The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
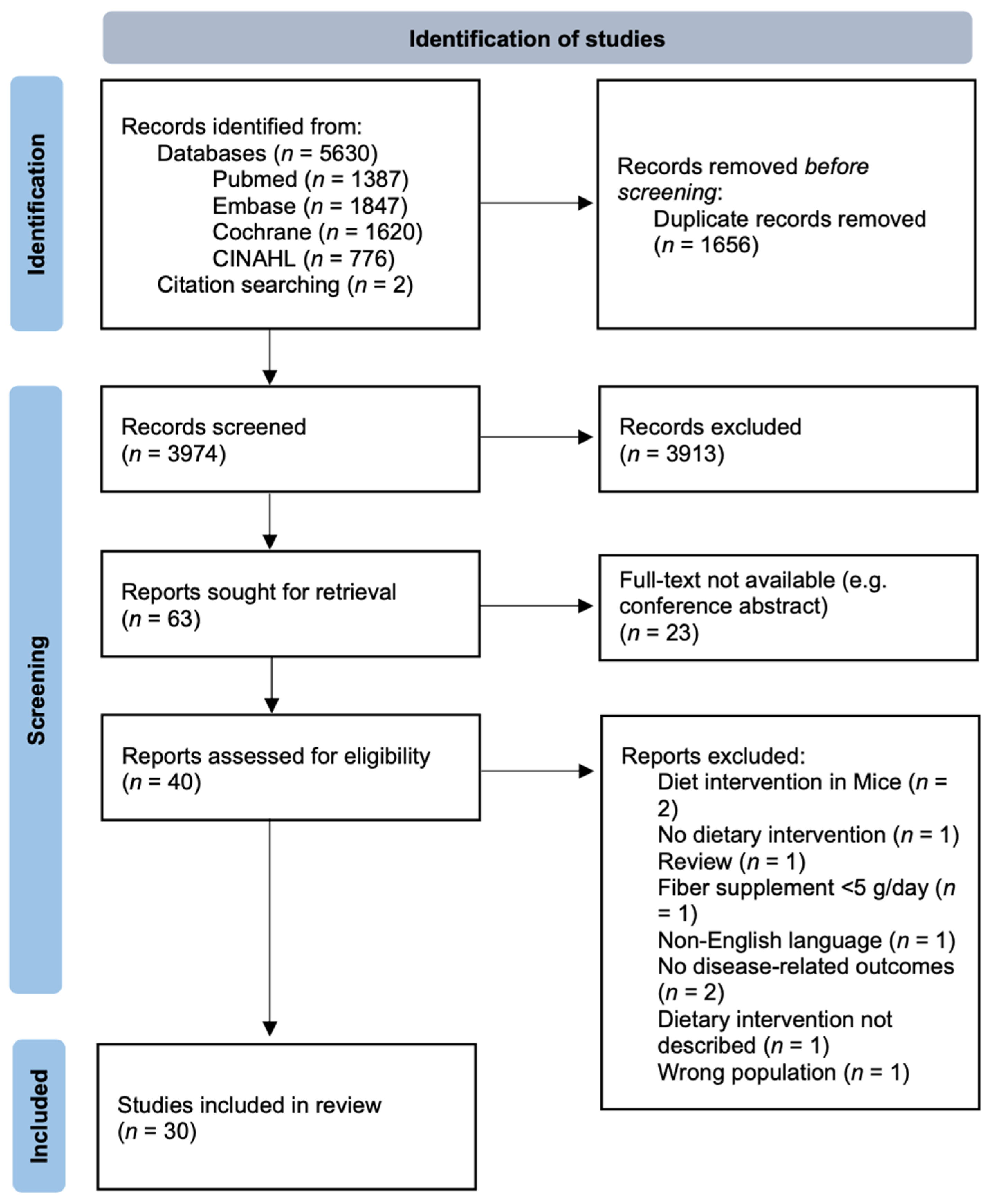
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Presentation
3. Results
3.1. Effect of Dietary Interventions on Disease-Specific Outcomes and the Microbiome
3.1.1. Low-Fiber Dietary Interventions
3.1.2. High-Fiber Dietary Interventions
Mediterranean Diet
High-Fiber Diet
Vegetarian and Vegan Diet
3.1.3. Supplemental Fiber Dietary Interventions
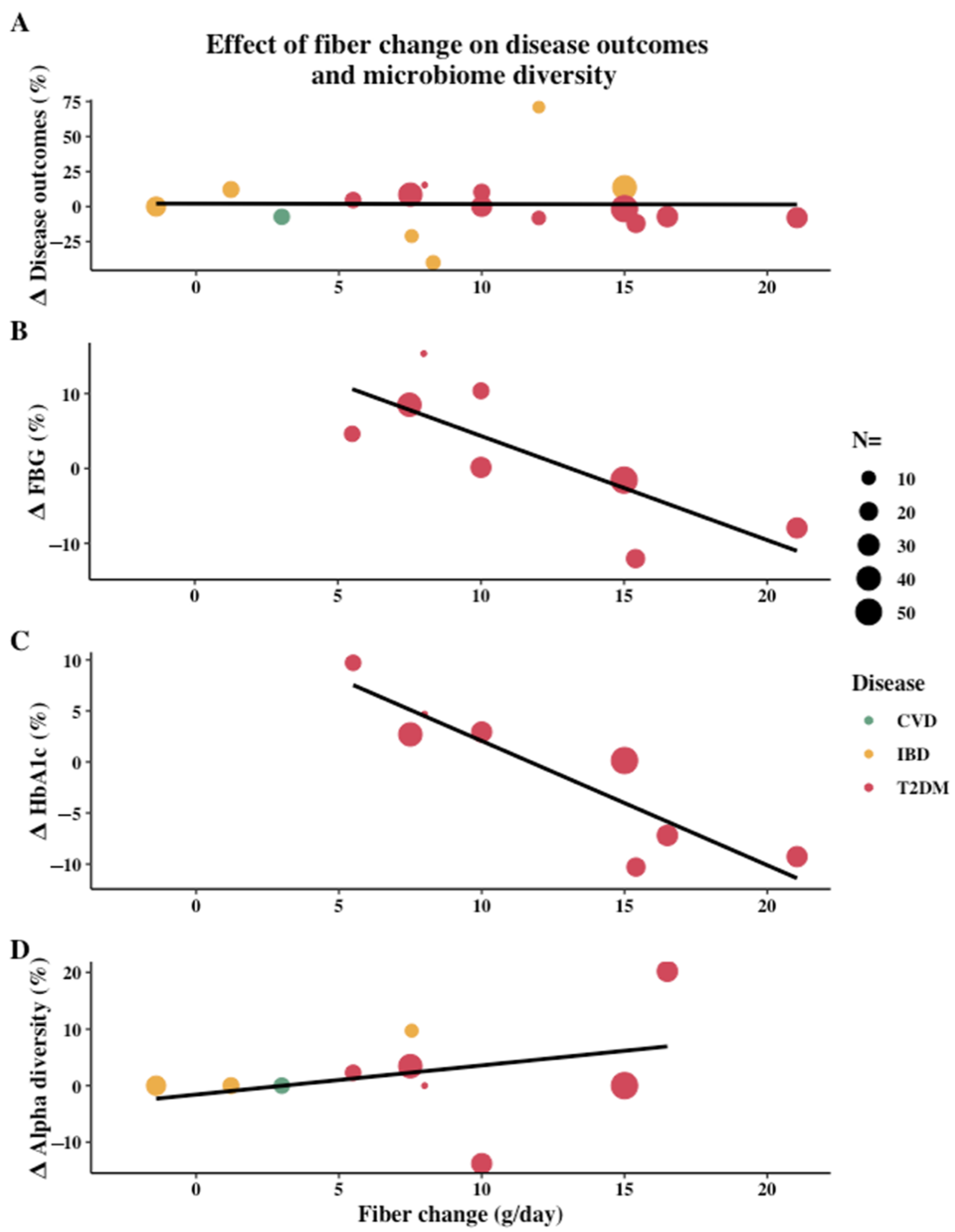
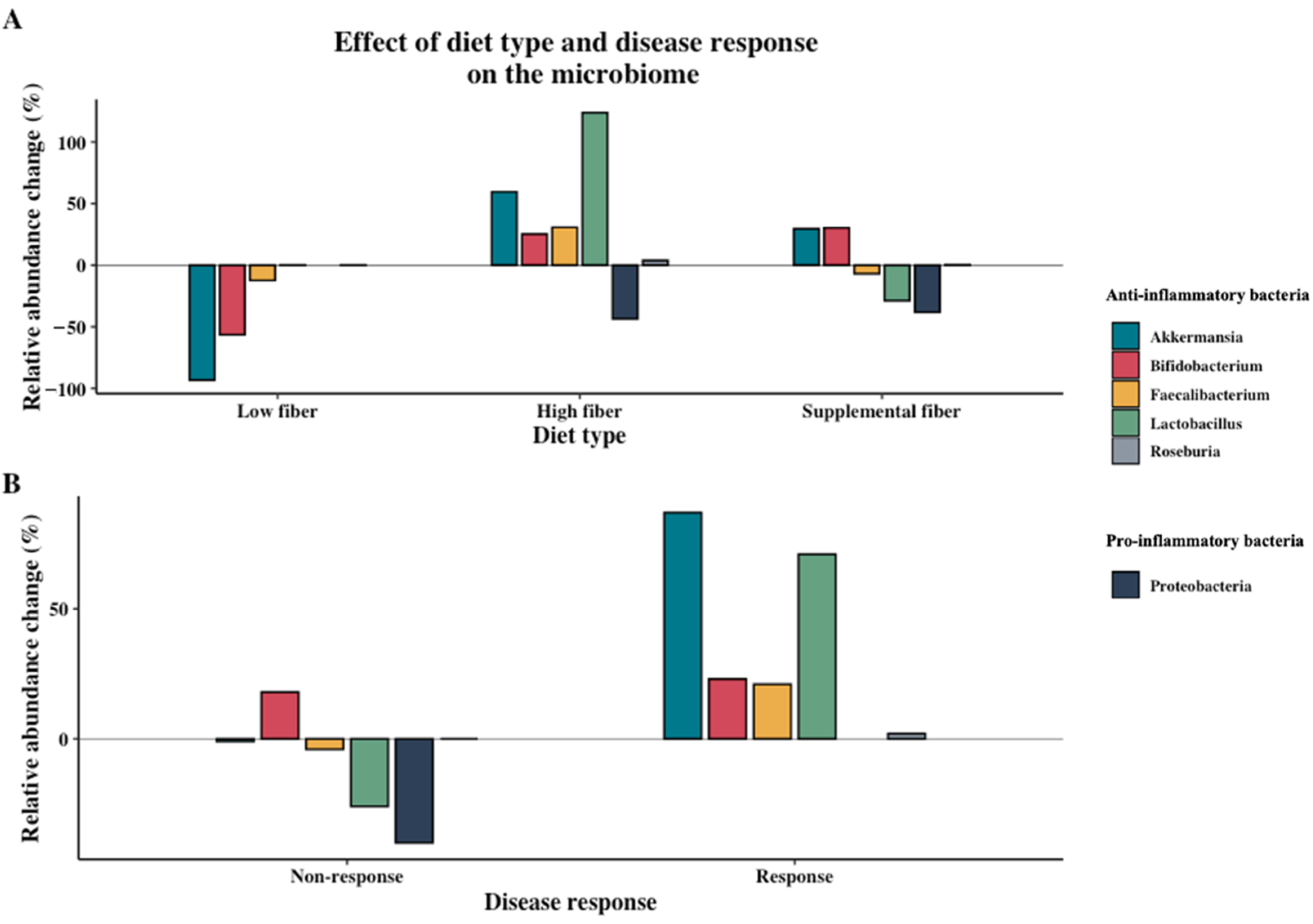
3.2. Effect of Dietary Intervention Type and Fiber
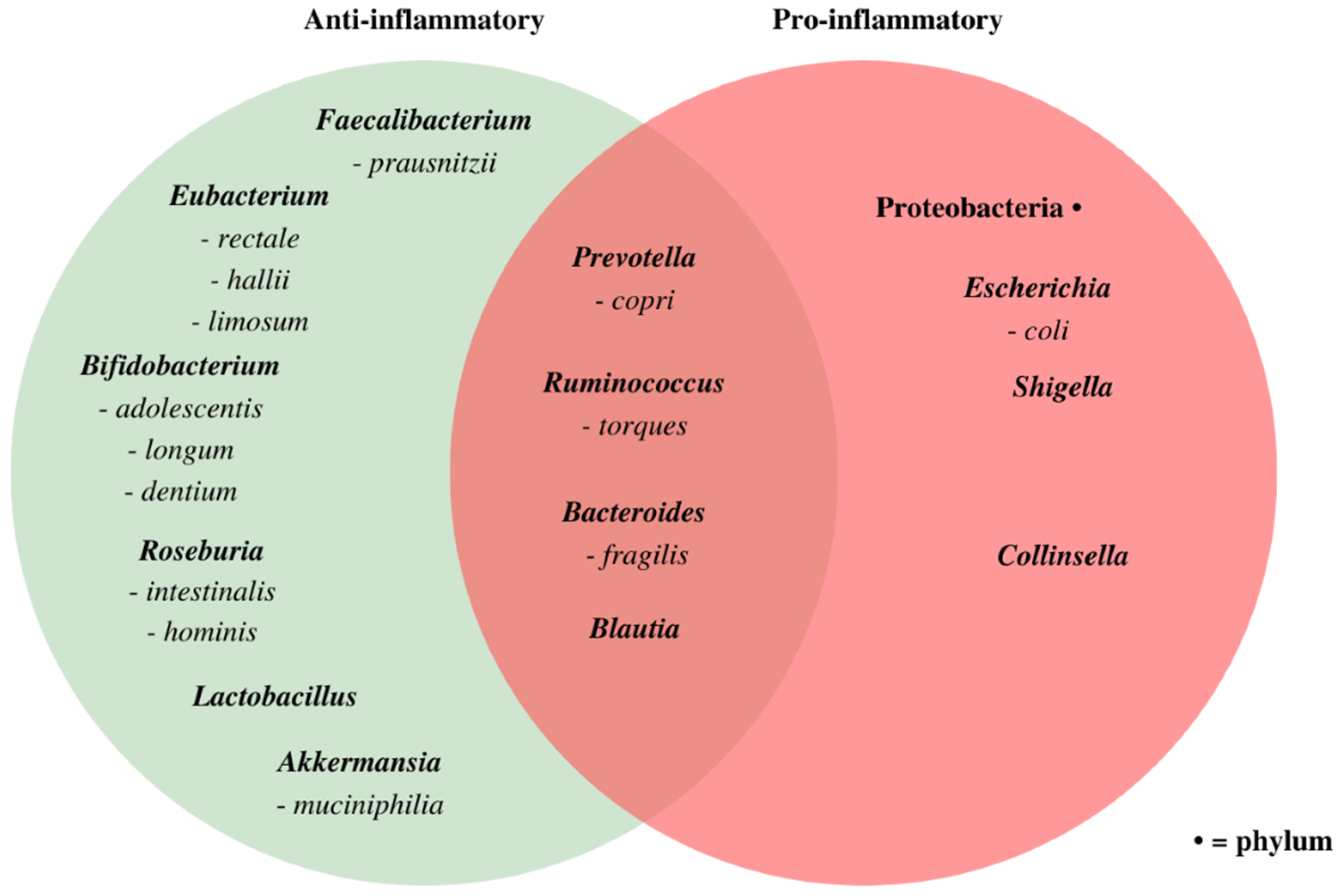
3.3. Associations between Microbiome Composition and Disease
4. Discussion
4.1. Clinical Benefits Are Mainly Seen with High-Fiber Diets
4.2. High-fiber Diets Can Increase Microbial Diversity and SCFA-Producing Bacteria
4.3. Methodological Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol. 2013, 149, 84–91. [Google Scholar] [CrossRef]
- Dregan, A.; Charlton, J.; Chowienczyk, P.; Gulliford, M.C. Chronic Inflammatory Disorders and Risk of Type 2 Diabetes Mellitus, Coronary Heart Disease, and Stroke. Circulation 2014, 130, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fu, L.; Shi, J.; Chen, X.; Li, Y.; Ma, B.; Zhnag, Y. The risk of metabolic syndrome in patients with rheumatoid arthritis: A me-ta-analysis of observational studies. PLoS ONE 2013, 8, e78151. [Google Scholar] [CrossRef] [Green Version]
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Proctor, M.J.; McMillan, D.; Horgan, P.G.; Fletcher, C.D.; Talwar, D.; Morrison, D. Systemic Inflammation Predicts All-Cause Mortality: A Glasgow Inflammation Outcome Study. PLoS ONE 2015, 10, e0116206. [Google Scholar] [CrossRef] [Green Version]
- Burska, A.N.; Sakthiswary, R.; Sattar, N. Effects of Tumour Necrosis Factor Antagonists on Insulin Sensitivity/Resistance in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0128889. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Willett, W.C. Balancing Life-Style and Genomics Research for Disease Prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Birbeck, G.; Burstein, R.; Chou, D.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Smidowicz, A.; Regula, J. Effect of Nutritional Status and Dietary Patterns on Human Serum C-Reactive Protein and Interleukin-6 Concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G., 3rd; Al-Solaiman, Y.; Jesri, A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C.; Rinninella, E.; Raoul, P.; Cintoni, M.; et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2019, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; Faria, A.V.D.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C.; Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, J.; Yuan, W.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Astbury, S.; Atallah, E.; Vijay, A.; Aithal, G.P.; I Grove, J.; Valdes, A.M. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes 2019, 11, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2017, 49, 140–147. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [Green Version]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prev-alence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; del Pugar, E.M.G.; López-Almela, I.; Moya-Pérez, A.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5, e00857-19. [Google Scholar] [CrossRef] [Green Version]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2015, 65, 1812–1821. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Holven, K.B.; Ulven, S.M. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018, 10, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, C.T.M.; Buurma-Rethans, E.J.M.; Dinissen, C.S.; Brands, H.A.M.; Dekkers, A.L.M.; Ocké, M.C. The Diet of the Dutch—Results of the Dutch National Food Consumptions Survey 2012–2016; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2020; p. 119. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.; Hernán, M.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.S. How to institute the low-FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 8–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorathia, A.Z.; Sorathia, S.J. Low Residue Diet; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [Green Version]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota with Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Walters, S.S.; Quiros, A.; Rolston, M.; Grishina, I.; Li, J.; Fenton, A.; DeSantis, T.Z.; Thai, A.; Andersen, G.L.; Papathakis, P.; et al. Analysis of Gut Microbiome and Diet Modification in Patients with Crohn’s Disease. SOJ Microbiol. Infect. Dis. 2014, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.E.; Cai, C.; Deehan, E.C.; et al. A Diversified Dietary Pattern Is Associated with a Balanced Gut Microbial Composition of Faecalibacterium and Escherichia/Shigella in Patients with Crohn’s Disease in Remission. J. Crohn?s Coliti 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Suga, T.; Tochihara, M.; Hibi, T.; Naganuma, M.; Homma, T.; Asakura, H.; Nakano, H.; Takahama, K.; Fujiyama, Y.; et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: First report of a multicenter open control trial. J. Gastroenterol. 2002, 37, 67–72. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, place-bo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, R.; Kjeldsen-Kragh, J.; Haugen, M.; Tuominen, J.; Toivanen, P.; Førre, Ø.; Eerola, E. Changes of Faecal flora in Rheumatoid arthritis during fasting and one-year vegetarian diet. Rheumatology 1994, 33, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, R.; Nenonen, M.; Helve, T.; Hanninen, O.; Toivanen, P.; Eerola, E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Rheumatology 1997, 36, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Michalsen, A.; Riegert, M.; Lüdtke, R.; Bäcker, M.; Langhorst, J.; Schwickert, M.; Dobos, G.J. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: An observational study. BMC Complement. Altern. Med. 2005, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Abendroth, A.; Michalsen, A.; Lüdtke, R.; Rüffer, A.; Musial, F.; Dobos, G.J.; Langhorst, J. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch. Komplementmed. 2010, 17, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Montes-Borrego, M.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Delgado, F.G.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; García, A.C.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; Lopez-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects With Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shao, W.; Gao, M.; Liu, J.; Guo, Q.; Jin, J.; Meng, F. Changes in intestinal flora in patients with type 2 diabetes on a low-fat diet during 6 months of follow-up. Exp. Ther. Med. 2020, 20, 40. [Google Scholar] [CrossRef]
- Ismael, S.; Silvestre, M.; Vasques, M.; Araújo, J.; Morais, J.; Duarte, M.; Pestana, D.; Faria, A.; Pereira-Leal, J.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host–microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef] [Green Version]
- Gonai, M.; Shigehisa, A.; Kigawa, I.; Kurasaki, K.; Chonan, O.; Matsuki, T.; Yoshida, Y.; Aida, M.; Hamano, K.; Terauchi, Y. Galacto-oligosaccharides ameliorate dysbiotic Bifidobacteriaceae decline in Japanese patients with type 2 diabetes. Benef. Microbes 2017, 8, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Saadat, Y.R.; Alamdari, N.M.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Jafarabadi, M.A.; Alizadeh, E.; Ghavami, A.; Saadat, Y.R.; Alamdari, N.M.; Dastouri, M.R.; Alipour, S.; Ostadrahimi, A. The effects of sodium butyrate and high-performance inulin supplementation on the promotion of gut bacterium Akkermansia muciniphila growth and alterations in miR-375 and KLF5 expression in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Eur. J. Integr. Med. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Lee, S.-E.; Choi, Y.; Jun, J.E.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Ko, G.P.; Lee, M.-K. Additional Effect of Dietary Fiber in Patients with Type 2 Diabetes Mellitus Using Metformin and Sulfonylurea: An Open-Label, Pilot Trial. Diabetes Metab. J. 2019, 43, 422–431. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sánchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2018, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2019, 59, 2969–2983. [Google Scholar] [CrossRef] [Green Version]
- Reimer, R.A.; Wharton, S.; Green, T.J.; Manjoo, P.; Ramay, H.R.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2020, 1–15. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Borchgrevink, C.; Laerum, E.; Haugen, M.; Eek, M.; Førre, Ø.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Slavin, J., Jr.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Threapleton, D.; Greenwood, D.C.; Evans, C.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, L.; Brown, L.; Forrester, T.E.; Plange-Rhule, J.; Bovet, P.; Lambert, E.V.; Layden, B.T.; Luke, A.; Dugas, L.R. The Association of Dietary Fiber Intake with Cardiometabolic Risk in Four Countries across the Epidemiologic Transition. Nutrients 2018, 10, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Orlich, M.J.; E Fraser, G. Vegetarian diets in the Adventist Health Study 2: A review of initial published findings. Am. J. Clin. Nutr. 2014, 100, 353S–358S. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low car-bohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lacoba, R.; Pardo-Garcia, I.; Amo-Saus, E.; Sotos, F.E. Mediterranean diet and health outcomes: A systematic meta-review. Eur. J. Public Health 2018, 28, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2013, 99, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Alwarith, J.; Rembert, E.; Brandon, L.; Nguyen, M.; Goergen, A.; Horne, T.; Nascimento, G.F.D.; Lakkadi, K.; Tura, A.; et al. A Mediterranean Diet and Low-Fat Vegan Diet to Improve Body Weight and Cardiometabolic Risk Factors: A Randomized, Cross-over Trial. J. Am. Coll. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Nestle, M. Mediterranean diets: Historical and research overview. Am. J. Clin. Nutr. 1995, 61, 1313S–1320S. [Google Scholar] [CrossRef]
- McRorie, J.W. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2020, 79, 410–428. [Google Scholar] [CrossRef]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollakova, D.; Andreadi, A.; Pacifici, F.; Della-Morte, D.; Lauro, D.; Tubili, C. The Impact of Vegan Diet in the Prevention and Treatment of Type 2 Diabetes: A Systematic Review. Nutrients 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet Advice for Crohn’s Disease: FODMAP and Beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 58, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Heal. Aging 2016, 4, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhrstad, M.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, T. Pitfalls in short-chain fatty acid research: A methodological review. Anim. Sci. J. 2018, 90, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-García, O.; Castro-Alarcón, N.; Pérez-Rubio, G.; Guzmán-Guzmán, I.P. DMARDs–Gut Microbiota Feedback: Implications in the Response to Therapy. Biomolecules 2020, 10, 1479. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of indi-viduals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Debelius, J.; Song, S.J.; Vazquez-Baeza, Y.; Xu, Z.Z.; Gonzalez, A.; Knight, R. Tiny microbes, enormous impacts: What matters in gut microbiome studies? Genome Biol. 2016, 17, 217. [Google Scholar] [CrossRef] [Green Version]
- Iyer, N. Methods in microbiome research. Lab Anim. 2016, 45, 323–326. [Google Scholar] [CrossRef] [Green Version]

