Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview
Abstract
:1. Introduction
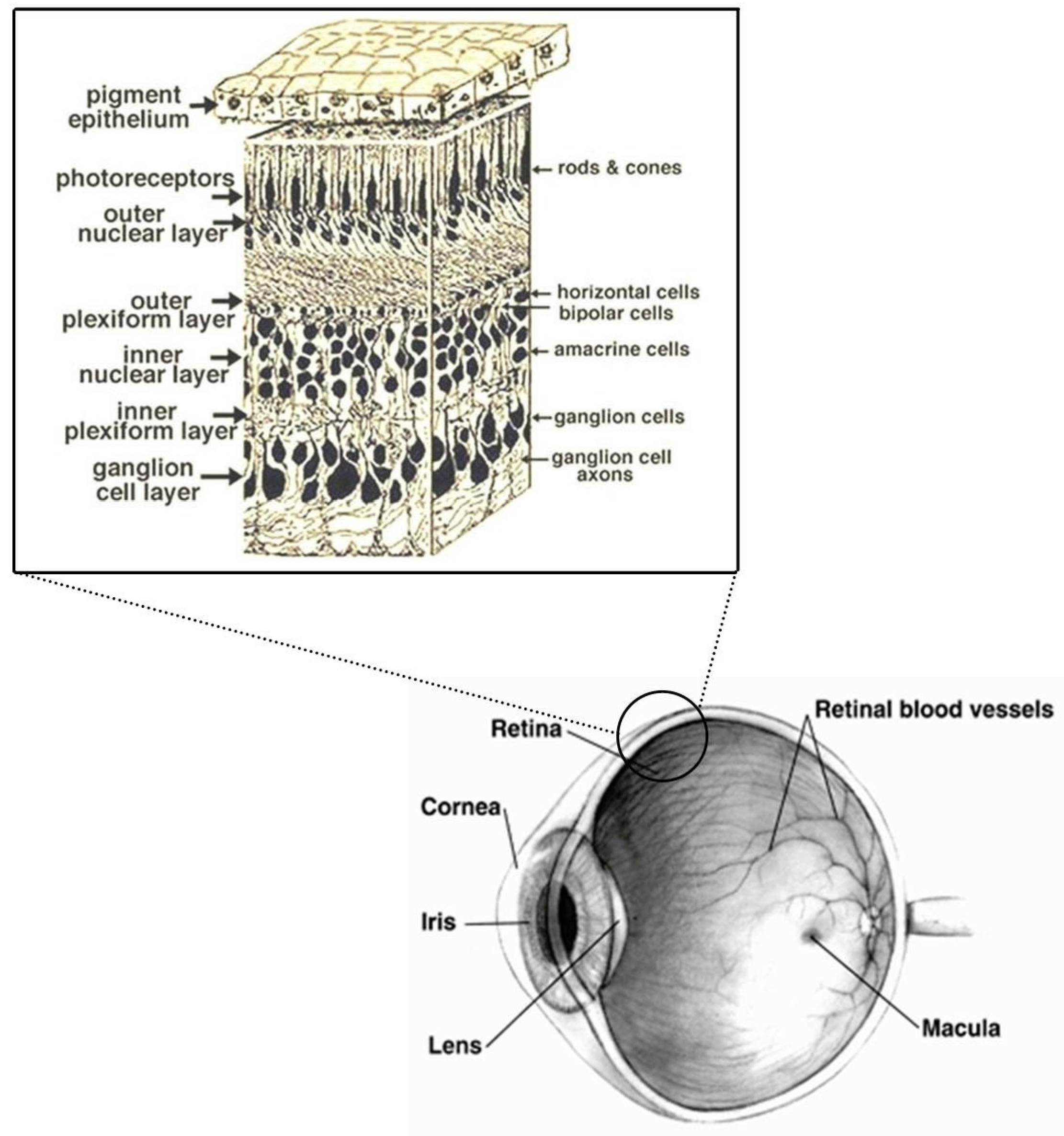
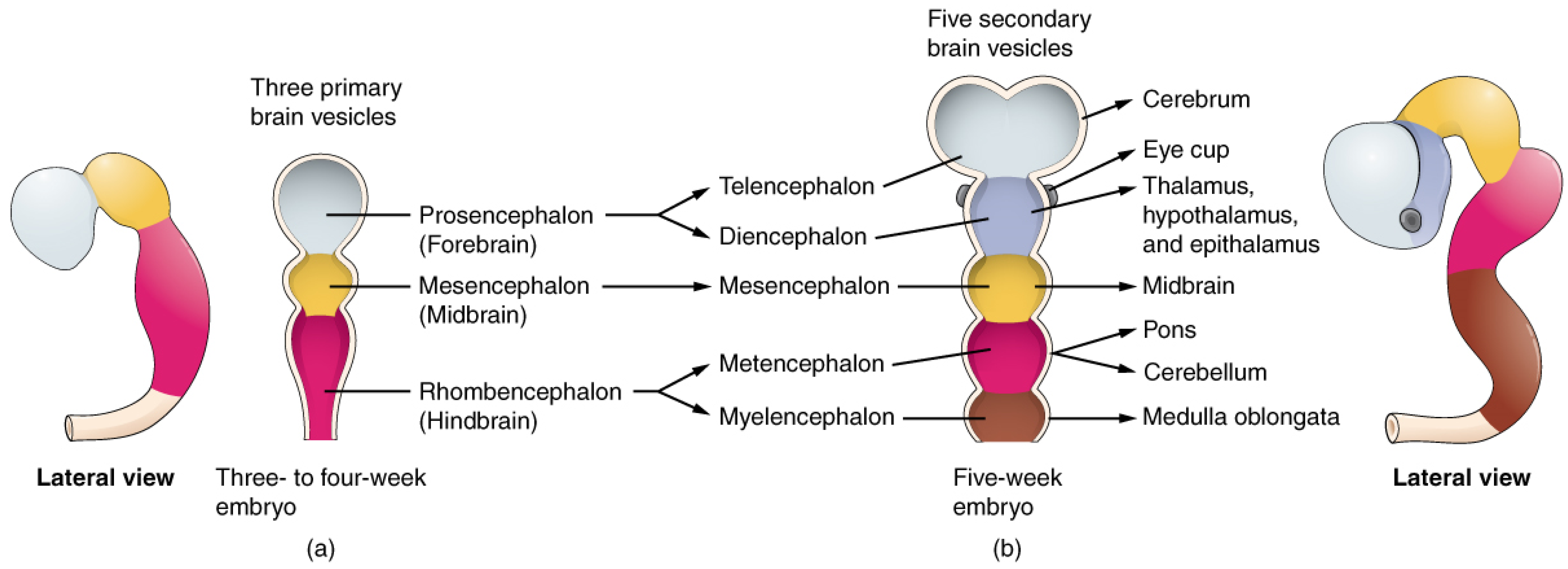
1.1. Retina and Brain Development
1.2. Oxidative Stress in the Term and Preterm Newborn
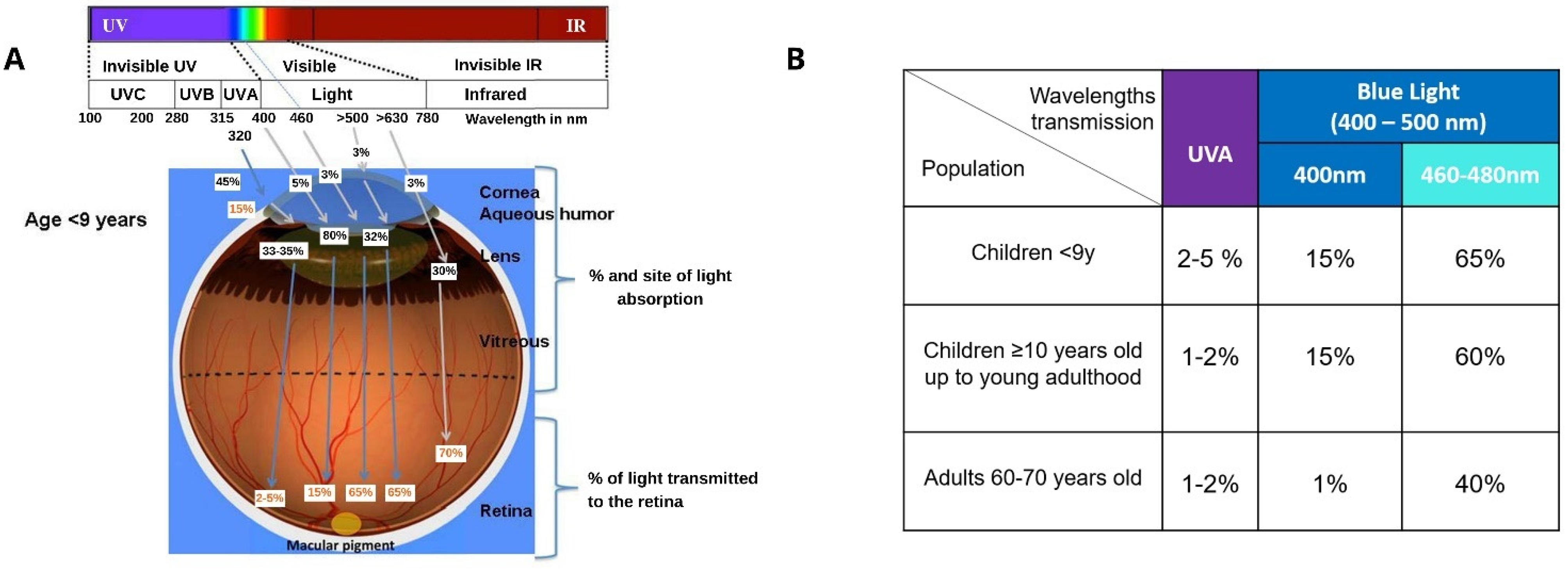
1.3. Light and the Eye
2. Lutein
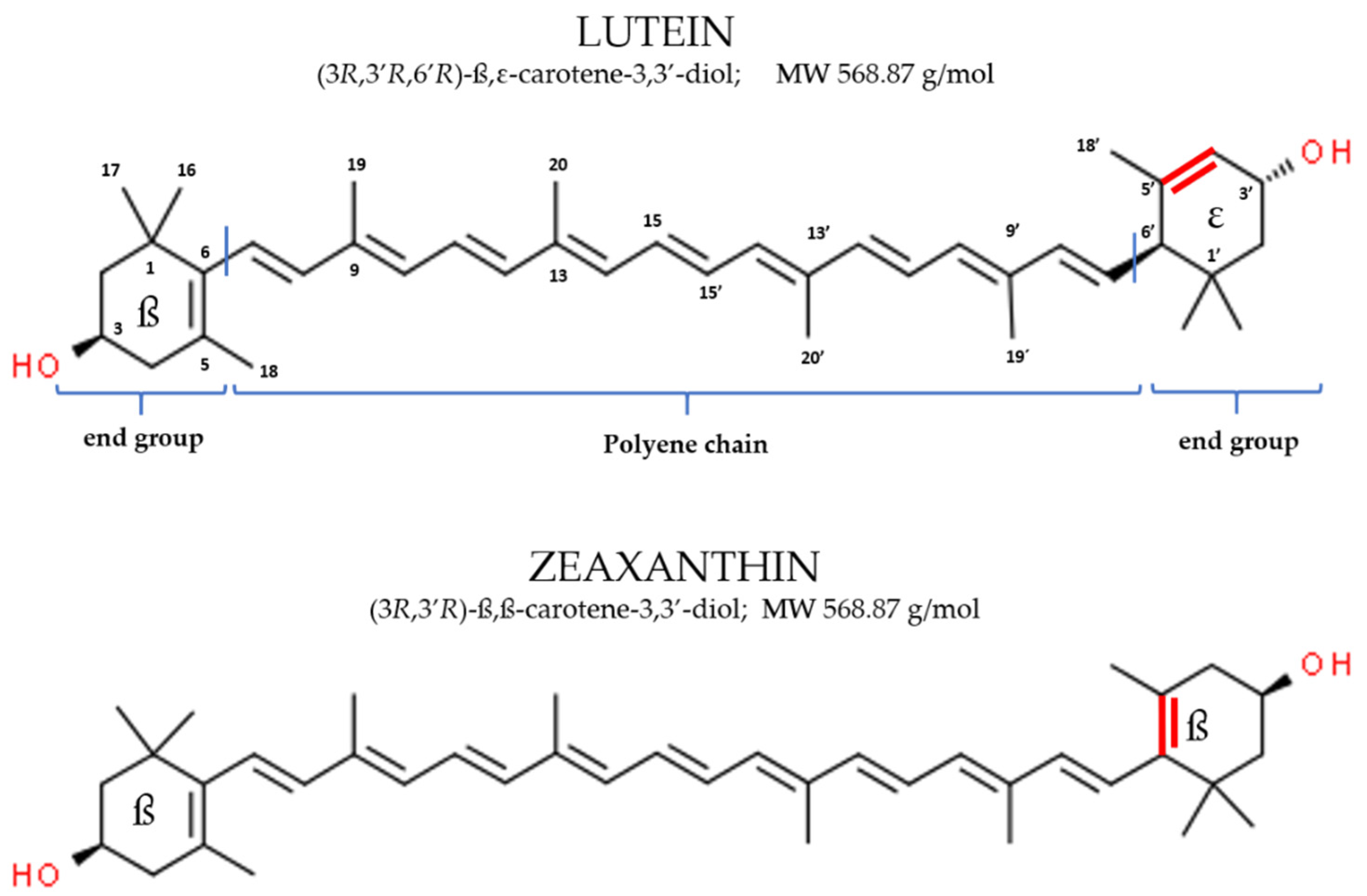
2.1. Physico-Chemical Characteristics
2.2. Dietary Sources, Absorption and Bioavailability
2.3. Mechanism of Action
2.4. Safety
3. Lutein in Pregnancy and Breastfeeding
4. Lutein in Eye and Brain Development and Function
4.1. Search Method
4.2. Lutein, Eye Development and Visual Function
4.2.1. Eye Deposition
4.2.2. Pre-Clinical Research in Non-Human Primates
4.2.3. Lutein, Oxidative Stress and Visual Function in Humans
4.2.4. Lutein in Premature Infants
4.3. Lutein, Brain Develeopment and Cognitive Function
4.3.1. Brain Deposition
4.3.2. Pre-Clinical Research in Non-Human Primates
4.3.3. Lutein Status and Cognitive Function in Humans
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zielińska, M.A.; Wesołowska, A.; Pawlus, B.; Hamułka, J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients 2017, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Cena, H.; Verduci, E.; Bosetti, A.; Pelizzo, G.; Zuccotti, G.V. Nutritional Surveillance for the Best Start in Life, Promoting Health for Neonates, Infants and Children. Nutrients 2020, 12, 3386. [Google Scholar] [CrossRef] [PubMed]
- WHO E-Library of Evidence for Nutrition Actions (ELENA). Available online: https://www.who.int/elena/titles/nutrition_counselling_pregnancy/en/ (accessed on 9 August 2021).
