Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization
2.3. Study Protocol
2.4. Body Composition Protocol
2.5. Data Collection
2.6. Data Analysis and Simple Size Calculation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Body composition |
| BCE Classification | Body Composition Elche Classification |
| BMI | Body Mass Index |
| FT | Fluid therapy |
| PPN | Peripheral parenteral nutrition |
| SMI | Skeletal muscle index |
References
- Kroenke, C.H.; Neugebauer, R.; Meyerhardt, J.; Prado, C.M.; Weltzien, E.; Kwan, M.L.; Xiao, J.; Caan, B.J. Analysis of Body Mass Index and Mortality in Patients with Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016, 2, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.I.; Doleman, B.; Scott, S.; Lund, J.N.; Williams, J.P. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015, 17, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Cespedes Feliciano, E.M.; Avrutin, E.; Caan, B.J.; Boroian, A.; Mourtzakis, M. Screening for low muscularity in colorectal cancer patients: A valid, clinic-friendly approach that predicts mortality. J. Cachexia Sarcopenia Muscle 2018, 9, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.; Trevisan, C.; Veronese, N.; Lucato, P.; Manzato, E. Imaging of sarcopenia. Eur. J. Radiol. 2016, 85, 1519–1524. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, G.; Perry, R.; Andersen, H.K.; Atkinson, C.; Penfold, C.; Lewis, S.J.; Ness, A.R.; Thomas, S. Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications. Cochrane Database Syst. Rev. 2019, 22, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Calder, P.C.; Cury-Boaventura, M.F.; De Waele, E.; Jakubowski, J.; Zaloga, G. Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion-A Review. Nutrients 2018, 10, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Guillén, L.; Soriano-Irigaray, L.; López-Rodríguez-Arias, F.; Barber, X.; Murcia, A.; Alcaide, M.J.; Aranaz-Ostáriz, V.; Soler-Silva, Á.; Navarro-Ruiz, A.; Arroyo, A. Effect of Early Peripheral Parenteral Nutrition Support in an Enhanced Recovery Program for Colorectal Cancer Surgery: A Randomized Open Trial. J. Clin. Med. 2021, 10, 3647. [Google Scholar] [CrossRef]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Perez, S.; McKeever, L.; Sheean, P. Tutorial: A Step-by-Step Guide (Version 2.0) for Measuring Abdominal Circumference and Skeletal Muscle from a Single Cross-Sectional Computed-Tomography Image Using the National Institutes of Health ImageJ. J. Parenter. Enter. Nutr. 2020, 44, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1-15. Available online: https://github.com/bethatkinson/rpart; https://cran.r-project.org/package=rpart (accessed on 12 February 2021).
- Govaert, J.A.; Fiocco, M.; van Dijk, W.A.; Dutch Value Based Healthcare Study Group. Costs of complications after colorectal cancer surgery in the Netherlands: Building the business case for hospitals. Eur. J. Surg. Oncol. 2015, 41, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Artinyan, A.; Orcutt, S.T.; Anaya, D.A.; Richardson, P.; Chen, G.J.; Berger, D.H. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann. Surg. 2015, 261, 497–505. [Google Scholar] [CrossRef]
- Ripollés-Melchor, J.; Ramírez-Rodríguez, J.M.; Casans-Francés, R. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery. The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg. 2019, 154, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Frasson, M.; Granero-Castro, P.; Ramos Rodríguez, J.L.; Flor-Lorente, B.; Braithwaite, M.; Martí Martínez, E.; Álvarez Pérez, J.A.; Codina Cazador, A.; Espí, A.; Garcia-Granero, E.; et al. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: Results from a prospective, multicentric study of 1102 patients. Int. J. Colorectal Dis. 2016, 31, 105–114. [Google Scholar] [CrossRef]