| First Author, Publication Year, Journal | Disease and Number of Cases (n = Enrolled (# of Dropouts)) | Study Design and Duration | Type of Intervention | Disease-Specific Outcome Baseline | Disease-Specific Outcome End Intervention | Change | Remarks | Gut Microbiome End Intervention | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | p-Value | Change | Remarks | |||||
| (a) Inflammatory Bowel Disease | |||||||||||
| Walters 2014 SOJ Microbiology and Infectious Diseases [54] | Crohn’s disease (n = 6 (2)) | Double-blinded cross-over RCT 12 weeks (4 weeks per intervention, 4-week wash-out) | Low fiber Specific carbohydrate diet (SCD) vs. low residue diet (LRD) | - | - | - | - | - | No numerical results. No significant clinical improvement in IBD patients receiving the SCD diet. | ↑ Diversity A general increase in diversity was observed mainly due to an increase of non-pathogenic clostridia species in SCD vs. LRD groups. | |
| Halmos 2016 Clinical and Translational Gastroenterology [53] | Crohn’s disease (n = 9) | Single-blinded (participant) cross-over RCT 9 weeks (3 weeks per intervention, 3-week wash-out) | Low fiber Low FODMAP diet with 8 g/day prebiotic vs. typical (Australian) diet | - | - | - | - | - | No numerical results. Figure 1 showed no observable difference in FC between groups. | ↔ SCFA ↑ Ruminococcus torques ↔ Bifidobacterium ↓ Clostridium coccoides group ↓ A. municinphilla ↔ F. prausnitzii | 8 participants collected fecal samples and n = 7 for microbial analysis of A. muciniphila. |
| Cox 2020 Gastroenterology [52] | Ulcerative colitis and Crohn’s disease (n = 52 (6)) | Single-blinded (participant) RCT 4 weeks | Low fiber Low FODMAP diet vs. Sham diet | FC (μg/g): 54.8 (84.8) | FC: 70.9 (117.3) | PMS: 0.2 [0.2] HBIS: 3.2 [0.4] FC: 53.3 (84.8) CRP (mg/L): 2.0 [0.3] | PMS: 0.2 [0.2] HBIS: 3.4 [0.5] FC: 66.9 (106.4) CRP: 1.6 [0.3] | p = 0.951 p = 0.841 p = 0.976 p = 0.246 | p-value of FC based on intervention (60.0 [9.4]) vs. control (59.6 [9.8] (μg/g)) | ↔Alpha diversity ↔ Total SCFA ↔ Total Bifidobacterium ↓ B. Adolescentis ↓ B. Longum ↑ B. Dentium ↓ F. Prausnitzii | Micobiome outcomes measured with quantitative metagenomic pipeline. Alpha diversity measured using Shannon index. Outcomes reported per protocol (n = 43). |
| Marlow 2013 Human Genomics [56] | Crohn’s disease (n = 8) | Uncontrolled trial 6 weeks | High fiber Mediterranean-inspired anti-inflammatory diet | - | NA | - | NA | - | No numerical results, CRP showed a trend of reduction (p = 0.39). | Clostridium leptum group and Bacteroidetes increased. Proteobacteria and Bacillaceae decreased. | No statistical analysis |
| Fritsch 2020 Clinical Gastroenterology and Hepatology [57] | Ulcerative colitis (n = 26 (9)) | Nonblinded cross-over RCT 10 weeks (4 weeks per intervention, 2-week wash-out) | High fiber Low fat, high fiber diet (LFD) vs. improved standard American diet (iSAD) | PMS: 1.41 [2] FC (μg/g): 88.7 (149) CRP (mg/L): 3.23 (3.88) | PMS: 1.41 [2] FC: 88.7 (149) CRP: 3.23 (3.88) | PMS: 0.6 [1.5] FC: 66.16 (61.52) CRP: 2.51 (1.61) | PMS: 0.76 [2] FC: 124.8 (141.5) CRP: 3.05 (2.92) | p = 0.63 p = 0.19 p = 0.21 | ↔ Alpha diversity ↑ F. Prausnitzii ↔ Prevotella Within LFD: ↔ Alpha diversity ↔ F. Prausnitzii ↑ Prevotella Within iSAD: ↔ Alpha diversity ↔ F. Prausnitzii ↔ Prevotella | Alpha diversity measured using Faith’s phylogenic diversity index | |
| Zhang 2020 Journal of Crohn’s and Colitis [58] | Crohn’s disease (n = 58 (18)) | Nonblinded non-randomized trial 12 weeks | High fiber non-diversified diet (NDD) group (Mediterranean-inspired diet) vs. diversified diet (DD) group (conventional management) | FC (μg/mg): 159.08 ± 277.9 | - | FC: 178.45 ± 224.1 | FC: 115.45 ± 159.4 | p = 0.56 | Within NDD group no significant difference baseline vs. end (p = 0.26) | ↔ Alpha diversity ↔ Beta diversity ↔ Faecalibacterium ↓ Proteobacteria ↓ Escherichia/Shigella Within NDD group: ↔ SCFA ↑ Faecalibacterium ↔ Proteobacteria ↔ Escherichia/Shigella ↔ Bifidobacterium ↔ Akkermansia | Alpha diversity measured using Shannon Index. Beta diversity measured using Manhattan and Gower distance matrices between bacterial communities. |
