Prevalence and Clinical Features of Celiac Disease in a Cohort of Italian Children with Autism Spectrum Disorders
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Celiac Disease Testing and Diagnosis
2.3. Statistical Analysis
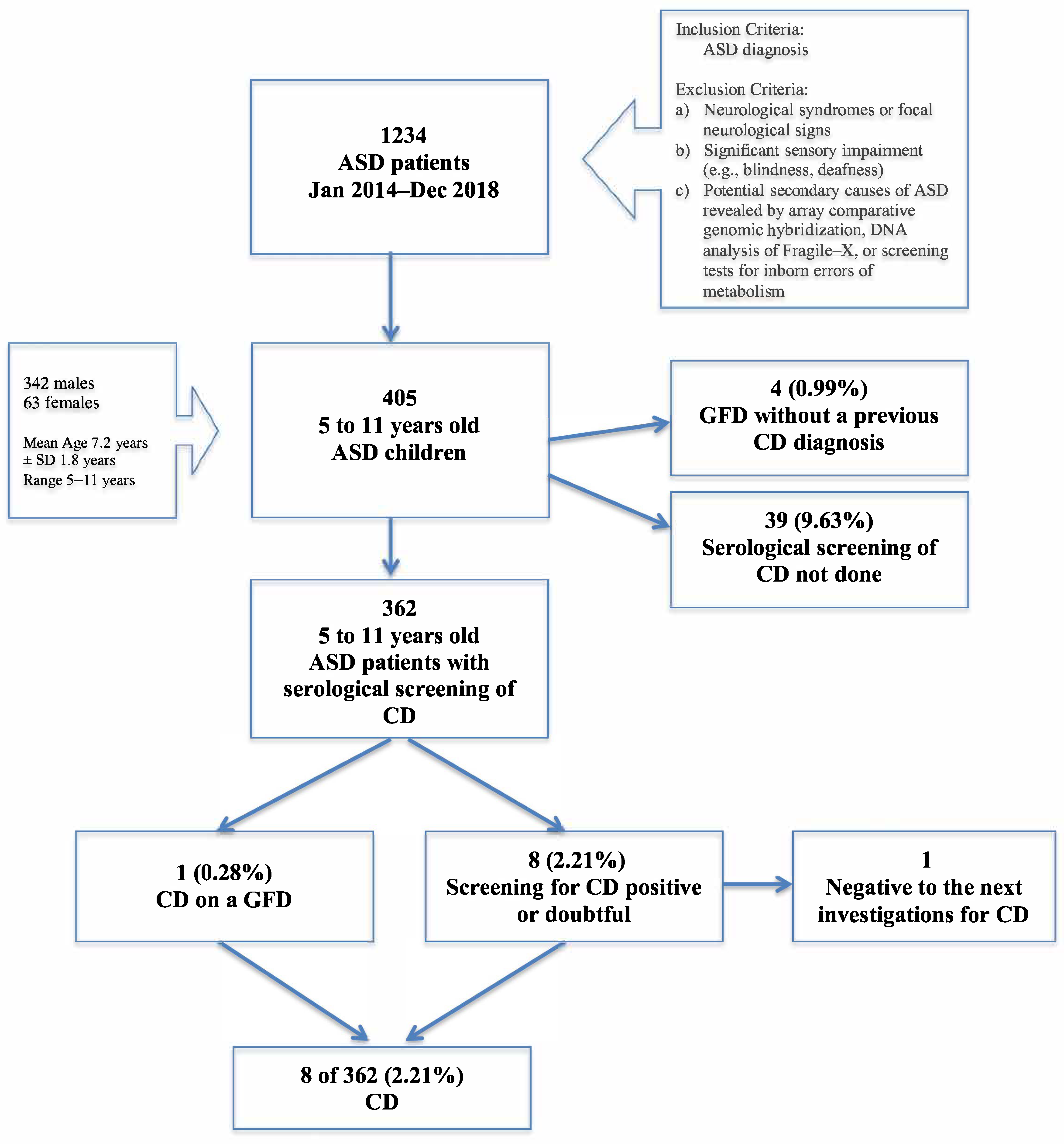
3. Results
4. Discussion
- Celiac disease (CD) is an immune-mediated disorder, elicited by gluten in susceptible individuals
- Studies assessing the prevalence of CD in patients with autism provide controversial results
- The study provides an updated estimate of the CD prevalence in Italian children with autism
- Our results support a frequent asymptomatic form of CD in autistic children
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a-CGH | array comparative genomic hybridization |
| ADOS-2 | Autism Diagnostic Observation Schedule-Second Edition |
| AGA | anti-gliadin antibodies |
| anti-tTG | anti-transglutaminase |
| ASD | autism spectrum disorder |
| CD | celiac disease |
| EMA | anti-endomysium |
| ESPGHAN | European Society for Paediatric Gastroenterology, Hepatology, and Nutrition |
| FEIA | fluorescent enzyme immunoassay |
| GFD | gluten-free diet |
| GI | gastrointestinal |
| Ig | immunoglobulin |
| IQR | interquartile range |
| MHC | major histocompatibility complex |
| SD | standard deviation |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors with Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef]
- Atladóttir, H.O.; Pedersen, M.G.; Thorsen, P.; Mortensen, P.B.; Deleuran, B.; Eaton, W.W.; Parner, E.T.; Sutton, R.M.; Niles, D.; Nysaether, J.; et al. Association of Family History of Autoimmune Diseases and Autism Spectrum Disorders. Pediatrics 2009, 124, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015, 46, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmiston, E.; Ashwood, P.; Van de Water, J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludvigsson, J.F.; Reichenberg, A.; Hultman, C.M.; Murray, J.A. A Nationwide Study of the Association Between Celiac Disease and the Risk of Autistic Spectrum Disorders. JAMA Psychiatry 2013, 70, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2012, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Fasano, A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005, 128 (Suppl. S1), S68–S73. [Google Scholar] [CrossRef]
- Nurminen, S.; Kivelä, L.; Taavela, J.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Factors associated with growth disturbance at celiac disease diagnosis in children: A retrospective cohort study. BMC Gastroenterol. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenna, R.; Tiberti, C.; Petrarca, L.; Lucantoni, F.; Mennini, M.; Luparia, R.P.; Panimolle, F.; Mastrogiorgio, G.; Pietropaoli, N.; Magliocca, F.M.; et al. The celiac iceberg: Characterization of the disease in primary schoolchildren. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Gujral, N.; Freeman, H.J.; Thomson, A.B.R. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Catassi, C.; Rätsch, I.-M.; Fabiani, E.; Rossini, M.; Bordicchia, F.; Candela, F.; Coppa, G.; Giorgi, P. Coeliac disease in the year 2000: Exploring the iceberg. Lancet 1994, 343, 200–203. [Google Scholar] [CrossRef]