- Walowski, C.O.; Braun, W.; Maisch, M.J.; Jensen, B.; Peine, S.; Norman, K.; Müller, M.J.; Bosy-Westphal, A. Reference Values for Skeletal Muscle Mass—Current Concepts and Methodological Considerations. Nutrients 2020, 12, 755. [Google Scholar] [CrossRef] [Green Version]
- Abbass, T.; Tsz Ho, Y.T.; Horgan, P.G.; Dolan, R.D.; McMillan, D.C. The relationship between computed tomography derived skeletal muscle index, psoas muscle index and clinical outcomes in patients with operable colorectal cancer. Clin. Nutr. ESPEN 2020, 39, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar]
- Almasaudi, A.S.; McSorley, S.T.; Edwards, C.A.; McMillan, D.C. The relationship between body mass index and short term postoperative outcomes in patients undergoing potentially curative surgery for colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 121, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Guillén, L.; Frasson, M.; Pellino, G.; Fornés-Ferrer, V.; Ramos, J.L.; Flor-Lorente, B.; García-Granero, Á.; Sierra, I.B.; Jiménez-Gómez, L.M.; Moya-Martínez, A.; et al. Nomograms for morbidity and mortality after oncologic colon resection in the enhanced recovery era: Results from a multicentric prospective national study. Int. J. Colorectal Dis. 2020, 35, 2227–2238. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Boelens, P.G.; Heesakkers, F.F.B.M.; Luyer, M.D.P. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: Prospective, randomized, controlled trial. Ann. Surg. 2014, 259, 649–655. [Google Scholar] [CrossRef]
- Zhuang, C.L.; Ye, X.Z.; Zhang, C.J.; Dong, Q.T.; Chen, B.C.; Yu, Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: A meta-analysis of randomized clinical trials. Dig. Surg. 2013, 30, 225–232. [Google Scholar] [CrossRef]
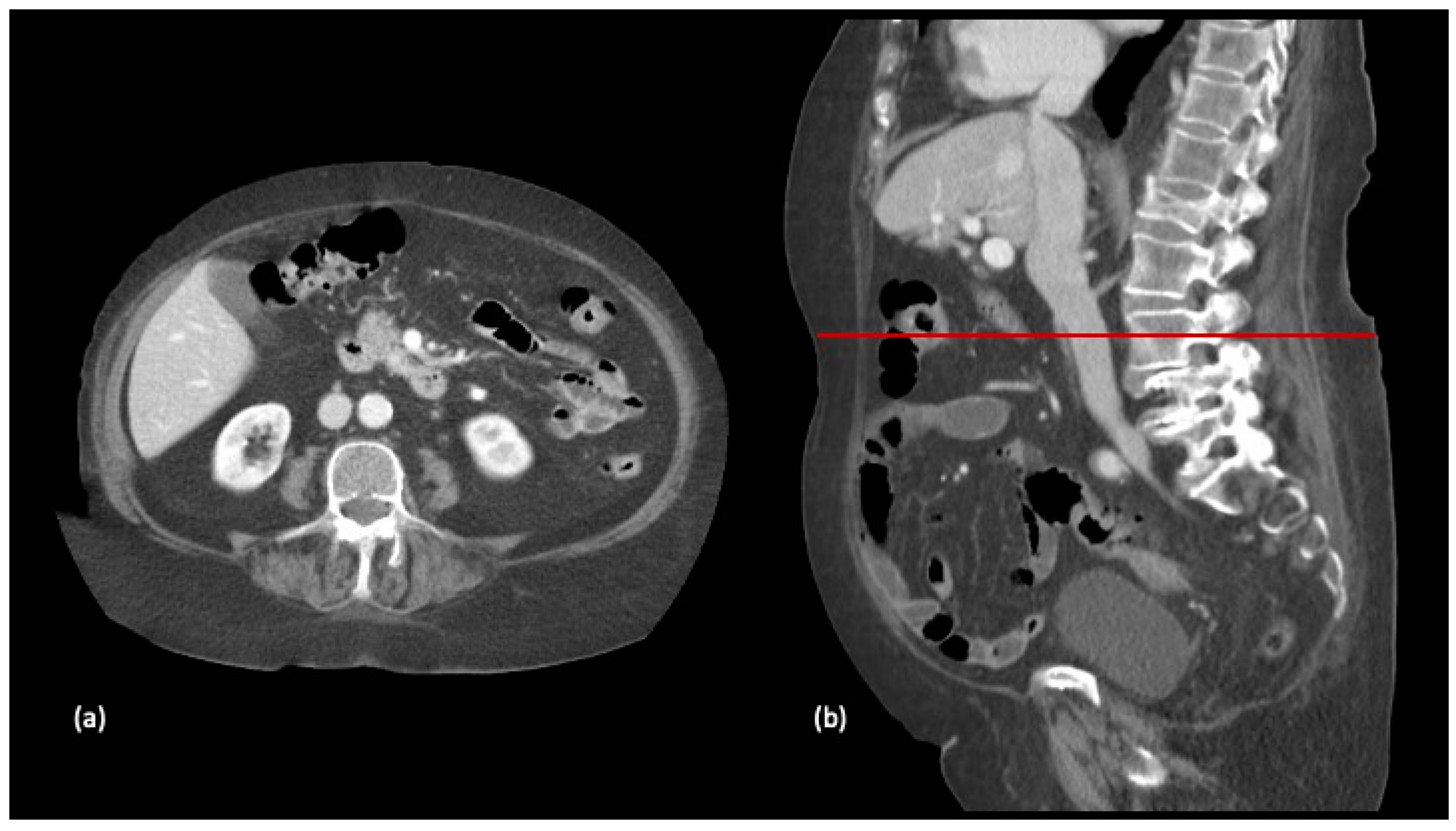
