz, A.; Komorowska, O. Łuszczyca i miażdżyca—Związek nieprzypadkowy. Dermatol. Dypl. 2014, 5, 18–23. [Google Scholar]
- Trojacka, E.; Zaleska, M.; Galus, R. Influence of exogenous and endogenous factors on the course of psoriasis. Pol. Merkur Lek. 2015, 38, 169–173. [Google Scholar]
- Holmannova, D.; Borska, L.; Andrys, C.; Borsky, P.; Kremlacek, J.; Hamakova, K.; Rehacek, V.; Malkova, A.; Svadlakova, T.; Palicka, V.; et al. The Impact of Psoriasis and Metabolic Syndrome on the Systemic Inflammation and Oxidative Damage to Nucleic Acids. J. Immunol. Res. 2020, 2020, 7352637. [Google Scholar] [CrossRef] [PubMed]
- Polic, M.V.; Miskulin, M.; Smolic, M.; Kralik, K.; Miskulin, I.; Berkovic, M.C.; Curcic, I.B. Psoriasis Severity-A Risk Factor of Insulin Resistance Independent of Metabolic Syndrome. Int. J. Environ. Res. Public. Health 2018, 15, 1486. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef]
- Ni, C.; Chiu, M.W. Psoriasis and comorbidities: Links and risks. Clin. Cosmet. Investig. Dermatol. 2014, 7, 119–132. [Google Scholar] [CrossRef]
- Baran, A.; Kiluk, P.; Mysliwiec, H.; Flisiak, I. The role of lipids in psoriasis. Prz. Dermatol. 2017, 104, 619–635. [Google Scholar] [CrossRef]
- Gupta, S.; Syrimi, Z.; Hughes, D.M.; Zhao, S.S. Comorbidities in psoriatic arthritis: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 275–284. [Google Scholar] [CrossRef]
- Choudhary, S.; Pradhan, D.; Pandey, A.; Khan, M.K.; Lall, R.; Ramesh, V.; Puri, P.; Jain, A.K.; Thomas, G. The Association of Metabolic Syndrome and Psoriasis: A Systematic Review and Meta-Analysis of Observational Study. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 703–717. [Google Scholar] [CrossRef]
- Antosik, K.; Krzęcio-Nieczyporuk, E.; Kurowska-Socha, B. Diet and nutrition in psoriasis treatment. Hyg. Pub. Health 2017, 52, 131–137. [Google Scholar]
- Gisondi, P.; Fostini, A.; Fossa, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018, 36, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Seminara, N.M.; Shin, D.B.; Troxel, A.B.; Kimmel, S.E.; Mehta, N.N.; Margolis, D.J.; Gelfand, J.M. Prevalence of metabolic syndrome in patients with psoriasis: A population-based study in the United Kingdom. J. Investig. Dermatol. 2012, 132, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. 2019, 24, 890–934. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef]
- Galluzzo, M.; Talamonti, M.; Perino, F.; Servoli, S.; Giordano, D.; Chimenti, S.; De Simone, C.; Peris, K. Bioelectrical impedance analysis to define an excess of body fat: Evaluation in patients with psoriasis. J. Dermatol. Treat. 2017, 28, 299–303. [Google Scholar] [CrossRef]
- Diniz, M.S.; Bavoso, N.C.; Kakehasi, A.M.; Lauria, M.W.; Soares, M.M.; Pinto, J.M. Assessment of adiposity in psoriatic patients by dual energy X-ray absorptiometry compared to conventional methods. An. Bras. Dermatol. 2016, 91, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Blake, T.; Gullick, N.J.; Hutchinson, C.E.; Barber, T.M. Psoriatic disease and body composition: A systematic review and narrative synthesis. PLoS ONE 2020, 15, e0237598. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Macchia, P.E.; Di Somma, C.; Napolitano, M.; Balato, A.; Falco, A.; Savanelli, M.C.; Balato, N.; Colao, A.; Savastano, S. Bioelectrical phase angle and psoriasis: A novel association with psoriasis severity, quality of life and metabolic syndrome. J. Transl. Med. 2016, 14, 130. [Google Scholar] [CrossRef]