- Catassi, C.; Fabiani, E.; Rätsch, I.M.; Coppa, G.V.; Giorgi, P.L.; Pierdomenico, R.; Alessandrini, S.; Iwanejko, G.; Domenici, R.; Mei, E.; et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr. 1996, 412, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C.; et al. Increased Prevalence of Celiac Disease in School-age Children in Italy. Clin. Gastroentorol. Hepatol. 2019, 18, 596–603. [Google Scholar] [CrossRef]
- Pavlovic, M.; Berenji, K.; Bukurov, M. Screening of celiac disease in Down syndrome—Old and new dilemmas. World J. Clin. Cases 2017, 5, 264–269. [Google Scholar] [CrossRef]
- Bai, J.C.; Fried, M.; Corazza, G.R.; Schuppan, D.; Farthing, M.; Catassi, C.; Greco, L.; Cohen, H.; Ciacci, C.; Eliakim, R.; et al. World Gastroenterology Organisation global guidelines on celiac disease. J. Clin. Gastroenterol. 2013, 47, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, S.M.; Johnson, C.P. Management of Children with Autism Spectrum Disorders. Pediatrics 2007, 120, 1162–1182. [Google Scholar] [CrossRef] [Green Version]
- Prosperi, M.; Santocchi, E.; Muratori, F.; Narducci, C.; Calderoni, S.; Tancredi, R.; Morales, M.A.; Guiducci, L. Vocal and motor be-haviors as a possible expression of gastrointestinal problems in preschoolers with Autism Spectrum Disorder. BMC Pediatr. 2019, 19, 466. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, S.; Santocchi, E.; Del Bianco, T.; Brunori, E.; Caponi, L.; Paolicchi, A.; Fulceri, F.; Prosperi, M.; Narzisi, A.; Cosenza, A.; et al. Serological screening for Celiac Disease in 382 pre-schoolers with Autism Spectrum Disorder. Ital. J. Pediatr. 2016, 42, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcia, G.; Posar, A.; Santucci, M.; Parmeggiani, A. Autism and Coeliac Disease. J. Autism Dev. Disord. 2008, 38, 407–408. [Google Scholar] [CrossRef]
- Bennabi, M.; Gaman, A.; Delorme, R.; Boukouaci, W.; Manier, C.; Scheid, I.; Mohammed, N.S.; Bengoufa, D.; Charron, D.; Krishnamoorthy, R.; et al. HLA-class II haplotypes and Autism Spectrum Disorders. Sci. Rep. 2018, 8, 7639. [Google Scholar] [CrossRef] [Green Version]
- Erickson, C.A.; Stigler, K.A.; Corkins, M.R.; Posey, D.J.; Fitzgerald, J.F.; McDougle, C.J. Gastrointestinal Factors in Autistic Disorder: A Critical Review. J. Autism Dev. Disord. 2005, 35, 713–727. [Google Scholar] [CrossRef]
- Butwicka, A.; Lichtenstein, P.; Frisén, L.; Almqvist, C.; Larsson, H.; Ludvigsson, J.F. Celiac disease is associated with childhood psychiatric disorders: A population-based study. J. Pediatr. 2017, 184, 87–93.e81. [Google Scholar] [CrossRef]
- Pavone, L.; Fiumara, A.; Bottaro, G.; Mazzone, D.; Coleman, M. Autism and celiac disease: Failure to validate the hypothesis that a link might exist. Biol. Psychiatry 1997, 42, 72–75. [Google Scholar] [CrossRef]
- Batista, I.C.; Gandolfi, L.; Nobrega, Y.K.M.; Almeida, R.C.; Almeida, L.M.; Campos Junior, D.; Pratesi, R. Autism spectrum disorder and celiac disease: No evidence for a link. Arq. Neuro-Psiquiatr. 2012, 70, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, K.; Perman, J.A. Autism and gastrointestinal symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.; Carcani-Rathwell, I.; Charman, T.; Pickles, A.; Loucas, T.; Meldrum, D.; Simonoff, E.; Sullivan, P.; Baird, G. Parent-reported gastro-intestinal symptoms in children with autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 2737–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneja, M.; Venkatakrishnan, A.; Kapoor, S.; Jain, R. Autism Spectrum Disorders and Celiac Disease: Is there an Association? Indian Pediatr. 2018, 55, 912–914. [Google Scholar] [PubMed]