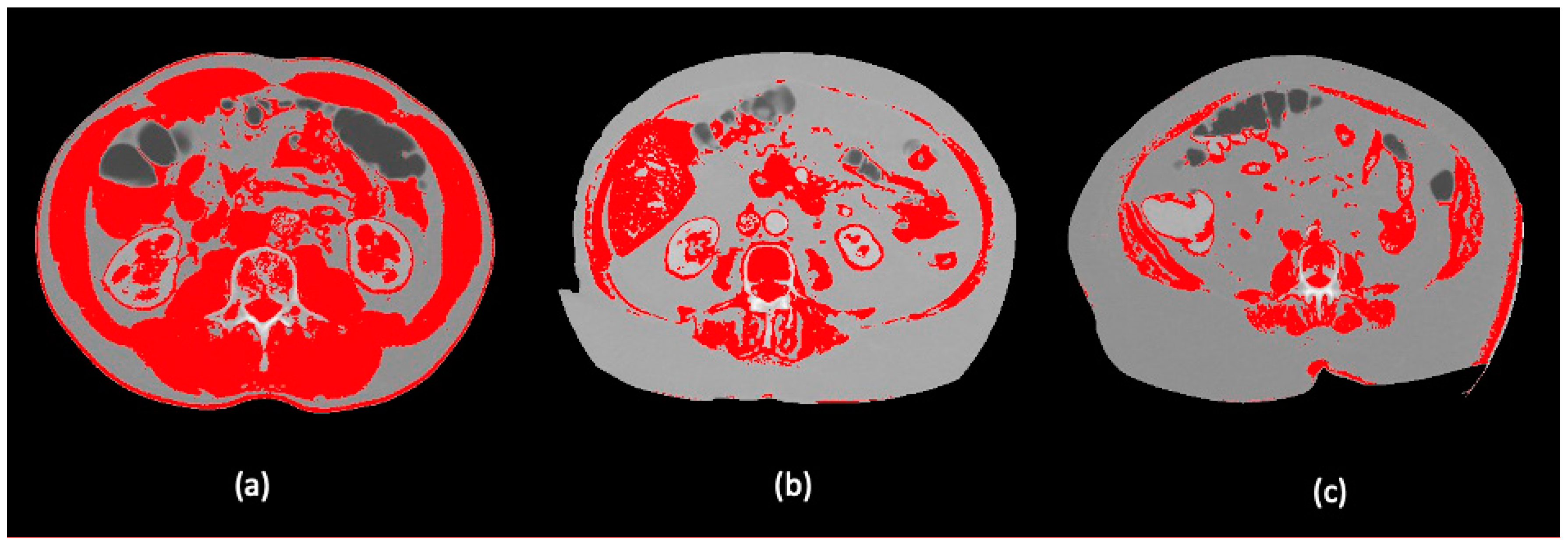
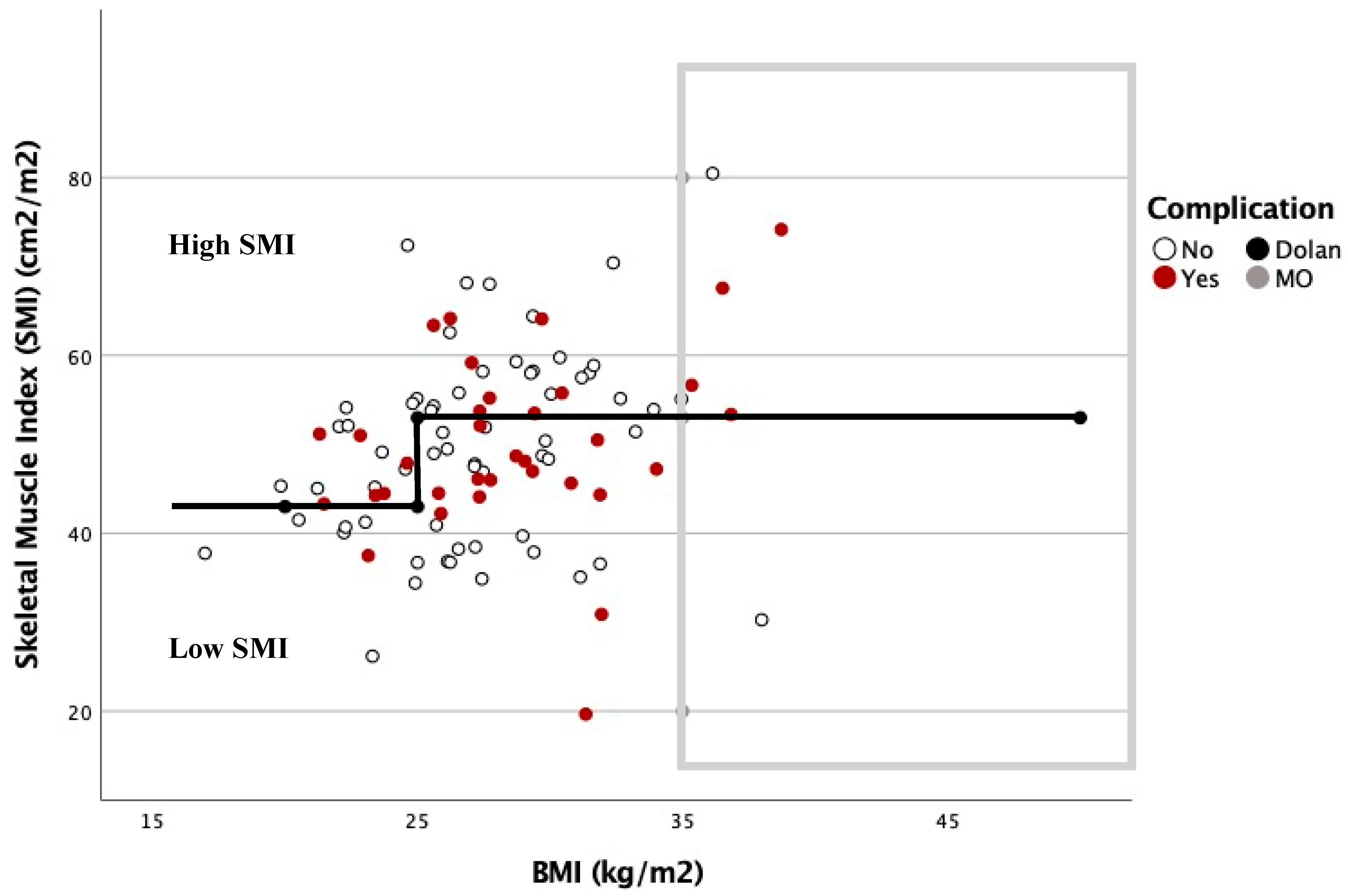

| Characteristics | All, n (%) n = 156 | High SMI, n (%) n = 78 (50) | Low SMI, n (%) n = 78 (50) | p-Value |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 | 55 (35.3) | 39 (50) | 16 (20.5) | |
| 65–75 | 40 (25.6) | 23 (29.5) | 17 (21.8) | |
| >75 | 61 (39.1) | 16 (20.5) | 45 (57.7) | |
| Sex | 0.999 | |||
| Male | 96 (61.5) | 48 (61.5) | 48 (61.5) | |
| Female | 60 (38.5) | 30 (38,5) | 30 (38.5) | |
| ASA score | <0.001 | |||
| I–II | 94 (60.3) | 60 (76.9) | 34 (43.6) | |
| III | 62 (39.7) | 18 (23.1) | 44 (56.4) | |
| BMI | 0.021 | |||
| <25 | 42 (26.9) | 24 (30.8) | 18 (23.1) | |
| 25–35 | 100 (64.1) | 43 (55.1) | 57 (73.1) | |
| >35 | 14 (9) | 11 (14.1) | 3 (3.8) | |
| Group | 0.999 | |||
| PPN | 82 (52.6) | 41 (52.6) | 41 (52.6) | |
| Control | 74 (47.4) | 37 (47.4) | 37 (47.4) | |
| Complications | 0.1 | |||
| Yes | 60 (38.5) | 25 (32.1) | 35 (44.9) | |
| No | 96 (61.5) | 53 (67.9) | 43 (55.1) | |
| Minor | 0.447 | |||
| Yes | 36 (23.1) | 16 (20.5) | 20 (25.6) | |
| No | 120 (76.9) | 62 (79.5) | 58 (74.4) | |
| Major | 0.183 | |||
| Yes | 24 (15.4) | 9 (11.5) | 15 (19.2) | |
| No | 132 (84.6) | 69 (88.5) | 63 (80.8) | |
| Postoperative Ileus | 0.076 | |||
| Yes | 24 (15.4) | 8 (0,1) | 16 (20.5) | |
| No | 132 (84.6) | 70 (0,9) | 62 (79.5) | |
| Length of hospital stay | 0.317 | |||
| ≤7 days | 100 (64.1) | 53 (67.9) | 47 (60.3) | |
| >7 days | 56 (35.9) | 25 (32.1) | 31 (39.7) | |
| Sitting in a chair (POD1) | 0.363 | |||
| Yes | 115 (73.7) | 60 (76.9) | 55 (70.5) | |
| No | 41 (26.3) | 18 (23.1) | 23 (29.5) | |
| Oral Tolerance (POD1) | 0.057 | |||
| Yes | 129 (82.7) | 69 (88.5) | 60 (76.9) | |
| No | 27 (17.3) | 9 (11.5) | 18 (23.1) |
| Characteristics | All, n (%) n = 156 | Low Risk BC, n (%) n = 68 (43.6) | High Risk BC, n (%) n = 88 (56.4) | p-Value |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 | 55 (35.3) | 32 (47.1) | 23 (26.1) | |
