Early Life Nutrition and Mental Health: The Role of DNA Methylation
Abstract
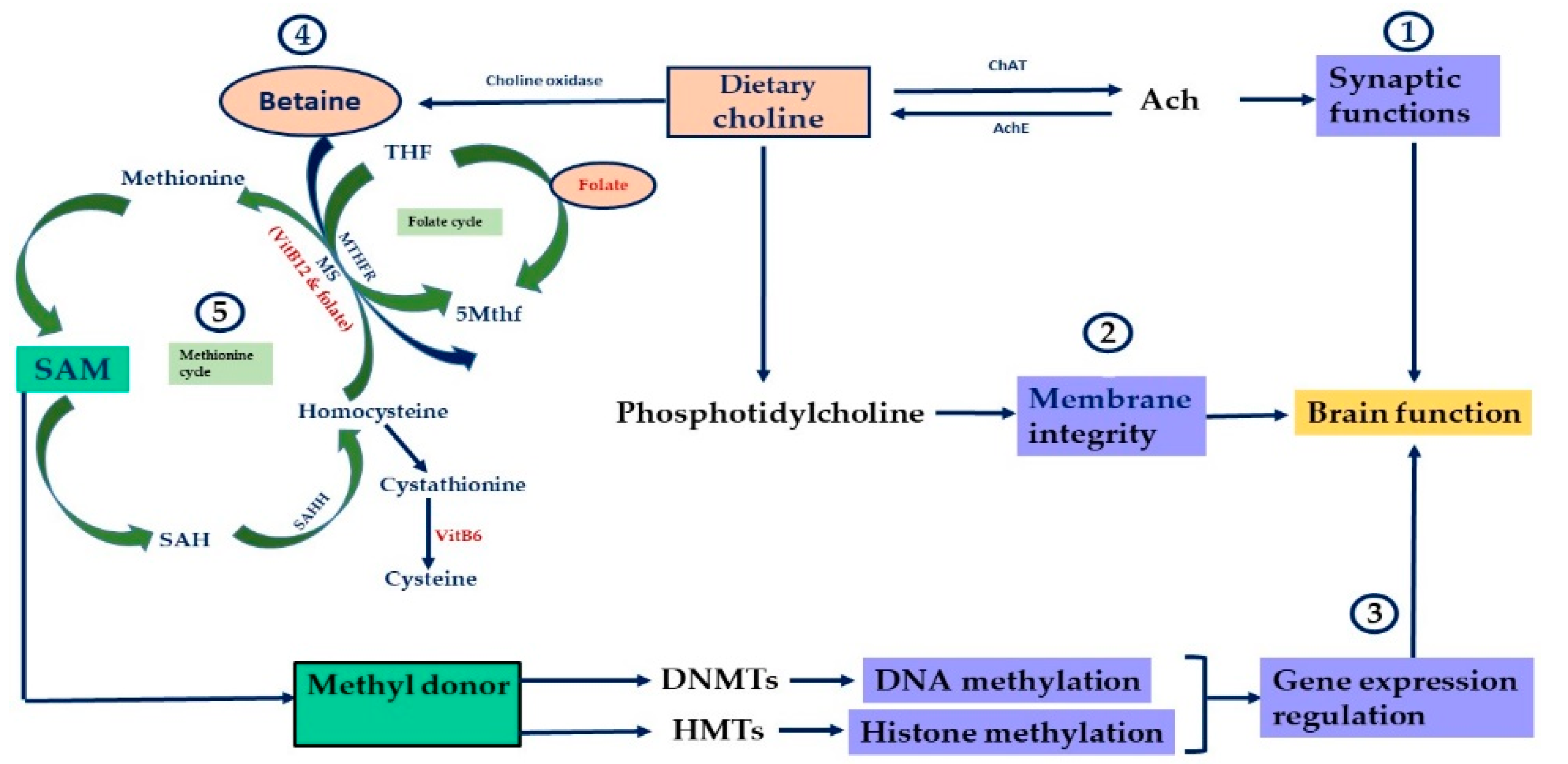
:1. Introduction
2. Nutrients, Genes and the Epigenome
3. Early-Life Nutrition and Mental Health
4. Micronutrients and Neurodegenerative Disorders
5. Optimization of Intake of One-Carbon-Metabolism-Associated Micronutrients
6. Limitations and Future Considerations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional Psychiatry: Towards Improving Mental Health by What You Eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.-Y.; Naninck, E.F.G.; Schmidt, M.V.; Lucassen, P.J.; Korosi, A. Early-Life Adversity Programs Emotional Functions and the Neuroendocrine Stress System: The Contribution of Nutrition, Metabolic Hormones and Epigenetic Mechanisms. Stress 2015, 18, 328–342. [Google Scholar] [CrossRef]
- Del Blanco, B.; Barco, A. Impact of Environmental Conditions and Chemicals on the Neuronal Epigenome. Curr. Opin. Chem. Biol. 2018, 45, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Blanco Rodríguez, J.; Camprubí Sánchez, C. Epigenetic Transgenerational Inheritance. Adv. Exp. Med. Biol. 2019, 1166, 57–74. [Google Scholar] [CrossRef]
- NIMH. Mental Illness. Available online: https://www.nimh.nih.gov/health/statistics/mental-illness.shtml (accessed on 31 March 2021).