- Pescosolido, N. Embriologia Dell’apparato Visivo Ed Aspetti Di Biologia Dello Sviluppo; Fabriano Editore: Moasca, Italy, 2003. [Google Scholar]
- Sasano, H.; Obana, A.; Sharifzadeh, M.; Bernstein, P.S.; Okazaki, S.; Gohto, Y.; Seto, T.; Gellermann, W. Optical Detection of Macular Pigment Formation in Premature Infants. Transl. Vis. Sci. Technol. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, G.D.; Fielder Alistair, D. Anatomy and physiology of the retina. In Pediatric Retina; Reynolds, J.D., Olitsky, S.E., Eds.; Spinger: Berlin/Heidelberg, Germany, 2011; pp. 39–65. ISBN 9783642120404. [Google Scholar]
- Lien, E.L.; Hammond, B.R. Nutritional Influences on Visual Development and Function. Prog. Retin. Eye Res. 2011, 30, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S. Discovering the Brain; Institute of Medicine National Academy of Science, Ed.; National Academy Press: Washington, DC, USA, 1992; ISBN 9781847427984. [Google Scholar]
- Graven, S.N.; Browne, J.V. Visual Development in the Human Fetus, Infant, and Young Child. Newborn Infant Nurs. Rev. 2008, 8, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Dobbing, J.; Sands, J. Quantitative Growth and Development of Human Brain. Arch. Dis. Child. 1973, 48, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Perrone, S.; Tei, M.; Longini, M.; Buonocore, G. The Multiple Facets of Lutein: A Call for Further Investigation in the Perinatal Period. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kamlin, C.O.F.; O’Donnell, C.P.F.; Davis, P.G.; Morley, C.J. Oxygen Saturation in Healthy Infants Immediately after Birth. J. Pediatr. 2006, 148, 585–589. [Google Scholar] [CrossRef]
- Dawes, G.S.; Mott, J.C.; Widdicombe, J.G. The Foetal Circulation in the Lamb. J. Physiol. 1954, 126, 563–587. [Google Scholar] [CrossRef]
- Perrone, S.; Negro, S.; Tataranno, M.L.; Buonocore, G. Oxidative Stress and Antioxidant Strategies in Newborns. J. Matern. Neonatal Med. 2010, 23, 63–65. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, L.M.; Stazzoni, G.; Ramenghi, L.; Buonocore, G. Brain Susceptibility to Oxidative Stress in the Perinatal Period. J. Matern. Neonatal Med. 2015, 28, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, Regional, and National Estimates of Levels of Preterm Birth in 2014: A Systematic Review and Modelling Analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- Kockevar, I.E.; Pathak, M.A.; Parrish, J.A. Photophysics, Photochemistry and Photobiology. In Fitzpatrick’s Dermatology in General Medicine; Freedberg, I.M., Eisen, A.Z., Wolff, K., Austen, K.F., Goldsmith, L.A., Katz, S.I., Eds.; McGraw-Hill: New York, NY, USA, 1999; pp. 220–229. [Google Scholar]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Health Effects of Artificial Light; Scientific Committee on Emerging and Newly Identified Health Risks: Geneva, Switzerland, 2012; ISBN 9789279263149. [Google Scholar]
- Gaillard, E.R.; Zheng, L.; Merriam, J.C.; Dillon, J. Age-Related Changes in the Absorption Characteristics of the Primate Lens. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1454–1459. [Google Scholar]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety in Response to the Internally-Solicited Request Entitled Effects on Human Health and the Environment (Fauna and Flora) of Systems Using Light-Emitting Diodes (LED); ANSES: Paris, France, 2019.
- Roberts, J.E. Ocular Photoxicity. J. Photochem. Photobiol. B Biol. 2001, 64, 136–143. [Google Scholar] [CrossRef]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The Topography and Age Relationship of Lipofuscin Concentration in the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 601–607. [Google Scholar]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical Damage of the Retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef]
- Algvere, P.V.; Marshall, J.; Seregard, S. Age-Related Maculopathy and the Impact of Blue Light Hazard. Acta Ophthalmol. Scand. 2006, 84, 4–15. [Google Scholar] [CrossRef]
- Wooten, B.R.; Hammond, B.R. Macular Pigment: Influences on Visual Acuity and Visibility. Prog. Retin. Eye Res. 2002, 21, 225–240. [Google Scholar] [CrossRef]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of Blue Light on the Circadian System and Eye Physiology. Mol. Vis. 2016, 22, 61–72. [Google Scholar] [PubMed]
- Rechichi, C.; De Mojà, G.; Aragona, P. Video Game Vision Syndrome: A New Clinical Picture in Children? J. Pediatr. Ophthalmol. Strabismus 2017, 54, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hwang, Y.; Yu, H.G.; Park, S.K. Association between Exposure to Smartphones and Ocular Health in Adolescents. Ophthalmic Epidemiol. 2016, 23, 419. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W. Macular Carotenoids: Lutein and Zeaxanthin. Dev. Ophthalmol. 2005, 38, 70–88. [Google Scholar] [CrossRef]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.M.; Wenzel, A.J. The Value of Measurement of Macular Carotenoid Pigment Optical Densities and Distributions in Age-Related Macular Degeneration and Other Retinal Disorders. Vision Res. 2010, 50, 716–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, P.S.; Arunkumar, R. The Emerging Roles of the Macular Pigment Carotenoids throughout the Lifespan and in Prenatal Supplementation. J. Lipid Res. 2021, 62, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Widomsa, J.; Zareba, M.; Subczynski, W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and the Brain? Foods 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; De Lera, A.R.; Desmarchelier, C.; et al. From Carotenoid Intake to Carotenoid Blood and Tissue Concentrations-Implications for Dietary Intake Recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R.; Marilyn Buzzard, I.; Bhagwat, S.; Davis, C.S.; Douglass, L.W.; Gebhardt, S.; Haytowitz, D.; Schakel, S. Carotenoid Content of U.S. Foods: An Update of the Database. J. Food Compos. Anal. 1999, 12, 169–196. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Photoprotection by Dietary Carotenoids: Concept, Mechanisms, Evidence and Future Development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and Zeaxanthin Supplementation and Association with Visual Function in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long Term Effects of Lutein, Zeaxanthin and Omega-3-LCPUFAs Supplementation on Optical Density of Macular Pigment in AMD Patients: The LUTEGA Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Keenan, T.D.L.; Agron, E.; Malley, C.E.; Domalpally, A. The Results of the 10 Year Follow-on Study of the Age-Related Eye Disease Study 2 (AREDS2). Investig. Ophthalmol. Vis. Sci. 2001, 62, 1215. [Google Scholar]
- Stringham, J.M.; Hammond, B.R. Macular Pigment and Visual Performance under Glare Conditions. Optom. Vis. Sci. 2008, 85, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringham, J.M.; O’Brien, K.J.; Stringham, N.T. Macular Carotenoid Supplementation Improves Disability Glare Performance and Dynamics of Photostress Recovery. Eye Vis. 2016, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive Findings of an Exploratory Trial of Docosahexaenoic Acid and Lutein Supplementation in Older Women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Hammond, B.R.; Stephen Miller, L.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef]
- Renzi-Hammond, L.M.; Bovier, E.R.; Fletcher, L.M.; Miller, L.S.; Mewborn, C.M.; Lindbergh, C.A.; Baxter, J.H.; Hammond, B.R. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients 2017, 9, 1246. [Google Scholar] [CrossRef] [Green Version]
- Kruger, C.L.; Murphy, M.; DeFreitas, Z.; Pfannkuch, F.; Heimbach, J. An Innovative Approach to the Determination of Safety for a Dietary Ingredient Derived from a New Source: Case Study Using a Crystalline Lutein Product. Food Chem. Toxicol. 2002, 40, 1535–1549. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; Van den Berg, H.; Hininger, I.; Rousell, A.-M.; Chopra, M.; et al. A European Carotenoid Database to Assess Carotenoid Intakes and Its Use in a Five-Country Comparative Study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef]
- Johnson, E.J.; Maras, J.E.; Rasmussen, H.M.; Tucker, K.L. Intake of Lutein and Zeaxanthin Differ with Age, Sex, and Ethnicity. J. Am. Diet. Assoc. 2010, 110, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Lanzi, S.; D’Evoli, L.; Aguzzi, A.; Lombardi-Boccia, G. Intake of Vitamin A and Carotenoids from the Italian Population—Results of an Italian total diet study. Int. J. Vitam. Nutr. Res. 2006, 76, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Granado, F.; Blásquez, S.; Olmedilla, B. Changes in Carotenoid Intake from Fruit and Vegetables in the Spanish Population over the Period 1964–2004. Public Health Nutr. 2007, 10, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wang, Z. Investigation on Dietary Carotenoids of Adults. Chin. J. Public Health 2007, 0580, 2006–2008. [Google Scholar]
- Nagai, N.; Izumi-Nagai, K.; Suzuki, M.; Shinoda, H.; Koto, T.; Uchida, A.; Mochimaru, H.; Tomita, Y.; Miyake, S.; Kobayashi, S.; et al. Association of Macular Pigment Optical Density with Serum Concentration of Oxidized Low-Density Lipoprotein in Healthy Adults. Retina 2015, 35, 820–826. [Google Scholar] [CrossRef]
- EUROSTAT. Do You Eat Fruit and Vegetables Daily? Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190401-1 (accessed on 20 January 2005).