- Budu-Aggrey, A.; Brumpton, B.; Tyrrell, J.; Watkins, S.; Modalsli, E.H.; Celis-Morales, C.; Ferguson, L.D.; Vie, G.; Palmer, T.; Fritsche, L.G.; et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PLoS Med. 2019, 16, e1002739. [Google Scholar] [CrossRef] [PubMed]
- Sahi, F.M.; Masood, A.; Danawar, N.A.; Mekaiel, A.; Malik, B.H. Association between Psoriasis and Depression: A Traditional Review. Cureus 2020, 12, e9708. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Balbás, G.M.; Regaña, M.S.; Millet, P.U. Study on the use of omega-3 fatty acids as a therapeutic supplement in treatment of psoriasis. Clin. Cosmet. Investig. Dermatol. 2011, 4, 73–77. [Google Scholar] [CrossRef]
- Barrea, L.; Nappi, F.; Di Somma, C.; Savanelli, M.C.; Falco, A.; Balato, A.; Balato, N.; Savastano, S. Environmental Risk Factors in Psoriasis: The Point of View of the Nutritionist. Int. J. Environ. Res. Public Health 2016, 13, 743. [Google Scholar] [CrossRef]
- Gołąbek, K.; Regulska-Ilow, B. Dietary support of pharmacological psoriasis treatment. Hyg. Pub. Health 2017, 52, 335–342. [Google Scholar]
- Jensen, P.; Zachariae, C.; Christensen, R.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Effect of weight loss on the severity of psoriasis: A randomized clinical study. JAMA Dermatol. 2013, 149, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Christensen, R.; Zachariae, C.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: A prospective observational follow-up study. Am. J. Clin. Nutr. 2016, 104, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Del Giglio, M.; Di Francesco, V.; Zamboni, M.; Girolomoni, G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: A randomized, controlled, investigator-blinded clinical trial. Am. J. Clin. Nutr. 2008, 88, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Wasiluk, D.; Ostrowska, L.; Stefańska, E. Can an adequate diet be helpful in the treatment of psoriasis vulgaris? Med. Og. Nauki. Zdr. 2012, 18, 405–408. [Google Scholar]
- Sicinska, P.; Pytel, E.; Kurowska, J.; Koter-Michalak, M. Supplementation with omega fatty acids in various diseases. Postepy Hig. I Med. Dosw. 2015, 69, 838–852. [Google Scholar] [CrossRef]
- Millsop, J.W.; Bhatia, B.K.; Debbaneh, M.; Koo, J.; Liao, W. Diet and psoriasis, part III: Role of nutritional supplements. J. Am. Acad. Dermatol. 2014, 71, 561–569. [Google Scholar] [CrossRef]
- Adil, M.; Singh, P.; Maheshwari, K. Clinical evaluation of omega-3 fatty acids in psoriasis. Prz. Dermatol. 2017, 104, 314–323. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Purzycka-Bohdan, D.; Nedoszytko, B.; Reich, A.; Szczerkowska-Dobosz, A.; Bartosinska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.; Dobrucki, L.; et al. Pathogenesis of psoriasis in the “omic” era. Part III. Metabolic disorders, metabolomics, nutrigenomics in psoriasis. Postepy Dermatol. I Alergol. 2020, 37, 452–467. [Google Scholar] [CrossRef]
- Ashcroft, F.J.; Mahammad, N.; Midtun Flatekvål, H.; Jullumstrø Feuerherm, A.; Johansen, B. cPLA2α Enzyme Inhibition Attenuates Inflammation and Keratinocyte Proliferation. Biomolecules 2020, 10, 1402. [Google Scholar] [CrossRef]
- Shao, S.; Chen, J.; Swindell, W.R.; Tsoi, L.C.; Xing, X.; Ma, F.; Uppala, R.; Sarkar, M.K.; Plazyo, O.; Billi, A.C.; et al. Phospholipase A2 enzymes represent a shared pathogenic pathway in psoriasis and pityriasis rubra pilaris. JCI Insight 2021, 6, e151911. [Google Scholar] [CrossRef]
- Barrea, L.; Macchia, P.E.; Tarantino, G.; Di Somma, C.; Pane, E.; Balato, N.; Napolitano, M.; Colao, A.; Savastano, S. Nutrition: A key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J. Transl. Med. 2015, 13, 303. [Google Scholar] [CrossRef]