- CDC. Data and Statistics on Children’s Mental Health|CDC. Available online: https://www.cdc.gov/childrensmentalhealth/data.html (accessed on 31 March 2021).
- Reik, W.; Dean, W.; Walter, J. Epigenetic Reprogramming in Mammalian Development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, D.; Pietrobelli, A. Micronutrients and Brain Development. Curr. Nutr. Rep. 2019, 8, 99–107. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Goll, M.G.; Bestor, T.H. Eukaryotic Cytosine Methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friso, S.; Udali, S.; De Santis, D.; Choi, S.-W. One-Carbon Metabolism and Epigenetics. Mol. Asp. Med. 2017, 54, 28–36. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and Epigenetics: An Interplay of Dietary Methyl Donors, One-Carbon Metabolism and DNA Methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle-Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.M.; Guéant, J.-L. B Vitamins and One Carbon Metabolism Micronutrients in Health and Disease. Biochimie 2020, 173, 1–2. [Google Scholar] [CrossRef]
- Hughes, C.F.; Ward, M.; Tracey, F.; Hoey, L.; Molloy, A.M.; Pentieva, K.; McNulty, H. B-Vitamin Intake and Biomarker Status in Relation to Cognitive Decline in Healthy Older Adults in a 4-Year Follow-Up Study. Nutrients 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Nurk, E.; Refsum, H.; Bjelland, I.; Drevon, C.A.; Tell, G.S.; Ueland, P.M.; Vollset, S.E.; Engedal, K.; Nygaard, H.A.; Smith, D.A. Plasma Free Choline, Betaine and Cognitive Performance: The Hordaland Health Study. Br. J. Nutr. 2013, 109, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agam, G.; Taylor, Z.; Vainer, E.; Golan, H.M. The Influence of Choline Treatment on Behavioral and Neurochemical Autistic-like Phenotype in Mthfr-Deficient Mice. Transl. Psychiatry 2020, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Borro, M.; Cavallaro, R.A.; Gentile, G.; Nicolia, V.; Fuso, A.; Simmaco, M.; Scarpa, S. One-Carbon Metabolism Alteration Affects Brain Proteome Profile in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 22, 1257–1268. [Google Scholar] [CrossRef]
- Bagnyukova, T.V.; Powell, C.L.; Pavliv, O.; Tryndyak, V.P.; Pogribny, I.P. Induction of Oxidative Stress and DNA Damage in Rat Brain by a Folate/Methyl-Deficient Diet. Brain Res. 2008, 1237, 44–51. [Google Scholar] [CrossRef]
- Gabory, A.; Attig, L.; Junien, C. Developmental Programming and Epigenetics. Am. J. Clin. Nutr. 2011, 94 (Suppl. S6), 1943S–1952S. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.J.; Rucklidge, J.J.; Kennedy, M.A. Epigenetics, Nutrition and Mental Health. Is There a Relationship? Nutr. Neurosci. 2018, 21, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Rader, J.I. Folic Acid Fortification, Folate Status and Plasma Homocysteine. J. Nutr. 2002, 132 (Suppl. S8), 2466S–2470S. [Google Scholar] [CrossRef] [Green Version]
- Imbard, A.; Benoist, J.-F.; Blom, H.J. Neural Tube Defects, Folic Acid and Methylation. Int. J. Environ. Res. Public Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef] [Green Version]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, M.D.; Zeisel, S.H. Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. J. Nutr. 2002, 132 (Suppl. S8), 2333S–2335S. [Google Scholar] [CrossRef]
- Dunlevy, L.P.E.; Burren, K.A.; Mills, K.; Chitty, L.S.; Copp, A.J.; Greene, N.D.E. Integrity of the Methylation Cycle Is Essential for Mammalian Neural Tube Closure. Birth Defects Res. Part A: Clin. Mol. Teratol. 2006, 76, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Essien, F.B.; Wannberg, S.L. Methionine but Not Folinic Acid or Vitamin B-12 Alters the Frequency of Neural Tube Defects in Axd Mutant Mice. J. Nutr. 1993, 123, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Carmichael, S.L.; Yang, W.; Selvin, S.; Schaffer, D.M. Periconceptional Dietary Intake of Choline and Betaine and Neural Tube Defects in Offspring. Am. J. Epidemiol. 