| Kanauchi 2002 Journal of Gastroenterology [59] | Ulcerative colitis (n = 18) | Single-blinded (observer) RCT 4 weeks | Supplemental fiber 20-30 g/day germinated barley foodstuff (GBF) with baseline medical treatment vs. baseline medical treatment | CAI (Lichtiger method): 8 [5] CRP (ng/mL): 0.96 [1.75] | CAI: 7 [4] CRP: 0.52 [0.63] | CAI: 6 [3] CRP: 0.6 [1] | CAI: 10 [5] CRP: 0.5 [0.5] | p = 0.045 N.S. | Numerical results estimated from Figure 2 for CAI and from Figure 3 for end of intervention CRP. | Within GBF group: Increased Bifidobacterium, Eubacterium limosum, and Lactobacillus. Decreased Bacteroides. | Microbiome outcomes only reported for intervention group and statistical significance not reported. |
| Furrie 2005 Gut [60] | Ulcerative colitis (n = 16 (4)) | Double-blinded RCT 4 weeks | Supplemental fiber 12 g/day of inulin-ogliofructose with Bifidobacterium longum synbiotic per day vs. placebo | CAI (Walmsley method): 5.6 ± 3.7 CRP (mg/L): 6.0 ± 6.5 | CAI: start 4.9 ± 3.2 CRP: 1.6 ± 3.6 | CAI: 5.3 ± 3.4 CRP: 1.8 ± 3.9 | CAI: 3.1 ± 2.5 - | Not reported. | No numerical results for CRP of control group at end intervention, but authors reported that none of the control patients had circulating levels of CRP. | Within prebiotic group: 42-fold increase in Bifidobacterium Within placebo group: 4.6-fold increase in Bifidobacterium | Microbiome outcomes measured using real time PCR in mucosal biopsies. Only within group outcomes reported, no statistical analysis was performed. |
| Benjamin 2011 Gut [61] | Crohn’s disease (n = 103 (18)) | Double-blinded RCT 4 weeks | Supplemental fiber 15 g/day fructo-oligosaccharides (FOS) vs. placebo | CDAI: 283 ± 61 FC (mg/kg): 621.4 ± 559.4 CRP (mg/L): 18.8 ± 21.6 | CDAI: 286 ± 62 FC: 647.9 ± 533.3 CRP: 20.8 ± 23.1 | CDAI: 250 ± 84.9 FC: 657.4 ± 592.5 CRP: 20.9 ± 26.4 | CDAI: 220 ± 88.7 FC: 829.8 ± 635.9 CRP: 20.2 ± 24.1 | p = 0.112 p = 0.322 p = 0.902 | FC reported per protocol (n = 60). | ↔ F. Prausnitzii ↔ Bifidobacterium | Microbiome outcomes measured with FISH technique. |
| (b) Rheumatoid Arthritis | |||||||||||
| Peltonen 1994 British Journal of Rheumatology [62] | Rheumatoid arthritis (n = 53 (19)) | Single-blinded (observer) RCT 56 weeks | High fiber 7 day fast followed by 1-year vegetarian diet (first 3.5 months vegan) vs. ordinary omnivore diet | DAS28†: 5.3 CRP (mg/L): 23 | DAS28: 4.7 CRP: 23 | DAS28: 3.6 CRP: 19 | DAS28: 5.5 CRP: 30 | - p = 0.0001 | DAS28 estimated from Figure 1 and Figure 2, CRP estimated from Figure 1. All components used in DAS28 were significantly lower in the intervention group vs. control at end intervention. | Significant difference in CFA profiles between baseline samples and end of intervention. | Microbiome outcomes measured using direct gas-liquid chromatography (GLC) to measure bacterial cellular fatty acid (CFA) profiles of stool samples. |
| Peltonen 1997 British Journal of Rheumatology [63] | Rheumatoid arthritis (n = 43 (7)) | Single-blinded (observer) RCT 4 weeks | High fiber Raw vegan diet vs. ordinary omnivore diet | - | - | RADII: 3.1 | RADII: 2.0 | p = 0.027 | No baseline results reported. | The raw vegan group had a significantly lower similarity index to the baseline samples than the control group. | Microbiome outcomes measured using direct gas-liquid chromatography (GLC) to measure bacterial cellular fatty acid (CFA) profiles of stool samples. |