- Black, C.; Kaye, J.A.; Jick, H. Relation of childhood gastrointestinal disorders to autism: Nested case-control study using data from the UK General Practice Research Database. BMJ 2002, 325, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.H.; Voigt, R.G.; Katusic, S.K.; Weaver, A.L.; Barbaresi, W.J. Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics 2009, 124, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, N.M.; Green, P.H.R.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef] [Green Version]
- Józefczuk, J.; Konopka, E.; Bierła, J.B.; Trojanowska, I.; Sowińska, A.; Czarnecki, R.; Sobol, L.; Józefczuk, P.; Surdy, W.; Cukrowska, B. The occurrence of antibodies against gluten in children with autism spectrum disorders does not correlate with serolog-ical markers of impaired intestinal permeability. J. Med. Food 2018, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Abolfazli, R.; Mirbagheri, S.; Zabihi, A.; Abouzari, M. Autism and Celiac Disease: Failure to Validate the Hypothesis of a Possible Link. Iran Red Crescent Med. J. 2009, 11, 442–444. [Google Scholar]
- Piwowarczyk, A.; Horvath, A.; Łukasik, J.; Pisula, E.; Szajewska, H. Gluten- and casein-free diet and autism spectrum disorders in children: A systematic review. Eur. J. Nutr. 2018, 57, 433–440. [Google Scholar] [CrossRef]
- Quan, J.; Panaccione, N.; Jeong, J.; Underwood, F.E.; Coward, S.; Windsor, J.W.; Ronksley, P.E.; Gidrewicz, D.; Debruyn, J.; Turner, J.M.; et al. Association Between Celiac Disease and Autism Spectrum Disorder: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. American College of Gastroenterology clinical guideline: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–677. [Google Scholar] [CrossRef] [Green Version]
- Luyster, R.; Gotham, K.; Guthrie, W.; Coffing, M.; Petrak, R.; Pierce, K.; Bishop, S.; Esler, A.; Hus, V.; Oti, R.; et al. The Autism Diagnostic Observation Schedule-toddler module: A new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1305–1320. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Franceschini, E.; Lionetti, M.E.; D’Adamo, G.; D’Angelo, E.; Gatti, S.; Naspi Catassi, G.; Malamisura, B.; Catassi, C. Misuse of serological screening tests for celiac disease in children: A prospective study in Italy. Dig. Liver Dis. 2019, 51, 1547–1550. [Google Scholar] [CrossRef] [Green Version]
- Tonutti, E.; Bizzaro, N. Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun. Rev. 2014, 13, 472–476. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, USA. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, R.; Lebwohl, B.; Ludvigsson, J.F.; Lewis, S.K.; Rizkalla-Reilly, N.; Green, P.H.R. Celiac Disease Is Diagnosed Less Frequently in Young Adult Males. Dig. Dis. Sci. 2014, 59, 1509–1512. [Google Scholar] [CrossRef]
- Bizzaro, N.; Tonutti, E. 56—Anti-gliadin Antibodies. In Autoantibodies, 2nd ed.; Shoenfeld, Y., Gershwin, M.E., Meroni, P.L., Eds.; Elsevier: Burlington, MA, USA, 2007; pp. 451–456. [Google Scholar]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less Hidden Celiac Disease But Increased Gluten Avoidance without a Diagnosis in the United States: Findings From the National Health and Nutrition Examination Surveys From 2009 to 2014. Mayo Clin. Proc. 2016, 92, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in Diagnosis of Celiac Disease without Biopsies in Clinical Practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125 (Suppl. S1), S1–S18. [Google Scholar] [CrossRef] [Green Version]