2004, 160, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, S.L.; Yang, W.; Shaw, G.M. Periconceptional Nutrient Intakes and Risks of Neural Tube Defects in California. Birth Defects Res. A. Clin. Mol. Teratol. 2010, 88, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Chandler, A.L.; Hobbs, C.A.; Mosley, B.S.; Berry, R.J.; Canfield, M.A.; Qi, Y.P.; Siega-Riz, A.M.; Shaw, G.M.; National Birth Defects Prevention Study. Neural Tube Defects and Maternal Intake of Micronutrients Related to One-Carbon Metabolism or Antioxidant Activity. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-P.; Shang, X.-X.; Zhao, Z.-T. Low Maternal Vitamin B(12) Is a Risk Factor for Neural Tube Defects: A Meta-Analysis. J. Matern. Fetal Neonatal Med. 2012, 25, 389–394. [Google Scholar] [CrossRef]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar]
- Li, F.; Watkins, D.; Rosenblatt, D.S. Vitamin B(12) and Birth Defects. Mol. Genet. Metab. 2009, 98, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Steen, M.T.; Boddie, A.M.; Fisher, A.J.; Macmahon, W.; Saxe, D.; Sullivan, K.M.; Dembure, P.P.; Elsas, L.J. Neural-Tube Defects Are Associated with Low Concentrations of Cobalamin (Vitamin B12) in Amniotic Fluid. Prenat. Diagn. 1998, 18, 545–555. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B12 Deficiencies during Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29 (Suppl. S2), S101–S111, Discussion S112–S115. [Google Scholar] [CrossRef]
- Sable, P.; Randhir, K.; Kale, A.; Chavan-Gautam, P.; Joshi, S. Maternal Micronutrients and Brain Global Methylation Patterns in the Offspring. Nutr. Neurosci. 2015, 18, 30–36. [Google Scholar] [CrossRef]
- McKee, S.E.; Zhang, S.; Chen, L.; Rabinowitz, J.D.; Reyes, T.M. Perinatal High Fat Diet and Early Life Methyl Donor Supplementation Alter One Carbon Metabolism and DNA Methylation in the Brain. J. Neurochem. 2018, 145, 362–373. [Google Scholar] [CrossRef]
- Mandaviya, P.R.; Joehanes, R.; Brody, J.; Castillo-Fernandez, J.E.; Dekkers, K.F.; Do, A.N.; Graff, M.; Hänninen, I.K.; Tanaka, T.; de Jonge, E.A.L.; et al. Association of Dietary Folate and Vitamin B-12 Intake with Genome-Wide DNA Methylation in Blood: A Large-Scale Epigenome-Wide Association Analysis in 5841 Individuals. Am. J. Clin. Nutr. 2019, 110, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; Irwin, R.E.; McNulty, H.; Strain, J.J.; Lees-Murdock, D.J.; McNulty, B.A.; Ward, M.; Walsh, C.P.; Pentieva, K. Gene-Specific DNA Methylation in Newborns in Response to Folic Acid Supplementation during the Second and Third Trimesters of Pregnancy: Epigenetic Analysis from a Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 107, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Ross, S.A.; Tryndyak, V.P.; Pogribna, M.; Poirier, L.A.; Karpinets, T.V. Histone H3 Lysine 9 and H4 Lysine 20 Trimethylation and the Expression of Suv4-20h2 and Suv-39h1 Histone Methyltransferases in Hepatocarcinogenesis Induced by Methyl Deficiency in Rats. Carcinogenesis 2006, 27, 1180–1186. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Tryndyak, V.P.; Muskhelishvili, L.; Rusyn, I.; Ross, S.A. Methyl Deficiency, Alterations in Global Histone Modifications, and Carcinogenesis. J. Nutr. 2007, 137 (Suppl. S1), 216S–222S. [Google Scholar] [CrossRef] [Green Version]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine Metabolism Regulates Maintenance and Differentiation of Human Pluripotent Stem Cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Garcia, B.A.; Luka, Z.; Loukachevitch, L.V.; Bhanu, N.V.; Wagner, C. Folate Deficiency Affects Histone Methylation. Med. Hypotheses 2016, 88, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Alonso, S.; Takai, D.; Lu, S.C.; Yamamoto, F.; Perucho, M.; Huang, S. Requirement of RIZ1 for Cancer Prevention by Methyl-Balanced Diet. PLoS ONE 2008, 3, e3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luka, Z.; Pakhomova, S.; Loukachevitch, L.V.; Calcutt, M.W.; Newcomer, M.E.; Wagner, C. Crystal Structure of the Histone Lysine Specific Demethylase LSD1 Complexed with Tetrahydrofolate. Protein Sci. 2014, 23, 993–998. [Google Scholar] [CrossRef] [Green Version]