- European Commission—Overview of Fruit and Vegetables Intake in European Countries. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/fruit-vegetables-5_en (accessed on 20 January 2005).
- Thoene, M.; Anderson-Berry, A.; Van Ormer, M.; Furtado, J.; Soliman, G.A.; Goldner, W.; Hanson, C. Quantification of Lutein + Zeaxanthin Presence in Human Placenta and Correlations with Blood Levels and Maternal Dietary Intake. Nutrients 2019, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Cena, H.; Roggi, C.; Turconi, G. Development and Validation of a Brief Food Frequency Questionnaire for Dietary Lutein and Zeaxanthin Intake Assessment in Italian Women. Eur. J. Nutr. 2008, 47, 1–9. [Google Scholar] [CrossRef]
- Cena, H.; Castellazzi, A.M.; Pietri, A.; Roggi, C.; Turconi, G. Lutein Concentration in Human Milk during Early Lactation and Its Relationship with Dietary Lutein Intake. Public Health Nutr. 2009, 12, 1878–1884. [Google Scholar] [CrossRef] [Green Version]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G.A.J. Dietary Factors That Affect the Bioavailability of Carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Bamedi, A.; Wirt, U. Carotenol Fatty Acid Esters: Easy Substrates for Digestive Enzymes? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 721–728. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Olmedilla-Alonso, B.; Blanco-Navarro, I.; Pérez-Sacristán, B. Lutein Bioavailability from Lutein Ester-Fortified Fermented Milk: In Vivo and in Vitro Study. J. Nutr. Biochem. 2010, 21, 133–139. [Google Scholar] [CrossRef]
- Roodenburg, A.J.C.; Leenen, R.; Van Het Hof, K.H.; Weststrate, J.A.; Tijburg, L.B.M. Amount of Fat in the Diet Affects Bioavailability of Lutein Esters but Not of α-Carotene, β-Carotene, and Vitamin E in Humans. Am. J. Clin. Nutr. 2000, 71, 1187–1193. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids, α -Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and Preterm Infants with Decreased Concentrations of Brain Carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary Identification of the Human Macular Pigment. Vis. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the Macular Pigment by HPLC: Retinal Distribution and Age Study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Sherry, C.L.; Oliver, J.S.; Renzi, L.M.; Marriage, B.J. Lutein Supplementation Increases Breast Milk and Plasma Lutein Concentrations in Lactating Women and Infant Plasma Concentrations but Does Not Affect Other Carotenoids. J. Nutr. 2014, 144, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipkie, T.E.; Morrow, A.L.; Jouni, Z.E.; McMahon, R.J.; Ferruzzi, M.G. Longitudinal Survey of Carotenoids in Human Milk from Urban Cohorts in China, Mexico, and the USA. PLoS ONE 2015, 10, e0127729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, V.C.; Mayes, C.B.D.; Tubman, T.R.J.; Northrop-Clewes, C.A.; Thurnham, D.I. A Comparison of Lutein and Zeaxanthin Concentrations in Formula and Human Milk Samples from Northern Ireland Mothers. Eur. J. Clin. Nutr. 2004, 58, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, T. Bioavailability of Non-Provitamin A Carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in Human Blood and Tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.J. The Role of Carotenoids in Human Health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef] [Green Version]
- Lindbergh, C.A.; Mewborn, C.M.; Hammond, B.R.; Renzi-Hammond, L.M.; Curran-Celentano, J.M.; Miller, L.S. Relationship of Lutein and Zeaxanthin Levels to Neurocognitive Functioning: An FMRI Study of Older Adults. J. Int. Neuropsychol. Soc. 2017, 23, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Bovier, E.R.; Hammond, B.R. A Randomized Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Visual Processing Speed in Young Healthy Subjects. Arch. Biochem. Biophys. 2015, 572, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Bovier, E.R.; Renzi, L.M.; Hammond, B.R. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Neural Processing Speed and Efficiency. PLoS ONE 2014, 9, e108178. [Google Scholar] [CrossRef]
- Lai, J.S.; Veetil, V.O.; Lanca, C.; Lee, B.L.; Godfrey, K.M.; Gluckman, P.D.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; et al. Maternal Lutein and Zeaxanthin Concentrations in Relation to Offspring Visual Acuity at 3 Years of Age: The GUSTO Study. Nutrients 2020, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal Intake of Lutein and Zeaxanthin during Pregnancy Is Positively Associated with Offspring Verbal Intelligence and Behavior Regulation in Mid-Childhood in the Project Viva Cohort. J. Nutr. 2021, 151, 615–627. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Sheppard, K.W. Synergistic Effects of Human Milk Nutrients in the Support of Infant Recognition Memory: An Observational Study. Nutrients 2015, 7, 9079–9095. [Google Scholar] [CrossRef] [Green Version]
- Barnett, S.M.; Khan, N.A.; Walk, A.M.; Raine, L.B.; Moulton, C.; Cohen, N.J.; Kramer, A.F.; Hammond, B.R.; Renzi-Hammond, L.; Hillman, C.H. Macular Pigment Optical Density Is Positively Associated with Academic Performance among Preadolescent Children. Nutr. Neurosci. 2018, 21, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and Zeaxanthin as Protectors of Lipid Membranes against Oxidative Damage: The Structural Aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Hammond, B.R. The Retina as a Nutritionally Responsive Tissue. Aging Health 2007, 3, 585–588. [Google Scholar] [CrossRef]
- Li, S.Y.; Lo, A.C.Y. Lutein Protects RGC-5 Cells against Hypoxia and Oxidative Stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilsiz, N.; Sahaboglu, A.; Yıldız, M.Z.; Reichenbach, A. Protective Effects of Various Antioxidants during Ischemia-Reperfusion in the Rat Retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 627–633. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M. Lutein Protects Dopaminergic Neurons against MPTP-Induced Apoptotic Death and Motor Dysfunction by Ameliorating Mitochondrial Disruption and Oxidative Stress. Nutr. Neurosci. 2016, 19, 237–246. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Bian, Q.; Qin, T.; Ren, Z.; Wu, D.; Shang, F. Lutein or Zeaxanthin Supplementation Suppresses Inflammatory Responses in Retinal Pigment Epithelial Cells and Macrophages. Adv. Exp. Med. Biol. 2012, 723, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein Effect on Retina and Hippocampus of Diabetic Mice. Free Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.H.; Park, J.G.; Yi, Y.S.; Park, K.W.; Rho, H.S.; Lee, M.S.; Yoo, J.W.; Kang, S.H.; Hong, Y.D.; et al. Radical Scavenging Activity-Based and AP-1-Targeted Anti-Inflammatory Effects of Lutein in Macrophage-like and Skin Keratinocytic Cells. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of Bv-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- Zamroziewicz, M.K.; Paul, E.J.; Zwilling, C.E.; Johnson, E.J.; Kuchan, M.J.; Cohen, N.J.; Barbey, A.K. Parahippocampal Cortex Mediates the Relationship between Lutein and Crystallized Intelligence in Healthy, Older Adults. Front. Aging Neurosci. 2016, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Stringham, J.M.; Hammond, B.R. The Glare Hypothesis of Macular Pigment Function. Optom. Vis. Sci. 2007, 84, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Loughman, J.; Nolan, J.M.; Howard, A.N.; Connolly, E.; Meagher, K.; Beatty, S. The Impact of Macular Pigment Augmentation on Visual Performance Using Different Carotenoid Formulations. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7871–7880. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Nicolai, S.; Briviba, K.; Hanusch, M.; Broszeit, G.; Peters, M.; Martin, H.D.; Sies, H. Biological Activities of Natural and Synthetic Carotenoids: Induction of Gap Junctional Communication and Singlet Oxygen Quenching. Carcinogenesis 1997, 18, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids Enhance Gap Junctional Communication and Inhibit Lipid Peroxidation in C3H/10T1/2 Cells: Relationship to Their Cancer Chemopreventive Action. Carcinogenesis 1991, 12, 2109–2114. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M. Influence of the Dietary Carotenoids Lutein and Zeaxanthin on Visual Performance: Application to Baseball. Am. J. Clin. Nutr. 2012, 96, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Evaluation of the Joint FAO/WHO Expect Committee on Food Additives (JECFA). Lutein from Tagetes Erecta; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the Re-Evaluation of Lutein (E 161b) as a Food Additive on Request of the European Commission. EFSA J. 2010, 8, 1678. [Google Scholar] [CrossRef]
- GRAS U.S. Food & Drug Administration. GRAS Notice Inventory; GRN No. 140, 221 and 390; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2004.
- European Food Safety Authority (EFSA). Safety, bioavailability and suitability of lutein for the particular nutritional use by infants and young children—Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2008, 6, 1–24. [Google Scholar] [CrossRef]
- Dagnelie, G.; Zorge, I.; McDonald, T.M. Lutein Improves Visual Function in Some Patients with Retinal Degeneration_a Pilot Study via the Internet. Optometry 2000, 71, 147–164. [Google Scholar] [PubMed]
- Kiely, M.; Cogan, P.F.; Kearney, P.J.; Morrissey, P.A. Concentrations of Tocopherols and Carotenoids in Maternal and Cord Blood Plasma. Eur. J. Clin. Nutr. 1999, 53, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Yeum, K.J.; Ferland, G.; Patry, J. Russel Relationship of Plasma Carotenoids, Retinol, and Tocopherols in Mothers and Newborn Infants. J. Am. Coll. Nutr. 1998, 17, 442–447. [Google Scholar] [CrossRef]
- Yamini, S.; West, K.P.; Wu, L.; Dreyfuss, M.L.; Yang, D.X.; Khatry, S.K. Circulating Levels of Retinol, Tocopherol and Carotenoid in Nepali Pregnant and Postpartum Women Following Long-Term β-Carotene and Vitamin A Supplementation. Eur. J. Clin. Nutr. 2001, 55, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picone, S.; Ritieni, A.; Fabiano, A.; Graziani, G.; Paolillo, P.; Livolti, G.; Galvano, F.; Gazzolo, D. Lutein Levels in Arterial Cord Blood Correlate with Neuroprotein Activin A in Healthy Preterm and Term Newborns: A Trophic Role for Lutein? Clin. Biochem. 2018, 52, 80–84. [Google Scholar] [CrossRef]
- Oostenbrug, G.S.; Mensink, R.P.; Al, M.D.M.; van Houwelingen, A.C.; Hornstra, G. Maternal and Neonatal Plasma Antioxidant Levels in Normal Pregnancy, and the Relationship with Fatty Acid Unsaturation. Br. J. Nutr. 1998, 80, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Connor, S.L.; Connor, W.E.; Bezzerides, E.A.; Wang, Y. The Maternal Transfer of Lutein and Zeaxanthin into the Fetus during Pregnancy. FASEB J. 2021, 22, 9–10. [Google Scholar]
- Gossage, C.P.; Deyhim, M.; Yamini, S.; Douglass, L.W.; Moser-Veillon, P.B. Carotenoid Composition of Human Milk during the First Month Postpartum and the Response to β-Carotene Supplementation. Am. J. Clin. Nutr. 2002, 76, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Schweigert, F.J.; Bathe, K.; Chen, F.; Büscher, U.; Dudenhausen, J.W. Effect of the Stage of Lactation in Humans on Carotenoid Levels in Milk, Blood Plasma and Plasma Lipoprotein Fractions. Eur. J. Nutr. 2004, 43, 39–44. [Google Scholar] [CrossRef]
- Patton, S.; Canfield, L.M.; Huston, G.E.; Ferris, A.M.; Jensen, R.G. Carotenoids of Human Colostrum. Lipids 1990, 25, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, T.; Mao, Y.; Tian, F.; Cai, X.; Kuchan, M.J.; Zhang, L.; Zhao, Y.; Chen, J. Carotenoid Profile in Breast Milk and Maternal and Cord Plasma: A Longitudinal Study in Southwest China. Br. J. Nutr. 2021, 1–7. [Google Scholar] [CrossRef]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; Derusso, P.A. Serum Lutein Concentrations in Healthy Term Infants Fed Human Milk or Infant Formula with Lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, E.; Demmelmair, H.; Horak, J.; Holdt, L.; Grote, V.; Maar, K.; Neuhofer, C.; Teupser, D.; Thiel, N.; Goeckeler-Leopold, E.; et al. Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial. Nutrients 2020, 12, 2849. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Neuringer, M.; Johnson, E.E.; Kuchan, M.J.; Pereira, S.L.; Johnson, E.J.; Erdman, J.W. Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein. Nutrients 2017, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Ranard, K.M.; Neuringer, M.; Johnson, E.E.; Renner, L.; Kuchan, M.J.; Pereira, S.L.; Johnson, E.J.; Erdman, J.W. Lutein Is Differentially Deposited across Brain Regions Following Formula or Breast Feeding of Infant Rhesus Macaques. J. Nutr. 2018, 148, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Addo, E.K.; Gorusupudi, A.; Allman, S.; Bernstein, P.S. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) Study—carotenoid Supplementation during Pregnancy: Ocular and Systemic Effects—study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Z.; Jiang, K.; Zhu, J.; He, G.; Ke, B. Macular Pigment Optical Density and Its Relationship with Refractive Status and Foveal Thickness in Chinese School-Aged Children. Curr. Eye Res. 2013, 38, 168–173. [Google Scholar] [CrossRef]