- Chen, X.; Hong, S.; Sun, X.; Xu, W.; Li, H.; Ma, T.; Zheng, Q.; Zhao, H.; Zhou, Y.; Qiang, Y.; et al. Efficacy of fish oil and its components in the management of psoriasis: A systematic review of 18 randomized controlled trials. Nutr. Rev. 2020, 78, 827–840. [Google Scholar] [CrossRef]
- Mendivil, C.O. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Front. Nutr. 2020, 7, 617652. [Google Scholar] [CrossRef] [PubMed]
- Ingkapairoj, K.; Chularojanamontri, L.; Chaiyabutr, C.; Silpa-Archa, N.; Wongpraparut, C.; Bunyaratavej, S. Dietary habits and perceptions of psoriatic patients: Mediterranean versus Asian diets. J. Dermatol. Treat. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Chi, C.C. Effects of fish oil supplement on psoriasis: A meta-analysis of randomized controlled trials. BMC Complement. Altern. Med. 2019, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Mieczan, T.; Wójcik, G. Importance of Redox Equilibrium in the Pathogenesis of Psoriasis-Impact of Antioxidant-Rich Diet. Nutrients 2020, 12, 1841. [Google Scholar] [CrossRef]
- Ratajczak, M.; Gietka-Czernel, M. The influence of selenium to human health. Post N Med. 2016, 29, 929–933. [Google Scholar]
- Janda, K.; Kasprzak, M.; Wolska, J. Vitamin C—Structure, properties, occurrence and functions. Pomeranian J. Life Sci. 2015, 61, 419–425. [Google Scholar]
- Zalega, J.; Szostak-Węgierek, D. Nutrition in cancer prevention. Part II. Minerals, vitamins, polyunsaturated fatty acids, probiotics, prebiotics. Probl. Hig. Epidemiol. 2013, 94, 50–58. [Google Scholar]
- Halamek, D. Anti-aging properties of vitamin D. Acad. Aesthet. Anti-Aging Med. 2016, 1, 30–44. [Google Scholar]
- Wu, Q.; Xu, Z.; Dan, Y.L.; Zhao, C.N.; Mao, Y.M.; Liu, L.N.; Pan, H.F. Seasonality and global public interest in psoriasis: An infodemiology study. Postgrad. Med. J. 2020, 96, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermato-Endocrinol. 2013, 5, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Gaál, J.; Lakos, G.; Szodoray, P.; Kiss, J.; Horváth, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta. Dermatol. Venereol. 2009, 89, 140–144. [Google Scholar] [CrossRef][Green Version]
- Tajjour, R.; Baddour, R.; Redwan, F.; Hassan, F. The relationship between psoriasis and serum levels of vitamin D. JAMMR 2018, 26, 1–12. [Google Scholar] [CrossRef]
- Faraji, S.; Alizadeh, M. Mechanistic Effects of Vitamin D Supplementation on Metabolic Syndrome Components in Patients with or without Vitamin D Deficiency. J. Obes. Metab. Syndr. 2020, 29, 270–280. [Google Scholar] [CrossRef]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012, 10, 2813. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, K.; Abe, S.; Yamada, K.; Suzuki, M.; Masahisa, M.; Endo, M.; Abe, K.; Inoue, R.; Hoshi, H. Improvement of Psoriasis by Alteration of the Gut Environment by Oral Administration of Fucoidan from. Mar. Drugs 2020, 18, 154. [Google Scholar] [CrossRef]
- Codoñer, F.M.; Ramírez-Bosca, A.; Climent, E.; Carrión-Gutierrez, M.; Guerrero, M.; Pérez-Orquín, J.M.; Horga de la Parte, J.; Genovés, S.; Ramón, D.; Navarro-López, V.; et al. Gut microbial composition in patients with psoriasis. Sci. Rep. 2018, 8, 3812. [Google Scholar] [CrossRef]
- Eppinga, H.; Sperna Weiland, C.J.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium prausnitzii in Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J. Crohn’s Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef]
- Huang, L.; Gao, R.; Yu, N.; Zhu, Y.; Ding, Y.; Qin, H. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci. China Life Sci. 2019, 62, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2018, 27, 144–149. [Google Scholar] [CrossRef]