- Luka, Z.; Moss, F.; Loukachevitch, L.V.; Bornhop, D.J.; Wagner, C. Histone Demethylase LSD1 Is a Folate-Binding Protein. Biochemistry 2011, 50, 4750–4756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esse, R.; Florindo, C.; Imbard, A.; Rocha, M.S.; de Vriese, A.S.; Smulders, Y.M.; Teerlink, T.; Tavares de Almeida, I.; Castro, R.; Blom, H.J. Global Protein and Histone Arginine Methylation Are Affected in a Tissue-Specific Manner in a Rat Model of Diet-Induced Hyperhomocysteinemia. Biochim. Biophys. Acta 2013, 1832, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Jiao, Y.; Yang, S.; Deng, M.; Yang, X.; Mao, C.; Sun, Y.; Ding, N.; Li, N.; Zhang, M.; et al. Homocysteine Activates Autophagy by Inhibition of CFTR Expression via Interaction between DNA Methylation and H3K27me3 in Mouse Liver. Cell Death Dis. 2018, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H. Nutritional Importance of Choline for Brain Development. J. Am. Coll. Nutr. 2004, 23 (Suppl. S6), 621S–626S. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Niculescu, M.D. Perinatal Choline Influences Brain Structure and Function. Nutr. Rev. 2006, 64, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Needed for Normal Development of Memory. J. Am. Coll. Nutr. 2000, 19 (Suppl. S5), 528S–531S. [Google Scholar] [CrossRef]
- Teather, L.A.; Wurtman, R.J. Dietary CDP-Choline Supplementation Prevents Memory Impairment Caused by Impoverished Environmental Conditions in Rats. Learn. Mem. 2005, 12, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Mehedint, M.G.; Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Choline Deficiency Alters Global Histone Methylation and Epigenetic Marking at the Re1 Site of the Calbindin 1 Gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Reinberg, D. Transcription Regulation by Histone Methylation: Interplay between Different Covalent Modifications of the Core Histone Tails. Genes Dev. 2001, 15, 2343–2360. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal Choline Intake Alters the Epigenetic State of Fetal Cortisol-Regulating Genes in Humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.M.; Smith, R.; Collins, C.E.; Mossman, D.; Wong-Brown, M.W.; Chan, E.-C.; Evans, T.-J.; Attia, J.R.; Smith, T.; Butler, T.; et al. Methyl-Donor and Cofactor Nutrient Intakes in the First 2-3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study. Nutrients 2018, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Ramaekers, V.T.; Hansen, S.I.; Holm, J.; Opladen, T.; Senderek, J.; Häusler, M.; Heimann, G.; Fowler, B.; Maiwald, R.; Blau, N. Reduced Folate Transport to the CNS in Female Rett Patients. Neurology 2003, 61, 506–515. [Google Scholar] [CrossRef]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational Choline Supplementation Normalized Fetal Alcohol-Induced Alterations in Histone Modifications, DNA Methylation, and Proopiomelanocortin (POMC) Gene Expression in β-Endorphin-Producing POMC Neurons of the Hypothalamus. Alcohol. Clin. Exp. Res. 2013, 37, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillycrop, K.A.; Slater-Jefferies, J.L.; Hanson, M.A.; Godfrey, K.M.; Jackson, A.A.; Burdge, G.C. Induction of Altered Epigenetic Regulation of the Hepatic Glucocorticoid Receptor in the Offspring of Rats Fed a Protein-Restricted Diet during Pregnancy Suggests That Reduced DNA Methyltransferase-1 Expression Is Involved in Impaired DNA Methylation and Changes in Histone Modifications. Br. J. Nutr. 2007, 97, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Naninck, E.F.G.; Oosterink, J.E.; Yam, K.-Y.; de Vries, L.P.; Schierbeek, H.; van Goudoever, J.B.; Verkaik-Schakel, R.