| Michalsen 2005 BMC Complementary and alternative medicine [64] | Rheumatoid arthritis (n = 16) | Nonblinded non-randomized trial 2 weeks | High fiber Mediterranean-inspired (Med) diet vs. fasting | DAS28: 5.2 ± 1.9 | DAS28: 5.2 ± 2.0 | DAS28: 5.0 ± 2.1 | DAS28: 4.5 ± 2.0* | p = 0.09 | Results estimated from Figure 2. | Within both Med diet and fasting groups: ↔ E. coli ↔ Enterococcus ↔ Lactobacillus ↔ Clostridium ↔ Bifidobacterium | No results reported for between groups. |
| Abendroth 2010 Forschende Komplementarmedizin [65] | Rheumatoid arthritis (n = 50) | Nonblinded non-randomized trial 2 weeks | High fiber Mediterranean-inspired (Med) diet vs. fasting | DAS28: 5.4 ± 1.4 CRP (mg/L): 20 ± 27 | DAS28: 5.6 ± 1.1 CRP: 8 ± 1 | DAS28: 4.6 ± 1.1 * CRP: 16 ± 22 | DAS28: 4.1 ± 1.3 * CRP: 7 ± 7 | p = 0.115 p = 0.59 | Results estimated from Figure 1. | ↔ SCFA Within Med diet group: SCFA: 22.9 ± 13.8 to 20.4 ± 9.8 Within Fasting group: SCFA: 26.5 ± 12.9 to 30.0 ± 23.9 | Within group results were not statistically analyzed |
| (c) Cardiovascular Disease | |||||||||||
| Haro 2016 Journal of Clinical Endocrinology and Metabolism [66] | Cardiovascular disease (n = 20) | Single-blinded (observer) RCT 52 weeks | High fiber Low-fat high complex carbohydrate diet vs. Mediterranean-inspired diet | LDL (mg/dL): 83.7 [5.7] HDL (mg/dL): 40.3 [2.4] TC (mg/dL): 149.8 [6.9] TG (mg/dL): 102.2 [7.9] | LDL: 88.0 [5.7] HDL: 42.1 [2.4] TC: 150.2 [6.9] TG: 98.7 [7.9] | LDL: 81.0 [5.1] HDL: 39.7 [2.0] TC: 142.2 [5.8] TG: 104.8 [11.5] | LDL: 85.9 [5.1] HDL: 44.8 [2.0] TC: 148.2 [5.8] TG: 85.8 [11.5] | p = 0.917 p = 0.250 p = 0.501 p = 0.313 | No numerical results available for SBP, no significant difference was found. No significant differences were found within the groups between baseline and end. | ↔ Alpha diversity ↔ F. prausnitzii ↑ Prevotella Within low-fat diet group: ↑ F. prausnitzii ↓ Roseburia Within Mediterranean diet group: ↑ Roseburia | |
| Haro 2017 Molecular Nutrition and Food Research [67] | Cardiovascular disease (n = 33) | Single-blinded (observer) RCT 104 weeks | High fiber Low-fat high complex carbohydrate diet vs. Mediterranean-inspired diet | - | - | - | - | - | No numerical results. Both diets lowered TG levels (p <0.001). CRP was reported together for the two diet groups. Compared to baseline there was no significant change in CRP. | Within both low-fat and Mediterranean diet groups: ↑ Bacteroides ↑ Prevotella ↑ Faecalibacterium | No results reported for between groups. |
| Djekic 2020 Journal of the American Heart Association [68] | Cardiovascular disease (n = 31 (4)) | Nonblinded cross-over RCT 12 weeks | High fiber Vegetarian diet vs. conventional Swedish diet | LDL (mg/dL): 61.9 (55.7–68.4) HDL (mg/dL): 47.6 (42.9–53.0) TC (mg/dL): 134.6 (124.9–144.2) TG (mg/dL): 86.8 (76.2–98.3) SBP (mmHg): 136 (129–143) CRP (mg/L): 0.73 (0.51–1.03) | LDL: 63.8 (58.0–69.6) HDL: 49.1 (44.5–54.1) TC: 136.9 (129.9–145.0) TG: 87.7 (77.1– 99.2) SBP: 140 (133–146) CRP: 0.81 (0.60–1.09) | LDL: 54.5 (49.5–59.6) * HDL: 44.5 (39.8–49.9) * TC: 124.1 (116.00–131.9) * TG: 92.1 (83.3–102.7) SBP: 133 (127–140) CRP: 0.74 (0.50–1.09) | LDL: 58.8 (52.6–65.0) * HDL: 46.1 (41.4–51.43)* TC: 129.2 (120.6–137.6)* TG: 86.8 (77.1–98.3) SBP: 136 (129–142) CRP: 0.81 (0.55–1.18) | p = 0.02 p = 0.2 p = 0.01 p = 0.1 p = 0.1 p = 0.6 | ↔ Alpha diversity ↔ Beta diversity ↔ Total SCFA ↔ Lactobacillus ↔ Bacteroides ↔ Roseburia ↔ Collinsella ↔ Bifidobacterium ↔ Faecalibacterium ↑ Akkermansia | Microbiome outcomes only available for n = 20. | |
| (d) Type 2 Diabetes | |||||||||||
| Ren 2020 Nutrients [55] | Type 2 diabetes (n = 50) (5)) | Nonblinded RCT 12 weeks | Low fiber Low-carb diet with 56 g/day almonds vs. low-fat diet | HbA1c (%): 7.64 ± 1.50 | HbA1c: 7.54 ± 1.25 | HbA1c: 6.91 ± 1.00 * | HbA1c: 7.38 ± 1.24* | p <0.01 | ↔ Alpha diversity ↔ Beta diversity ↑ Roseburia ↓ Eubacterium ↑ Ruminococcus ↔ Lactobacillus ↔ Bacteroides Within low-carb diet group: ↑ Eubacterium ↓ Bacteroides ↑ Roseburia Within low-fat diet group: ↓ Ruminococcus ↓ Roseburia | ||