- Koper, M.; Wozniacka, A.; Robak, E. The intestinal microbiota in psoriasis. Postepy Hig. I Med. Dosw. 2020, 74, 236–246. [Google Scholar] [CrossRef]
- Szabo-Fodor, J.; Bonai, A.; Bota, B.; Egyed, L.; Lakatos, F.; Papai, G.; Zsolnai, A.; Glavits, R.; Horvatovich, K.; Kovacs, M. Physiological Effects of Whey- and Milk-Based Probiotic Yogurt in Rats. Pol. J. Microbiol. 2017, 66, 483–490. [Google Scholar] [CrossRef][Green Version]
- Kariyawasam, K.M.G.M.; Lee, N.K.; Paik, H.D. Fermente.ed dairy products as delivery vehicles of novel probiotic strains isolated from traditional fermented Asian foods. J. Food Sci. Technol. 2021, 58, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; Agüera-Santos, J.; Navarro-Moratalla, L. Probiotics in the Therapeutic Arsenal of Dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Dermatol. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- Dalheim, L.; Svenning, J.B.; Olsen, R.L. In vitro intestinal digestion of lipids from the marine diatom Porosira glacialis compared to commercial LC n-3 PUFA products. PLoS ONE 2021, 16, e0252125. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Gargan, C.; Hayes, M.; Bastiaens, L. Nutritional Profiling and Preliminary Bioactivity Screening of Five Micro-Algae Strains Cultivated in Northwest Europe. Foods 2021, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.J.; Black, L.J.; Sherriff, J.L.; Dunlop, E.; Strobel, N.; Lucas, R.M.; Bornman, J.F. Vitamin D Content of Australian Native Food Plants and Australian-Grown Edible Seaweed. Nutrients 2018, 10, 876. [Google Scholar] [CrossRef]
- Göring, H. Vitamin D in Nature: A Product of Synthesis and/or Degradation of Cell Membrane Components. Biochemistry 2018, 83, 1350–1357. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Brenden, H.; Felsner, I.; Aue, N.; Brynjolfsdottir, A.; Krutmann, J. Blue Lagoon Algae Improve Uneven Skin Pigmentation: Results from in vitro Studies and from a Monocentric, Randomized, Double-Blind, Vehicle-Controlled, Split-Face Study. Ski. Pharm. Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Annunziata, G.; Megna, M.; Falco, A.; Balato, A.; Colao, A.; Savastano, S. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018, 92, 1831–1845. [Google Scholar] [CrossRef]
- Baspinar, B.; Eskici, G.; Ozcelik, A.O. How coffee affects metabolic syndrome and its components. Food Funct. 2017, 8, 2089–2101. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Ambrósio, A.F.; Santiago, A.R. Having a Coffee Break: The Impact of Caffeine Consumption on Microglia-Mediated Inflammation in Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 4761081. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76, 626–636. [Google Scholar] [CrossRef]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. Associations between coffee consumption and inflammatory markers in healthy persons: The ATTICA study. Am. J. Clin. Nutr. 2004, 80, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, J.; Qureshi, A.A. No association between coffee and caffeine intake and risk of psoriasis in US women. Arch. Dermatol. 2012, 148, 395–397. [Google Scholar] [CrossRef][Green Version]
- Favari, C.; Righetti, L.; Tassotti, M.; Gethings, L.A.; Martini, D.; Rosi, A.; Antonini, M.; Rubert, J.; Manach, C.; Dei Cas, A.; et al. Metabolomic Changes after Coffee Consumption: New Paths on the Block. Mol. Nutr. Food Res. 2021, 65, e2000875. [Google Scholar] [CrossRef]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Kittanamongkolchai, W. Psoriasis and Risk of Celiac Disease: A Systematic Review and Meta-analysis. Indian J. Dermatol. 2017, 62, 41–46. [Google Scholar] [CrossRef]