-N.; Plantinga, J.A.; Plosch, T.; Lucassen, P.J.; et al. Early Micronutrient Supplementation Protects against Early Stress-Induced Cognitive Impairments. FASEB J. 2017, 31, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.P.; Henzel, K.S.; Pearson, B.L.; Siwek, M.E.; Papazoglou, A.; Guo, L.; Paesler, K.; Yu, M.; Müller, R.; Xie, K.; et al. A Paternal Methyl Donor-Rich Diet Altered Cognitive and Neural Functions in Offspring Mice. Mol. Psychiatry 2018, 23, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Sahara, Y.; Matsuzawa, D.; Ishii, D.; Fuchida, T.; Goto, T.; Sutoh, C.; Shimizu, E. Paternal Methyl Donor Deficient Diets during Development Affect Male Offspring Behavior and Memory-Related Gene Expression in Mice. Dev. Psychobiol. 2019, 61, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, R.; Gupta, S. Epigenetic Modifications in Cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients That Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef] [Green Version]
- Dauncey, M.J. Recent Advances in Nutrition, Genes and Brain Health. Proc. Nutr. Soc. 2012, 71, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. Brain on Stress: How the Social Environment Gets under the Skin. Proc. Natl. Acad. Sci. USA 2012, 109 (Suppl. S2), 17180–17185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. In Pursuit of Resilience: Stress, Epigenetics, and Brain Plasticity. Ann. N. Y. Acad. Sci. 2016, 1373, 56–64. [Google Scholar] [CrossRef]
- McEwen, B.S.; Gianaros, P.J. Stress- and Allostasis-Induced Brain Plasticity. Annu. Rev. Med. 2011, 62, 431–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- McEwen, B.S. The Neurobiology of Stress: From Serendipity to Clinical Relevance. Brain Res. 2000, 886, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Owen, L.; Corfe, B. The Role of Diet and Nutrition on Mental Health and Wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenberg, S.J.; Georgieff, M.K.; Nutrition, C.O. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.L.; Dewey, K.G. Nutrition and Brain Development in Early Life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimov, S.; Mundy, L.K.; Bayer, J.K.; Jacka, F.N.; Canterford, L.; Patton, G.C. Diet Quality and Mental Health Problems in Late Childhood. Nutr. Neurosci. 2021, 24, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gonzalo, L.; Turner, A.I.; Torres, S.J.; Esteban-Cornejo, I.; Castro-Piñero, J.; Delgado-Alfonso, Á.; Marcos, A.; Gómez-Martínez, S.; Veiga, Ó.L. Diet Quality and Well-Being in Children and Adolescents: The UP&DOWN Longitudinal Study. Br. J. Nutr. 2019, 121, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.D.; Warren, M.J.; Refsum, H. Chapter Six—Vitamin B12. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; New Research and Developments of Water-Soluble Vitamins; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 215–279. [Google Scholar]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S.A. Vitamins, Minerals, and Mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Enderami, A.; Zarghami, M.; Darvishi-Khezri, H. The Effects and Potential Mechanisms of Folic Acid on Cognitive Function: A Comprehensive Review. Neurol. Sci. 2018, 39, 1667–1675. [Google Scholar] [CrossRef]
- Black, M.M. Effects of Vitamin B12 and Folate Deficiency on Brain Development in Children. Food Nutr. Bull. 2008, 29 (Suppl. S2), S126–S131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucklidge, J.J.; Eggleston, M.J.F.; Johnstone, J.M.; Darling, K.; Frampton, C.M. Vitamin-Mineral Treatment Improves Aggression and Emotional Regulation in Children with ADHD: A Fully Blinded, Randomized, Placebo-Controlled Trial. J. Child. Psychol. Psychiatry 2018, 59, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.; Lumey, L.H.; Fleming, T.P.; Sapienza, C.; Hoyo, C.; Aronica, L.; Thompson, J.; Nichol, P.F. RW-2018-Research Workshop: The Effect of Nutrition on Epigenetic Status, Growth, and Health. J. Parenter. Enteral Nutr. 2019, 43, 627–637. [Google Scholar] [CrossRef]