| 65–75 | 40 (25.6) | 22 (32.4) | 18 (20.5) | |
| >75 | 61 (39.1) | 14 (20.6) | 47 (53.4) | |
| Sex | 0.475 | |||
| Male | 96 (61.5) | 44 (64.7) | 52 (59.1) | |
| Female | 60 (38.5) | 24 (35.3) | 36 (40.9) | |
| ASA | <0.001 | |||
| I–II | 94 (60.3) | 53 (77.9) | 41 (46.6) | |
| III–V | 62 (39.7) | 15 (22.1) | 47 (53.4) | |
| BMI | <0.001 | |||
| <25 | 42 (26.9) | 25 (36.8) | 27 (30.7) | |
| 25–35 | 100 (64.1) | 43 (63.2) | 57 (64.8) | |
| >35 | 14 (9) | 0 | 14 (15.9) | |
| Group | 0.81 | |||
| PPN | 82 (52.6) | 35 (0.5) | 41 (46.6) | |
| Control | 74 (47.4) | 33 (0.5) | 47 (53.4) | |
| Complications | 0.041 | |||
| Yes | 60 (38.5) | 20 (29.4) | 40 (45.5) | |
| No | 96 (61.5) | 48 (70.6) | 48 (54.5) | |
| Minor | 0.517 | |||
| Yes | 36 (23.1) | 14 (20.6) | 22 (25) | |
| No | 120 (76.9) | 54 (79.4) | 66 (75) | |
| Major n, (SD) | 0.046 | |||
| Yes | 24 (15.4) | 6 (8.8) | 18 (20.5) | |
| No | 132 (84.6) | 62 (91.2) | 70 (79.5) | |
| Postoperative Ileus | 0.004 | |||
| Yes | 24 (15.4) | 4 (5.9) | 20 (22.7) | |
| No | 132 (84.6) | 64 (94.1) | 68 (77.3) | |
| Length of hospital stay | 0.069 | |||
| ≤7 days | 100 (64.1) | 49 (72.1) | 51 (58) | |
| >7 days | 56 (35.9) | 19 (27.9) | 37 (42) | |
| Sitting in a chair (POD1) | 0.156 | |||
| Yes | 115 (73.7) | 54 (79.4) | 61 (69.3) | |
| No | 41 (26.3) | 14 (20.6) | 27 (30.7) | |
| Oral Tolerance (POD1) | 0.042 | |||
| Yes | 129 (82.7) | 61 (89.7) | 68 (77.3) | |
| No | 27 (17.3) | 7 (10.3) | 20 (22.7) |
| All n = 156 | Low-Risk BC, n (%) n = 68 (43.6%) | High-Risk BC, n (%) n = 88 (56.4%) | p-Value | ||
|---|---|---|---|---|---|
| Group n, (%) | PPN n = 35 (51.5) | Control n = 33 (48.5) | PPN n = 47 (53.4) | Control n = 41 (46.6) | |
| Complications n, (%) | 10 (28.57) | 10 (30.30) | 18 (38.3) | 22 (53.66) | 0.041 |