- Bhatia, B.K.; Millsop, J.W.; Debbaneh, M.; Koo, J.; Linos, E.; Liao, W. Diet and psoriasis, part II: Celiac disease and role of a gluten-free diet. J. Am. Acad. Dermatol. 2014, 71, 350–358. [Google Scholar] [CrossRef]
- Dhattarwal, N.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Yadav, R.S.; Sharma, S.B.; Sharma, A.; Sharma, R.; Rana, A.; Sondhi, M. The association of anti-gliadin and anti-transglutaminase antibodies and chronic plaque psoriasis in Indian patients: Preliminary results of a descriptive cross-sectional study. Australas. J. Dermatol. 2020, 61, e378–e382. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Dominguez, P.L.; Choi, H.K.; Han, J.; Curhan, G. Alcohol intake and risk of incident psoriasis in US women: A prospective study. Arch. Dermatol. 2010, 146, 1364–1369. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The risk of stroke in patients with psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef]
- Korovesi, A.; Dalamaga, M.; Kotopouli, M.; Papadavid, E. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: A cross-sectional observational study. Int. J. Dermatol. 2019, 58, e164–e165. [Google Scholar] [CrossRef]
- Phan, C.; Touvier, M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Wolkenstein, P.; Chosidow, O.; Ezzedine, K.; Sbidian, E. Association Between Mediterranean Anti-inflammatory Dietary Profile and Severity of Psoriasis: Results From the NutriNet-Santé Cohort. JAMA Dermatol. 2018, 154, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, A.; Cuenca-Barrales, C.; Vega-Castillo, J.J.; Ruiz-Carrascosa, J.C.; Ruiz-Villaverde, R. Adherence to Mediterranean diet in Spanish patients with psoriasis: Cardiovascular benefits? Dermatol. Ther. 2019, 32, e12810. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Navarini, L.; Carubbi, F.; Picchianti-Diamanti, A.; Chimenti, M.S.; Tasso, M.; Currado, D.; Ruscitti, P.; Ciccozzi, M.; Annarumma, A.; et al. Mediterranean diet and Psoriatic Arthritis activity: A multicenter cross-sectional study. Rheumatol. Int. 2020, 40, 951–958. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Honda, T.; Adachi, A.; Nagatake, T.; Kunisawa, J.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nomura, T.; Ginhoux, F.; et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci. Rep. 2017, 7, 14076. [Google Scholar] [CrossRef]
- Locker, F.; Leitner, J.; Aminzadeh-Gohari, S.; Weber, D.D.; Sanio, P.; Koller, A.; Feichtinger, R.G.; Weiss, R.; Kofler, B.; Lang, R. The Influence of Ketogenic Diets on Psoriasiform-Like Skin Inflammation. J. Investig. Dermatol. 2020, 140, 707–710.e7. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Megna, M.; Cacciapuoti, S.; Frias-Toral, E.; Fabbrocini, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: An update for dermatologists and nutritionists. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef]
- Castaldo, G.; Pagano, I.; Grimaldi, M.; Marino, C.; Molettieri, P.; Santoro, A.; Stillitano, I.; Romano, R.; Montoro, P.; D’Ursi, A.M.; et al. Effect of Very-Low-Calorie Ketogenic Diet on Psoriasis Patients: A Nuclear Magnetic Resonance-Based Metabolomic Study. J. Proteome Res. 2021, 20, 1509–1521. [Google Scholar] [CrossRef]
- Castaldo, G.; Rastrelli, L.; Galdo, G.; Molettieri, P.; Rotondi Aufiero, F.; Cereda, E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: A proof-of-concept, single-arm, open-label clinical trial. Nutrition 2020, 74, 110757. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Batycka-Baran, A.; Bieniek, A.; Baran, W. Folate supplementation in patients with psoriasis treated with methotrexate—Effect on safety and efficacy. Prz. Dermatol. 2014, 101, 409–417. [Google Scholar] [CrossRef]