- Weaver, I.C.G. Shaping Adult Phenotypes through Early Life Environments. Birth Defects Res. Part C Embryo Today 2009, 87, 314–326. [Google Scholar] [CrossRef]
- Weaver, I.C.G. Integrating Early Life Experience, Gene Expression, Brain Development, and Emergent Phenotypes: Unraveling the Thread of Nature via Nurture. Adv. Genet. 2014, 86, 277–307. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.-W. Epigenetics: The Link between Nature and Nurture. Mol. Aspects Med. 2013, 34, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekamper, P.; van Poppel, F.; Stein, A.D.; Lumey, L.H. Independent and Additive Association of Prenatal Famine Exposure and Intermediary Life Conditions with Adult Mortality between Age 18-63 Years. Soc. Sci. Med. 2014, 119, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Lumey, L.H.; Stein, A.D.; Kahn, H.S.; van der Pal-de Bruin, K.M.; Blauw, G.J.; Zybert, P.A.; Susser, E.S. Cohort Profile: The Dutch Hunger Winter Families Study. Int J. Epidemiol. 2007, 36, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Ekamper, P.; van Poppel, F.; Stein, A.D.; Bijwaard, G.E.; Lumey, L.H. Prenatal Famine Exposure and Adult Mortality from Cancer, Cardiovascular Disease, and Other Causes through Age 63 Years. Am. J. Epidemiol. 2015, 181, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lumey, L.H. Exposure to the Chinese Famine of 1959-61 in Early Life and Long-Term Health Conditions: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2017, 46, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent Epigenetic Differences Associated with Prenatal Exposure to Famine in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D.; Slagboom, P.E.; Heijmans, B.T. DNA Methylation Differences after Exposure to Prenatal Famine Are Common and Timing- and Sex-Specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA Methylation Signatures Link Prenatal Famine Exposure to Growth and Metabolism. Nat. Commun 2014, 5, 5592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hodgson, N.W.; Trivedi, M.S.; Abdolmaleky, H.M.; Fournier, M.; Cuenod, M.; Do, K.Q.; Deth, R.C. Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PLoS ONE 2016, 11, e0146797. [Google Scholar] [CrossRef] [Green Version]
- Bottiglieri, T. Folate, Vitamin B₁₂, and S-Adenosylmethionine. Psychiatr. Clin. North. Am. 2013, 36, 1–13. [Google Scholar] [CrossRef]
- Kim, J.-M.; Stewart, R.; Kim, S.-W.; Yang, S.-J.; Shin, I.-S.; Yoon, J.-S. Predictive Value of Folate, Vitamin B12 and Homocysteine Levels in Late-Life Depression. Br. J. Psychiatry 2008, 192, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.M.; Fernandez-Ballart, J.D.; Molloy, A.M.; Canals, J. Moderately Elevated Maternal Homocysteine at Preconception Is Inversely Associated with Cognitive Performance in Children 4 Months and 6 Years after Birth. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef]
- Zhu, Y.; Mordaunt, C.E.; Durbin-Johnson, B.P.; Caudill, M.A.; Malysheva, O.V.; Miller, J.W.; Green, R.; James, S.J.; Melnyk, S.B.; Fallin, M.D.; et al. Expression Changes in Epigenetic Gene Pathways Associated With One-Carbon Nutritional Metabolites in Maternal Blood From Pregnancies Resulting in Autism and Non-Typical Neurodevelopment. Autism Res. 2021, 14, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.W.M.; Lim, W.M.; Ma, D.; Rosales, F.J.; Goh, E.L.K. Choline Rescues Behavioural Deficits in a Mouse Model of Rett Syndrome by Modulating Neuronal Plasticity. Mol. Neurobiol. 