- Mulder, K.A.; Innis, S.M.; Rasmussen, B.F.; Wu, B.T.; Richardson, K.J.; Hasman, D. Plasma Lutein Concentrations Are Related to Dietary Intake, but Unrelated to Dietary Saturated Fat or Cognition in Young Children. J. Nutr. Sci. 2014, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassevoort, K.M.; Khazoum, S.E.; Walker, J.A.; Barnett, S.M.; Raine, L.B.; Hammond, B.R.; Renzi-Hammond, L.M.; Kramer, A.F.; Khan, N.A.; Hillman, C.H.; et al. Macular Carotenoids, Aerobic Fitness, and Central Adiposity Are Associated Differentially with Hippocampal-Dependent Relational Memory in Preadolescent Children. J. Pediatr. 2017, 183, 108–114.e1. [Google Scholar] [CrossRef]
- Walk, A.M.; Khan, N.A.; Barnett, S.M.; Raine, L.B.; Kramer, A.F.; Cohen, N.J.; Moulton, C.J.; Renzi-Hammond, L.M.; Hammond, B.R.; Hillman, C.H. From Neuro-Pigments to Neural Efficiency: The Relationship between Retinal Carotenoids and Behavioral and Neuroelectric Indices of Cognitive Control in Childhood. Int. J. Psychophysiol. 2017, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.E.; Renzi-Hammond, L.M.; Khan, N.A.; Hillman, C.H.; Frick, J.E.; Hammond, B.R. The Macular Carotenoids Are Associated with Cognitive Function in Preadolescent Children. Nutrients 2018, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Yakovleva, M.A.; Panova, I.G.; Fel’dman, T.B.; Zak, P.P.; Tatikolov, A.S.; Sukhikh, G.T.; Ostrovsky, M.A. Finding of Carotenoids in the Vitreous Body of Human Eye during Prenatal Development. Russ. J. Dev. Biol. 2007, 38, 317–321. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [Green Version]
- Vachali, P.; Li, B.; Nelson, K.; Bernstein, P.S. Surface Plasmon Resonance (SPR) Studies on the Interactions of Carotenoids and Their Binding Proteins. Arch. Biochem. Biophys. 2012, 519, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Borel, P.; De Edelenyi, F.S.; Vincent-Baudry, S.; Malezet-Desmoulin, C.; Margotat, A.; Lyan, B.; Gorrand, J.M.; Meunier, N.; Drouault-Holowacz, S.; Bieuvelet, S. Genetic Variants in BCMO1 and CD36 Are Associated with Plasma Lutein Concentrations and Macular Pigment Optical Density in Humans. Ann. Med. 2011, 43, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hannon, B.A.; Robinson, K.N.; Raine, L.B.; Hammond, B.R.; Renzi-Hammond, L.M.; Cohen, N.J.; Kramer, A.F.; Hillman, C.H.; Teran-Garcia, M.; et al. Single Nucleotide Polymorphisms in CD36 Are Associated with Macular Pigment among Children. J. Nutr. 2021, 1–2. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the Human Macular Carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Malinow, M.R.; Feeney-Burns, L.; Peterson, L.H.; Klein, M.L.; Neuringer, M. Diet-Related Macular Anomalies in Monkeys. Investig. Ophthalmol. Vis. Sci. 1980, 19, 857–863. [Google Scholar]
- Leung, I.Y.F.; Sandstrom, M.M.; Zucker, C.L.; Neuringer, M.; Snodderly, D.M. Nutritional Manipulation of Primate Retinas, II: Effects of Age, n-3 Fatty Acids, Lutein, and Zeaxanthin on Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3244–3256. [Google Scholar] [CrossRef] [PubMed]
- Neuringer, M.; Sandstrom, M.M.; Johnson, E.J.; Snodderly, D.M. Nutritional Manipulation of Primate Retinas, I: Effects of Lutein or Zeaxanthin Supplements on Serum and Macular Pigment in Xanthophyll-Free Rheus Monkeys. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3234–3243. [Google Scholar] [CrossRef] [Green Version]
- Barker, F.M.; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional Manipulation of Primate Retinas, V: Effects of Lutein, Zeaxanthin, and n-3 Fatty Acids on Retinal Sensitivity to Blue-Light-Induced Damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Feeney-Burns, L.; Neuringer, M.; Gao, C. Macular Pathology in Monkeys Fed Semi-Purified Diets. Prog. Clin. Biol. Res. 1989, 214, 601–622. [Google Scholar]
- Lorenzoni, F.; Giampietri, M.; Ferri, G.; Lunardi, S.; Madrigali, V.; Battini, L.; Boldrini, A.; Ghirri, P. Lutein Administration to Pregnant Women with Gestational Diabetes Mellitus Is Associated to a Decrease of Oxidative Stress in Newborns. Gynecol. Endocrinol. 2013, 29, 901–903. [Google Scholar] [CrossRef]
- Henriksen, B.S.; Chan, G.; Hoffman, R.O.; Sharifzadeh, M.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S. Interrelationships between Maternal Carotenoid Status and Newborn Infant Macular Pigment Optical Density and Carotenoid Status. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5568–5578. [Google Scholar] [CrossRef]
- Neuringer, M.; Bone, R.A.; Jeffrey, B.; Bettler, J.; Zimmer, J.P.; Wallace, P.; DeRusso, P.A. Lutein in Breastmilk and Infant Formula: Effects on Serum Lutein, Macular Pigment and Visual Function. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1707. [Google Scholar]
- Liu, Z.; Meyers, K.J.; Johnson, E.J.; Snodderly, M.; Tinker, L.; Wallace, R.; Sarto, G.; Mares, J.A. Exposure to Lutein in Infancy via Breast Milk and Later Life Macular Pigment Optical Density. Investig. Ophthalmol. Vis. Sci. 2015, 56, 192. [Google Scholar]
- Renzi, L.M.; Hammond, B.R. The Relation between the Macular Carotenoids, Lutein and Zeaxanthin, and Temporal Vision. Ophthalmic Physiol. Opt. 2010, 30, 351–357. [Google Scholar] [CrossRef]
- Loughman, J.; Nolan, J.M.; Beatty, S. Impact of Dietary Carotenoid Deprivation on Macular Pigment and Serum Concentrations of Lutein and Zeaxanthin. Br. J. Nutr. 2012, 108, 2102–2103. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Lin, X.M.; Zou, Z.Y.; Xu, X.R.; Li, Y.; Xu, R. A 12-Week Lutein Supplementation Improves Visual Function in Chinese People with Long-Term Computer Display Light Exposure. Br. J. Nutr. 2009, 102, 186–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juturu, V.; Stringham, J.M. Lutein and Zeaxanthin Isomers (L/Zi) Supplementation Improves Visual Function, Performance and Sleep Quality in Individuals Using Computer Devices (CDU)–A Double Blind Randomized Placebo Controlled Study. Biomed. J. Sci. Tech. Res. 2018, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; MacKey, A.D.; Dimmit, R.A.; Hartmann, E.E.; et al. Effect of Carotenoid Supplementation on Plasma Carotenoids, Inflammation and Visual Development in Preterm Infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Capeding, R.; Gepanayao, C.P.; Calimon, N.; Lebumfacil, J.; Davis, A.M.; Stouffer, N.; Harris, B.J. Lutein-Fortified Infant Formula Fed to Healthy Term Infants: Evaluation of Growth Effects and Safety. Nutr. J. 2010, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mackey, A.D.; Albrecht, D.; Oliver, J.; Williams, T.; Long, A.C.; Price, P.T. Plasma Carotenoid Concentrations of Infants Are Increased by Feeding a Milk-Based Infant Formula Supplemented with Carotenoids. J. Sci. Food Agric. 2013, 93, 1945–1952. [Google Scholar] [CrossRef]