| Vitamin A | Vitamin C | Vitamin E | Carotenoids | Flavonoids | Selenium |
|---|---|---|---|---|---|
| fish fat, liver, cheese, eggs, butter, Vitamin A is also made from provitamins—carotenoids | raw vegetables and fruits, e.g.,: peppers, parsley leaves, brussels sprouts, broccoli, rosehips, currants, strawberries, citruses | vegetable oils (rapeseed oil, soybean oil, corn oil), nuts, sunflower seeds, wheat germ | vegetables with orange, yellow and green colour: kale, broccoli, brussels sprouts, lettuce, cauliflower, spinach, carrots, red pepper, tomato, white and red cabbage | tomatoes, peppers, onions, broccoli, citrus fruits, apples, grapes, blackcurrants, some cereals (wheat, oats), legumes, red wine, green tea, coffee, cocoa | meat, fish, whole grains (e.g., oats, brown rice), dairy products, brassica vegetables, garlic, onion, asparagus, legumes, nuts (mainly Brazil nuts), sunflower seeds, mushrooms |
| Dietary Aspect | Recommendations |
|---|---|
| Energy | The energy value of the diet should be individually adapted to the patient. In overweight and obese people, calorie reductions should be implemented to reduce body weight. |
| Fatty-acids | Limit the supply of saturated fatty acids (found in fatty meats and animal fats such as lard, butter, cream) and trans fats (found in stick margarines, highly processed foods, confectionery products). Increase the supply of omega-3 polyunsaturated fatty acids (especially DHA and EPA), of which marine fish are the best source. The ratio of omega-3 to omega-6 fatty acids should be 1:1.80–1:5. Supplementation with omega-3 fatty acids may be considered. |
| Carbohydrates | Patients should choose carbohydrate products with a low glycaemic index or glycaemic load. It is recommended to limit the intake of sugar, sweets, fruit preserves, and sweet drinks. Instead of white bread, plain pasta, and white rice, patients should choose whole grain cereals. |
| Antioxidants | Increase the intake of products that are sources of natural antioxidants (mainly raw fruit and vegetables). |
| Vitamin D | In case of vitamin D deficiency, supplementation is recommended. |
| Alcohol | Consumption of alcohol is contraindicated. |
| Alternative diets | The following alternative diets may be considered: Gluten-free diet Vegetarian diet Mediterranean diet Ketogenic diet |
| The diet for patients with psoriasis should be tailored to the individual needs of the patient, their comorbidities, and the treatment they are receiving. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbicz, J.; Całyniuk, B.; Górski, M.; Buczkowska, M.; Piecuch, M.; Kulik, A.; Rozentryt, P. Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients 2022, 14, 119. https://doi.org/10.3390/nu14010119
Garbicz J, Całyniuk B, Górski M, Buczkowska M, Piecuch M, Kulik A, Rozentryt P. Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients. 2022; 14(1):119. https://doi.org/10.3390/nu14010119
Chicago/Turabian StyleGarbicz, Jagoda, Beata Całyniuk, Michał Górski, Marta Buczkowska, Małgorzata Piecuch, Aleksandra Kulik, and Piotr Rozentryt. 2022. "Nutritional Therapy in Persons Suffering from Psoriasis" Nutrients 14, no. 1: 119. https://doi.org/10.3390/nu14010119
APA StyleGarbicz, J., Całyniuk, B., Górski, M., Buczkowska, M., Piecuch, M., Kulik, A., & Rozentryt, P. (2022). Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients, 14(1), 119. https://doi.org/10.3390/nu14010119