| Candela 2016 British Journal of Nutrition [69] | Type 2 diabetes (n = 40) | Single-blinded (observer) RCT 3 weeks | High fiber Macrobiotic (Ma-Pi 2) diet vs. Mediterranean-inspired (Med) diet | FBG (mg/dL): 126 (43) HbA1c (%): 6.5 (1.6) CRP (mg/L): 3.2 (10.6) | FBG: 138 (42) HbA1c: 6.9 (1.1) CRP: 2.7 (4.5) | FBG: 95 (15)* HbA1C: 6.1 (1.2) CRP: 1.0 (1.8) * | FBG: 108 (12.5)* HbA1C: 6.8 (0.9) CRP: 1.6 (3.8) | p <0.0002 p = 0.3 p = 0.03 | ↔ Beta diversity Within both Ma-Pi 2 and Mediterranean diet group: ↔ Alpha diversity | Bacterial outcomes reported as deviation in terms of fold change from the median profile of healthy subjects (Figure 4). Statistical significance not reported. | |
| Zhao 2018 Science [70] | Type 2 diabetes (n = 49) (6)) | Nonblinded RCT 12 weeks | High fiber High-fiber diet with 600 mg/day acarbose vs. usual care with 600 mg/day acarbose | FBG (mmol/L): 8.47 [0.42] HbA1c (%): 8.27 [0.27] | FBG: 8.91 [0.57] HbA1c: 8.31 [0.38] | FBG: 6.37 [0.20]* HbA1c: 6.36 [0.11]* | FBG: 6.92 [0.47]* HbA1c: 7.01 [0.27]* | p = 0.2233 p = 0.0122 | ↑ Beta diversity ↔ Total SCFA Higher abundance of F. prausnitzii and Eubacterium rectale, less Lactobacillus reuteri and Bifidobacterium longum. | Statistical significance not reported for bacteria species outcomes. | |
| Liu 2020 Experimental and Therapeutic Medicine [71] | Type 2 diabetes (n = 140) (124)) | Uncontrolled trial 26 weeks | High fiber Mediterranean-inspired diet | FBG (mmol/L): 8.2 HbA1c (mmol/L): 6.9 | NA | FBG: 7.1 HbA1c: 6.3 | NA | p <0.001 p <0.001 | Results estimated from Figure 2. | Within Med diet group: ↔ Roseburia ↔ Lachnospira ↑ Pseudomonas | |
| Ismael 2021 Nutrients [72] | Type 2 diabetes (n = 11) (3)) | Uncontrolled trial 12 weeks | High fiber Personalized Mediterranean diet based on participant’s dietary history and nutritional needs | FBG (mg/dL): 131.63 ± 8.53 HbA1c (%): 7.53 ± 1.07 CRP (mg/L): 2.0 ± 2.0 | NA | FBG: 122.50 ± 9.42 HbA1c: 6.86 ± 0.85 CRP: 2.5 ± 3.3 | NA | p = 0.581 p = 0.024 p = 0.21 | ↔ Alpha diversity ↔ Firmicutes: Bacteroidetes ↔ Prevotella: Bacteroides Increase of Bacteroides, Ruminococcus, Akkermansia, Roseburia and decrease of Faecalibacterium and Prevotella. | Statistical significance not reported for bacteria genus and species outcomes. | |
| Pedersen 2016 British Journal of Nutrition [73] | Type 2 diabetes (n = 32) (3)) | Double-blinded RCT 12 weeks | Supplemental fiber 5.5 g/day prebiotic fiber (GOS mixture) vs. placebo | FBG (mmol/L): 6.1 [0.4] HbA1c (mmol/mol): 51.2 [3.1] CRP (mg/L): 1.31 (0.97) | FBG: 6.2 [0.3] HbA1c: 46.3 [1.8] CRP: 1.65 (3.13) | FBG: 6.8 [0.4]* HbA1c: 53.1 [3.2] CRP: 1.26 (2.36) | FBG: 6.5 [0.3] HbA1c: 48.4 [2.4] CRP: 0.92 (1.37) | p = 0.227 p = 0.946 p = 0.444 | ↔ Alpha diversity ↔ Bifidobacterium ↔ Lactobacillus ↔ Roseburia ↔ Enterobacteriacea Within prebiotic group: ↑ Alpha diversity | ||
| Gonai 2017 Beneficial Microbes [74] | Type 2 diabetes (n = 55 (3)) | Double-blinded RCT 4 weeks | Supplemental fiber 10 g/day galacto-oligosaccharides (GOS) vs. placebo | FBG (mg/dL): 132.3 ± 26.1 HbA1c (%): 7.1 ± 1.2 | FBG 130.0 ± 27.3 HbA1c: 6.8 ± 0.9 | FBG 138.4 ± 31.0 HbA1c: 7.0 ± 1.1 | FBG:138.2 ± 33.6 HbA1c: 6.8 ± 1.1 | - | No significant differences were found. | ↓Alpha diversity ↑ Bifidobacteriacea ↔ Ruminococcacea ↓ Lachnospiraceae Within GOS group: ↓ Alpha diversity ↑ Bifidobacteriacea ↓ Ruminococcacea ↓ Lachnospiraceae Within placebo group: ↔ Alpha diversity ↔ Bifidobacteriacea ↔ Ruminococcacea | |
| Roshanravan 2017 Journal of Cardiovascular and Thoracic Research [75] Roshanravan 2018 European Journal of Integrative Medicine [76] | Type 2 diabetes (n = 30) | Double-blinded RCT 6.5 weeks | Supplemental fiber 10 g/day inulin vs. placebo | FBG (mg/dL): 167.07 ± 82.17 CRP (mg/L): 5.45 ± 2.28 | FBG: 129.53 ± 26.38 CRP: 5.40 ± 2.01 | - CRP: 3.80 ± 1.38* | - CRP: 5.91 ± 2.15* | p = 0.309 p <0.001 | No numerical results reported for end intervention, change from baseline levels depicted in Figure 4. | ↑ A. muciniphilia Within inulin group: ↑ A. muciniphilia Within placebo group: ↔ A. muciniphilia | |