- Kon, I.Y.; Gmoshinskaya, M.V.; Safronova, A.I.; Alarcon, P.; Vandenplas, Y. Growth and Tolerance Assessment of a Lutein-Fortified Infant Formula. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romagnoli, C.; Tirone, C.; Persichilli, S.; Gervasoni, J.; Zuppi, C.; Barone, G.; Zecca, E. Lutein Absorption in Premature Infants. Eur. J. Clin. Nutr. 2010, 64, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tei, M.; Longini, M.; Santacroce, A.; Turrisi, G.; Proietti, F.; Felici, C.; Picardi, A.; Bazzini, F.; Vasarri, P.; et al. Lipid and Protein Oxidation in Newborn Infants after Lutein Administration. Oxid. Med. Cell. Longev. 2014. [Google Scholar] [CrossRef] [Green Version]
- Perrone, S.; Longini, M.; Marzocchi, B.; Picardi, A.; Bellieni, C.V.; Proietti, F.; Rodriguez, A.; Turrisi, G.; Buonocore, G. Effects of Lutein on Oxidative Stress in the Term Newborn: A Pilot Study. Neonatology 2010, 97, 36–40. [Google Scholar] [CrossRef]
- Romagnoli, C.; Costa, S.; Giannantonio, C. Lutein and the Retinopathy of Prematurity. In Handbook of Nutrition, Diet and the Eye; Academic Press: Cambridge, MA, USA, 2014; pp. 459–464. [Google Scholar] [CrossRef]
- Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; Di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G.; et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J. Clin. Med. 2020, 9, 2711. [Google Scholar] [CrossRef]
- Jewell, V.C.; Northrop-Clewes, C.A.; Tubman, T.R.J.; Thurnham, D.I. Nutritional Factors and Visual Function in Premature Infants. Proc. Nutr. Soc. 2001, 60, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, P.S.; Sharifzadeh, M.; Liu, A.; Ermakov, I.; Nelson, K.; Sheng, X.; Panish, C.; Carlstrom, B.; Hoffman, R.O.; Gellermann, W. Blue-Light Reflectance Imaging of Macular Pigment in Infants and Children. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4034–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.; Giannantonio, C.; Romagnoli, C.; Vento, G.; Gervasoni, J.; Persichilli, S.; Zuppi, C.; Cota, F. Effects of Lutein Supplementation on Biological Antioxidant Status in Preterm Infants: A Randomized Clinical Trial. J. Matern. Neonatal Med. 2013, 26, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Bellettato, M.; et al. Lutein and Zeaxanthin Supplementation in Preterm Very Low-Birth-Weight Neonates in Neonatal Intensive Care Units: A Multicenter Randomized Controlled Trial. Am. J. Perinatol. 2013, 30, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, C.; Lori, I.; Favelli, F.; Frosini, S.; Messner, H.; Wanker, P.; De Marini, S.; Oretti, C.; Boldrini, A.; Ciantelli, M.; et al. Lutein and Zeaxanthin Supplementation in Preterm Infants to Prevent Retinopathy of Prematurity: A Randomized Controlled Study. J. Matern. Neonatal Med. 2012, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Cota, F.; Costa, S.; Giannantonio, C.; Purcaro, V.; Catenazzi, P.; Vento, G. Lutein Supplementation and Retinopathy of Prematurity: A Meta-Analysis. J. Matern. Neonatal Med. 2020, 1–6. [Google Scholar] [CrossRef]
- Craft, N.E.; Haitema, T.B.; Gaernett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoids, Tocopherol and Retinol Concentration in Elderly Human Brain. J. Nutr. 2004, 8, 156–162. [Google Scholar]
- Lieblein-Boff, J.C.; Johnson, E.J.; Kennedy, A.D.; Lai, C.S.; Kuchan, M.J. Exploratory Metabolomic Analyses Reveal Compounds Correlated with Lutein Concentration in Frontal Cortex, Hippocampus, and Occipital Cortex of Human Infant Brain. PLoS ONE 2015, 10, e0136904. [Google Scholar] [CrossRef] [Green Version]
- Tanprasertsuk, J.; Li, B.; Bernstein, P.S.; Vishwanathan, R.; Johnson, M.A.; Poon, L.; Johnson, E.J. Relationship between Concentrations of Lutein and Stard3 among Pediatric and Geriatric Human Brain Tissue. PLoS ONE 2016, 11, e0155488. [Google Scholar] [CrossRef] [Green Version]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular Pigment Carotenoids in the Retina and Occipital Cortex Are Related in Humans. Nutr. Neurosci. 2016, 19, 95–101. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Neuringer, M.; Max Snodderly, D.; Schalch, W.; Johnson, E.J. Macular Lutein and Zeaxanthin Are Related to Brain Lutein and Zeaxanthin in Primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohn, E.S.; Erdman, J.W.; Kuchan, M.J.; Neuringer, M.; Johnson, E.J. Lutein Accumulates in Subcellular Membranes of Brain Regions in Adult Rhesus Macaques: Relationship to DHA Oxidation Products. PLoS ONE 2017, 12, e0186767. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Wooten, B.R. CFF Thresholds: Relation to Macular Pigment Optical Density. Ophthalmic Physiol. Opt. 2005, 25, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Iannaccone, A.; Scott, T.M.; Kritchevsky, S.B.; Jennings, B.J.; Carboni, G.; Forma, G.; Satterfield, S.; Harris, T.; Johnson, K.C.; et al. Macular Pigment Optical Density Is Related to Cognitive Function in Older People. Age Ageing 2014, 43, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.; Coen, R.F.; Akuffo, K.O.; Beatty, S.; Dennison, J.; Moran, R.; Stack, J.; Howard, A.N.; Mulcahy, R.; Nolan, J.M. Cognitive Function and Its Relationship with Macular Pigment Optical Density and Serum Concentrations of Its Constituent Carotenoids. J. Alzheimer’s Dis. 2015, 48, 261–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajana, S.; Weber, D.; Helmer, C.; Merle, B.M.; Stuetz, W.; Dartigues, J.F.; Rougier, M.B.; Korobelnik, J.F.; Grune, T.; Delcourt, C.; et al. Plasma Concentrations of Lutein and Zeaxanthin, Macular Pigment Optical Density, and Their Associations with Cognitive Performances among Older Adults. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1828–1835. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Walk, A.M.; Edwards, C.G.; Jones, A.R.; Cannavale, C.N.; Thompson, S.V.; Reeser, G.E.; Holscher, H.D. Macular Xanthophylls Are Related to Intellectual Ability among Adults with Overweight and Obesity. Nutrients 2018, 10, 396. [Google Scholar] [CrossRef] [Green Version]
- Mewborn, C.M.; Terry, D.P.; Renzi-Hammond, L.M.; Hammond, B.R.; Miller, L.S. Relation of Retinal and Serum Lutein and Zeaxanthin to White Matter Integrity in Older Adults: A Diffusion Tensor Imaging Study. Arch. Clin. Neuropsychol. 2018, 33, 861–874. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Lindbergh, C.A.; Hammond, B.R.; Renzi-Hammond, L.M.; Miller, L.S. The Effects of Lutein and Zeaxanthin Supplementation on Brain Morphology in Older Adults: A Randomized, Controlled Trial. J. Aging Res. 2019, 2019. [Google Scholar] [CrossRef]