2019, 56, 3882–3896. [Google Scholar] [CrossRef] [Green Version]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary Protein Restriction of Pregnant Rats Induces and Folic Acid Supplementation Prevents Epigenetic Modification of Hepatic Gene Expression in the Offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Javelot, H.; Messaoudi, M.; Jacquelin, C.; Bisson, J.F.; Rozan, P.; Nejdi, A.; Lazarus, C.; Cassel, J.C.; Strazielle, C.; Lalonde, R. Behavioral and Neurochemical Effects of Dietary Methyl Donor Deficiency Combined with Unpredictable Chronic Mild Stress in Rats. Behav. Brain Res. 2014, 261, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Dauncey, M.J. New Insights into Nutrition and Cognitive Neuroscience. Proc. Nutr. Soc. 2009, 68, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Pogribny, I.P.; Karpf, A.R.; James, S.R.; Melnyk, S.; Han, T.; Tryndyak, V.P. Epigenetic Alterations in the Brains of Fisher 344 Rats Induced by Long-Term Administration of Folate/Methyl-Deficient Diet. Brain Res. 2008, 1237, 25–34. [Google Scholar] [CrossRef]
- Feng, Y.; Jankovic, J.; Wu, Y.-C. Epigenetic Mechanisms in Parkinson’s Disease. J. Neurol. Sci. 2015, 349, 3–9. [Google Scholar] [CrossRef]
- Chouliaras, L.; Rutten, B.P.F.; Kenis, G.; Peerbooms, O.; Visser, P.J.; Verhey, F.; van Os, J.; Steinbusch, H.W.M.; van den Hove, D.L.A. Epigenetic Regulation in the Pathophysiology of Alzheimer’s Disease. Prog. Neurobiol. 2010, 90, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F.; Mancuso, M.; Siciliano, G.; Migliore, L.; Murri, L. Genes and the Environment in Neurodegeneration. Biosci. Rep. 2006, 26, 341–367. [Google Scholar] [CrossRef]
- Kwok, J.B.J. Role of Epigenetics in Alzheimer’s and Parkinson’s Disease. Epigenomics 2010, 2, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Lardenoije, R.; Iatrou, A.; Kenis, G.; Kompotis, K.; Steinbusch, H.W.M.; Mastroeni, D.; Coleman, P.; Lemere, C.A.; Hof, P.R.; van den Hove, D.L.A.; et al. The Epigenetics of Aging and Neurodegeneration. Prog. Neurobiol. 2015, 131, 21–64. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Damiani, E. Epigenetics and Neurodegeneration: Role of Early-Life Nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Gröber, U.; Kisters, K.; Schmidt, J. Neuroenhancement with Vitamin B12-Underestimated Neurological Significance. Nutrients 2013, 5, 5031–5045. [Google Scholar] [CrossRef] [Green Version]
- van de Lagemaat, E.E.; de Groot, L.C.P.G.M.; van den Heuvel, E.G.H.M. Vitamin B12 in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Shea, T.B. Folate and Homocysteine Metabolism in Neural Plasticity and Neurodegenerative Disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Bhate, V.; Deshpande, S.; Bhat, D.; Joshi, N.; Ladkat, R.; Watve, S.; Fall, C.; de Jager, C.A.; Refsum, H.; Yajnik, C. Vitamin B12 Status of Pregnant Indian Women and Cognitive Function in Their 9-Year-Old Children. Food Nutr. Bull. 2008, 29, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Rathod, R.; Khaire, A.; Kemse, N.; Kale, A.; Joshi, S. Maternal Omega-3 Fatty Acid Supplementation on Vitamin B12 Rich Diet Improves Brain Omega-3 Fatty Acids, Neurotrophins and Cognition in the Wistar Rat Offspring. Brain Dev. 2014, 36, 853–863. [Google Scholar] [CrossRef]
- Rong, H.; Xi, Y.; An, Y.; Tao, L.; Zhang, X.; Yu, H.; Wang, Y.; Qin, Z.; Xiao, R. The Correlation between Early Stages of Life Exposed to Chinese Famine and Cognitive Decline in Adulthood: Nutrition of Adulthood Plays an Important Role in the Link? Front. Aging Neurosci. 2017, 9, 444. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the Ageing Brain: Towards Evidence for an Optimal Diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Quan, S.; Yang, G.; Ye, Q.; Chen, M.; Yu, H.; Wang, G.; Wang, Y.; Zeng, X.; Qiao, S. One Carbon Metabolism and Mammalian Pregnancy Outcomes. Mol. Nutr. Food Res. 2021, 65, e2000734. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; Otten, J.J., Hellwig, J.P., Meyers, L.D., Eds.; The National Academies Press: Washington, DC, USA, 2006; 560p. [Google Scholar]
- Home|Dietary Guidelines for Americans. Available online: https://www.dietaryguidelines.gov/ (accessed on 24 May 2021).