| Minor n, (%) | 8 (22.86) | 6 (18.18) | 10 (21.28) | 12 (29.27) | 0.517 |
| Major n, (%) | 2 (6.06) | 4 (12.12) | 8 (17.02) | 10 (24.39) | 0.046 |
| Independent Variables | β (95%CI) | p-Value |
|---|---|---|
| β0 | 6.06 (2.07,10.06) | 0.003 |
| β1 > 65 years | 2.22 (−1.7,6.13) | 0.269 |
| β2 Female gender | 0.28 (−2.72,3.28) | 0.855 |
| β3 PPN | −0.56 (−3.49,2.36) | 0.706 |
| β4 ASA III | 0.33 (−3.14,3.8) | 0.853 |
| β5 High risk BC | 3.6 (0.21,7) | 0.039 |
| β6 BMI 25–35 | 0.71 (−2.65,4.08) | 0.678 |
| β7 BMI >35 | 1.36 (−4.76,7.48) | 0.663 |
| Lenght hospital stays = β0 + β1 ≥ 65 years+⋯+ β7 BMI ≥ 35 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rodríguez-Arias, F.; Sánchez-Guillén, L.; Lillo-García, C.; Aranaz-Ostáriz, V.; Alcaide, M.J.; Soler-Silva, Á.; Soriano-Irigaray, L.; Barber, X.; Arroyo, A. Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program. Nutrients 2021, 13, 3245. https://doi.org/10.3390/nu13093245
López-Rodríguez-Arias F, Sánchez-Guillén L, Lillo-García C, Aranaz-Ostáriz V, Alcaide MJ, Soler-Silva Á, Soriano-Irigaray L, Barber X, Arroyo A. Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program. Nutrients. 2021; 13(9):3245. https://doi.org/10.3390/nu13093245
Chicago/Turabian StyleLópez-Rodríguez-Arias, Francisco, Luis Sánchez-Guillén, Cristina Lillo-García, Verónica Aranaz-Ostáriz, M José Alcaide, Álvaro Soler-Silva, Leticia Soriano-Irigaray, Xavier Barber, and Antonio Arroyo. 2021. "Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program" Nutrients 13, no. 9: 3245. https://doi.org/10.3390/nu13093245
APA StyleLópez-Rodríguez-Arias, F., Sánchez-Guillén, L., Lillo-García, C., Aranaz-Ostáriz, V., Alcaide, M. J., Soler-Silva, Á., Soriano-Irigaray, L., Barber, X., & Arroyo, A. (2021). Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program. Nutrients, 13(9), 3245. https://doi.org/10.3390/nu13093245