- Oliver, W.; Renzi-Hammond, L.M.; Thorne, S.A.; Clementz, B.; Miller, L.S.; Hammond, B.R. Neural Activation During Visual Attention Differs in Individuals with High versus Low Macular Pigment Density. Mol. Nutr. Food Res. 2019, 63, 1–8. [Google Scholar] [CrossRef]
- Ceravolo, S.A.; Hammond, B.R.; Oliver, W.; Clementz, B.; Miller, L.S.; Renzi-Hammond, L.M. Dietary Carotenoids Lutein and Zeaxanthin Change Brain Activation in Older Adult Participants: A Randomized, Double-Masked, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2019, 63, e1801051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringham, N.; Holmes, P.; Stringham, J. Effects of Macular Xanthophyll Supplementation on Brain-Derived Neurotrophic Factor, pro-Inflammatory Cytokines, and Cognitive Performance. Physiol. Behav. 2019, 1, 112650. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Rasmussen, H.M.; Chen, O.; Johnson, E.J. Avocado Consumption Increases Macular Pigment Density in Older Adults: A Randomized, Controlled Trial. Nutrients 2017, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.G.; Walk, A.M.; Thompson, S.V.; Reeser, G.E.; Dilger, R.N.; Erdman, J.W.; Burd, N.A.; Holscher, H.D.; Khan, N.A. Dietary Lutein plus Zeaxanthin and Choline Intake Is Interactively Associated with Cognitive Flexibility in Middle-Adulthood in Adults with Overweight and Obesity. Nutr. Neurosci. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Alzheimer’s Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.G.; Walk, A.M.; Thompson, S.V.; Reeser, G.E.; Erdman, J.W.; Burd, N.A.; Holscher, H.D.; Khan, N.A. Effects of 12-Week Avocado Consumption on Cognitive Function among Adults with Overweight and Obesity. Int. J. Psychophysiol. 2020, 148, 13–24. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Erdman, J.W.; Hathcock, J.; Ellwood, K.; Beatty, S.; Johnson, E.; Marchioli, R.; Lauritzen, L.; Rice, H.B.; Shao, A.; et al. Nutrient Reference Values for Bioactives: New Approaches Needed? A Conference Report. Eur. J. Nutr. 2013, 52, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W. Dietary Guidance for Lutein: Consideration for Intake Recommendations Is Scientifically Supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef] [Green Version]

| Population | Age | L + Z Estimated Intake Based on Recommended 3–5 Servings F&V/day | Average Daily L + Z Intakes |
|---|---|---|---|
| General population, USA [50] | 2 years and older | Mean 3.83 90th percentile 7.29 | - |
| General population, USA [52] | 1–18 years | - | Mean < 0.6 |
| Females of childbearing potential, USA [52] | 19–50 years | - | Mean < 2 |
| Pregnant women, USA [59] | 19–43 years | - | 2.48 |
| Pregnant women, Italy [60] | 20–25 years | - | 1 |
| Breastfeeding women, Italy [61] | 24–42 years | - | 1.2 |
| Outcome | Xanthophyll-Free Animals | L/Z, L or Z Supplemented Animals |
|---|---|---|
| Serum levels | Undetectable levels of L and Z | Increase levels of L and/or Z |
| Macular pigment | No yellow macular pigmentation | Accumulation of macular pigment |
| Retina | Distinct changes in the RPE cell profile (foveal dip) and density (increased cell density). Increase in macular hyperfluorescence and mottling of the RPE although in absence of major visual disturbances. Prominent presence of drusen-like bodies at the level of the pigment epithelium. | Attenuation of the structural changes in RPE cell profile (central foveal peak), presence of asymmetry in the RPE profile suggesting that L and Z could stimulate cell migration. |
| Blue Light sensitivity | Increased vulnerability to acute blue-light induced damage in the foveal region | Decreased foveal vulnerability to acute blue-light exposure |
| Author | Year | Age | n | Key Findings |
|---|---|---|---|---|
| Mahmassani [81] | 2021 | Pregnancy I trimester (median 9.9 WG) Pregnancy II trimester (median 27.9 WG) Infancy (5.2–10.0 months) Early-Childhood (2.8–6.2 years) Mid-Childhood (6.6–10.9 years) | 1580 mother-child pairs | Greater maternal L/Z intakes in the I-II trimester were associated with better verbal intelligence (main analysis) and better behavior regulation ability (secondary analyses) in mid-childhood. Higher maternal I trimester intake of L/Z-rich foods was associated with better social-emotional development and behavioral regulation ability in this same age group. No benefits of greater maternal L/Z intakes were observed in infancy and early childhood |
| Saint [124] | 2018 | 7–13 years | 51 | Link between higher carotenoid status and improved cognitive functioning. MPOD was significantly correlated to global Intelligence (Brief Intellectual Ability) and executive processes composite scores. Exploratory analysis also showed positive associations with spatial relations subtest. |
| Barnett [83] | 2018 | 8–9 years old | 56 | MPOD is positively related to academic achievement, mathematics, and written language composite standard scores in school children. |
| Walk [123] | 2017 | 8–10 years | 49 | MPOD is correlated (p < 0.05) with cognitive control performance. Children with higher MPOD present higher accuracy in performing tasks which require cognitive control processing (modified flanker task) and require the allocation of less attentional resources to perform the task (smaller P3 amplitudes in the EEG recordings). |
| Hassevoort [122] | 2017 | 7–10 years | 40 | MPOD is positively associated with a spatial reconstruction task designed to assess relational memory performance, a hippocampal-dependent function, even after accounting for IQ and aerobic fitness. |
| Cheatham [82] | 2015 | 6 month-old | 55 | High L & High Choline in maternal breast milk are associated with better infant recognition memory (difference in latency to peak amplitude scores at frontal and central areas in EEG recordings (p < 0.05 and p < 0.001; respectively) |
| Mulder [121] | 2014 | 5.6–5.9 years | 160 | L intake and L serum levels showed no association with child cognitive tests. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzolo, D.; Picone, S.; Gaiero, A.; Bellettato, M.; Montrone, G.; Riccobene, F.; Lista, G.; Pellegrini, G. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview. Nutrients 2021, 13, 3239. https://doi.org/10.3390/nu13093239
Gazzolo D, Picone S, Gaiero A, Bellettato M, Montrone G, Riccobene F, Lista G, Pellegrini G. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview. Nutrients. 2021; 13(9):3239. https://doi.org/10.3390/nu13093239
Chicago/Turabian StyleGazzolo, Diego, Simonetta Picone, Alberto Gaiero, Massimo Bellettato, Gerardo Montrone, Francesco Riccobene, Gianluca Lista, and Guido Pellegrini. 2021. "Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview" Nutrients 13, no. 9: 3239. https://doi.org/10.3390/nu13093239