| Lee 2019 Diabetes and Metabolism Journal [77] | Type 2 diabetes (n = 14) (4)) | Uncontrolled trial 4 weeks | Supplemental fiber 12 g/day psyllium supplement | QUICKI§: 0.34 (0.06) | NA | QUICKI: 0.31 (0.05) | NA | p >0.05 | ↓ Blautia ↓ Blautia wexlerae ↓ Eubacterium ↓ Bifidobacterium longum ↓ Enterobacter soli | ||
| Medina-Vera 2019 Diabetes and Metabolism [78] | Type 2 diabetes (n = 81) (28)) | Double-blinded RCT 12 weeks | Supplemental fiber 16.5 g/day fiber in high-fiber functional food supplement with reduced calorie diet vs. reduced calorie diet | HbA1c (%): 7.5 ± 1.3 | HbA1c: 6.9 ± 1 | - | - | p <0.05 p <0.01 | End intervention −7.20% difference of HbA1c and −13% difference CRP between groups. | ↑ Alpha diversity F. prausnitzii +34%, A. muciniphilia +125%, P. copri −13%, B. longum and B. fragilis increased. | Statistical significance not reported for bacteria species outcomes. |
| Horvath 2020 European Journal of Nutrition [79] | Type 2 diabetes (n = 26 (15)) | Double-blinded RCT 26 weeks | Supplemental fiber 8/g day GOS and FOS with Lactobacillus and Bifidobacterium spp. synbiotic vs. placebo | FBG (mg/dL): 177 (147–207) HbA1c (mmol/mol): 64 (53–74) | FBG: 174 (148–200) HbA1c: 62 (59–66) | FBG: 188 (142–235) HbA1c: 67 (54–80) | FBG: 163 (134–191) HbA1c: 64 (58–71) | p = 0.5 p = 0.8 | No statistical analysis performed within groups. | ↔ Alpha diversity ↔ Beta diversity Within both prebiotic and placebo group: ↔ Alpha diversity ↔ Beta diversity | |
| Reimer 2021 European Journal of Nutrition [80] | Type 2 diabetes (n = 290) (190)) | Double-blinded RCT 52 weeks | Supplemental fiber 15 g/day highly viscous polysaccharides with low calorie diet vs. placebo with low calorie diet | FBG (mmol/L): 7.4 ± 1.9 HbA1c (%): 7.2 ± 1.1 | FBG: 7.3 ± 1.9 HbA1c: 7.0 ± 0.9 | FBG: 7.59 HbA1c: 6.97* | FBG: 7.71 HbA1c: 6.96 | p = 0.955 p = 0.516 | ↔ Alpha diversity ↔ Beta diversity ↓ F. prausnitzii ↔ A. muciniphila ↔ Roseburia ↓ Lactobacillus ↑ Collinsella | n = 47 for diversity outcomes, n = 87 for microbial outcomes. | |
| Kanazawa 2021 Nutrients [81] | Type 2 diabetes (n = 88 (8)) | Nonblinded RCT 24 weeks | Supplemental fiber 7.5 g/day GOS with Lactobacillus parasei and Bifidobacterium breve synbiotic vs. no synbiotic | FBG (mg/dL): 140.5 ± 3 3.6 HbA1c (%): 7.4 ± 0.7 CRP (mg/dl): 603.5 (1515.5) | FBG: 131.7 ± 21.5 HbA1c: 7.3 ± 0.8 CRP: 1050 (1350) | FBG: 146.7 ± 41.1 HbA1c: 7.6 ± 1.0 CRP: 743.5 (1479) | FBG: 135.2 ± 29.9 HbA1c: 7.4 ± 0.8 CRP: 819 (1996) | N.S. N.S. N.S. | ↔ Alpha diversity ↔ Total SCFA ↑ A. muciniphilia ↔ Prevotella ↑ Lactobacillus ↑ Bifidobacterium ↔ Proteobacteria Within synbiotic group: ↔ Alpha diversity ↑ Total SCFA ↔ A. muciniphilia ↑ Prevotella ↑ Lactobacillus ↑ Bifidobacterium ↓ Proteobacteria | No statistically significant changes between baseline and end intervention within the control group. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208. https://doi.org/10.3390/nu13093208
Wagenaar CA, van de Put M, Bisschops M, Walrabenstein W, de Jonge CS, Herrema H, van Schaardenburg D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients. 2021; 13(9):3208. https://doi.org/10.3390/nu13093208
Chicago/Turabian StyleWagenaar, Carlijn A., Marieke van de Put, Michelle Bisschops, Wendy Walrabenstein, Catharina S. de Jonge, Hilde Herrema, and Dirkjan van Schaardenburg. 2021. "The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review" Nutrients 13, no. 9: 3208. https://doi.org/10.3390/nu13093208
APA StyleWagenaar, C. A., van de Put, M., Bisschops, M., Walrabenstein, W., de Jonge, C. S., Herrema, H., & van Schaardenburg, D. (2021). The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients, 13(9), 3208. https://doi.org/10.3390/nu13093208