- Ruxton, C.H.S.; Derbyshire, E.; Toribio-Mateas, M. Role of Fatty Acids and Micronutrients in Healthy Ageing: A Systematic Review of Randomised Controlled Trials Set in the Context of European Dietary Surveys of Older Adults. J. Hum. Nutr. Diet. 2016, 29, 308–324. [Google Scholar] [CrossRef]
- Roberts, S.B.; Silver, R.E.; Das, S.K.; Fielding, R.A.; Gilhooly, C.H.; Jacques, P.F.; Kelly, J.M.; Mason, J.B.; McKeown, N.M.; Reardon, M.A.; et al. Healthy Aging-Nutrition Matters: Start Early and Screen Often. Adv. Nutr. 2021, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
| Description | Outcomes | References |
|---|---|---|
| Prenatal choline and betaine intake in humans | Reduction in NTD risk | [26,28,29] |
| Maternal choline supplementation in humans | Attenuation of the stress axis in offspring Alteration in the methylation of Crh and Nr3c1 in placenta and in cord blood with a decrease in corticosterone levels in cord plasma (n = 29) | [55] |
| Maternal diet supplementation with folic acid during pregnancy | Changes in methylation of candidate genes related to brain development in blood samples of newborns (n = 86) with a genome-wide decrease in methylation | [39] |
| Maternal supplementation with folic acid in humans | Genome-wide decrease in methylation, alteration in the methylation status of genes in the offspring (n = 5841) | [38] |
| Methyl donor intake during early postnatal period (2–3 years) | Higher methylation in buccal cells of males compared to females in children at age 4 with no proven association between methyl donor intake and changes in global methylation (n = 73) | [56] |
| Supplementation of folinic acid in female Rett patients | Improved levels of 5-MTHF in CSF of female patients (n = 4) | [57] |
| Prenatal choline supplementation in alcohol-exposed pregnant rats | Increase in H3K4me3, decrease in H3K9me2 in β-endorphin-producing neurons in the hypothalamus of exposed offspring with a decrease in Pomc gene methylation and decrease in ACTH and corticosterone levels in the blood | [58] |
| Choline deficiency at E17 in mice | Decrease in G9a, decrease in H3K9me1 and H3K9me2 in the SVZ and ventricular zone in mice hippocampus with no changes in global levels of histone marks in the mouse fetal brain. Decrease in binding of REST on Calb1 gene promoter with an increase in Calb1 expression in NPC | [53] |
| Methionine intake in Axd mutant mice | Reduction in NTD | [27] |
| Imbalanced levels of VitB12 and folic acid in pregnant Wistar rats | Hypermethylation in the cortex of adult offspring | [36] |
| Methyl-balanced diet in mice | Increase in KMT8 expression and cancer prevention in liver | [44] |
| Folate deficient diet in mice | Increase in H3K4me in liver | [43] |
| Wistar rats fed with HM, LV or HMLV | Increase in homocysteine and SAM in the liver, and decrease in H3R8me2 in the brain | [47] |
| Mice-induced hyperhomocysteinemia | Increase in EZH2, increase in H3K27me3 along Cftr gene promoter and decrease in Cftr expression | [48] |
| Prolonged methionine deprivation in ESCs/iPSCs | Cellular apoptosis | [42] |
| Methionine deficiency in hESCs | Decrease is SAM levels, decrease in H3K4me3 Impacted differentiation of ESC | [42] |
| Maternal diet supplementation with methyl donors in viable yellow Agouti mice | Alteration in the methylation status of IAP and Agouti gene expression and shifting phenotype toward the brown color | [59] |
| Pregnant rats fed with diet low in methyl donors | Hypermethylation, increase in H3K9Ace along hepatic GR gene promoter and decrease in DNMT1 expression in the offspring | [60] |
| Maternal diet supplementation with methionine and B vitamins in ES mice | Normalization in methionine levels in plasma and hippocampus and stress axis in offspring with no changes in global DNA methylation | [61] |
| Elevated paternal dietary intake of methyl donors in mice | Changes in Mat2a expression and changes in methylation of Kcnmb2 gene promoter in offspring | [62] |
| Paternal diet deficient in methyl donors in mice (FMCD diet) | Changes in methylation of PP1 gene promoter in the hippocampus of offspring | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekdash, R.A. Early Life Nutrition and Mental Health: The Role of DNA Methylation. Nutrients 2021, 13, 3111. https://doi.org/10.3390/nu13093111
Bekdash RA. Early Life Nutrition and Mental Health: The Role of DNA Methylation. Nutrients. 2021; 13(9):3111. https://doi.org/10.3390/nu13093111
Chicago/Turabian StyleBekdash, Rola A. 2021. "Early Life Nutrition and Mental Health: The Role of DNA Methylation" Nutrients 13, no. 9: 3111. https://doi.org/10.3390/nu13093111
APA StyleBekdash, R. A. (2021). Early Life Nutrition and Mental Health: The Role of DNA Methylation. Nutrients, 13(9), 3111. https://doi.org/10.3390/nu13093111