- Oberlander, T.F.; Zeltzer, L.K. Pain in children with autism. In Mental Health and Pain: Somatic and Psychiatric Components of Pain in Mental Health; Marchand, S., Saravane, D., Gaumond, I., Eds.; Springer Publishing: Berlin/Heidelberg, Germany, 2014; pp. 191–209. [Google Scholar]
- Maenner, M.J.; Arneson, C.L.; Levy, S.E.; Kirby, R.S.; Nicholas, J.S.; Durkin, M.S. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J. Autism Dev. Disord. 2012, 42, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal abnormalities in children with au-tistic disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef]
- Holingue, C.; Newill, C.; Lee, L.-C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal Dysfunction in Autism: Parental Report, Clinical Evaluation, and Associated Factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Margolis, K.G.; Buie, T.M.; Turner, J.B.; Silberman, A.E.; Feldman, J.F.; Murray, K.F.; McSwiggan-Hardin, M.; Levy, J.; Bauman, M.L.; Veenstra-VanderWeele, J.; et al. Development of a Brief Parent-Report Screen for Common Gastrointestinal Disorders in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 349–362. [Google Scholar] [CrossRef]
- Auricchio, R.; Tosco, A.; Piccolo, E.; Galatola, M.; Izzo, V.; Maglio, M.; Paparo, F.; Troncone, R.; Greco, L. Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet. Am. J. Gastroenterol. 2014, 109, 913–921. [Google Scholar] [CrossRef]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
| No. | Age at First Evaluation (Years) | Sex | tTG IgA (U/mL) | Total IgA (mg/dL) | EMA | Risk Factor and Clinical Presentation | CD Diagnosis | Biopsy Type |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.8 | F | 277.0 | 72 | + | none | tTG+HLA+ | n.p. |
| 2 | 9.0 | F | 33.0 | 135 | + | none | tTG+SIB+ | MARSH 3A |
| 3 | 5.8 | F | >128.0 | 220 | + | hyporexia, poor weight gain | CL+tTG+HLA+ | n.p. |
| 4 a | 6.0 | F | n.a. | n.a. | n.a. | poor weight gain after the first year of life | tTG+SIB+ | n.a. |
| 5 | 11.6 | M | 522.0 | 128 | + | mild constipation, insomnia | tTG+SIB+ | mod MARSH 3B |
| 6 | 6.6 | F | 16.0 | 49 | + | severe constipation, insomnia, irritability | CL+tTG+HLA+ | refused |
| 7 | 6.9 | M | 137.0 | 161 | + | atopic dermatitis from birth on elbows and skin folds | CL+tTG+SIB+ | MARSH 3B |
| 8 | 10.3 | M | 55.0 | 58 | + | none | tTG+SIB+ | mod MARSH 3C |
| 9 | 6.2 | M | 17.0 | 131 | + | none | excluded, based on repetition of serological screening tests | |
| This Study | Control Study (Gatti et al., 2020) | |
|---|---|---|
| Type of study | Retrospective | Cross-sectional |
| Aim | To estimate the prevalence of CD in ASD children | To estimate the prevalence of CD in an Italian pediatric population |
| Evaluation period | January 2014–December 2018 | May 2015–December 2016 |
| Geographical origin | all over Italy | Ancona and Verona (Italy) |
| Initial sample size (n) | 405 | 5705 |
| CD screening not performed (n) | 43 | 1135 |
| CD screening performed (n) | 362 | 4570 |
| Previous diagnosis of CD (n) | 1 | 23 |
| Children with CD (n) | 7 | 54 |
| No symptoms of CD (n) | 3 | 23 |
| Supposed children with CD in the subgroup who not performed the screening (n) | 1 | 13 |
| Age range (years) | 5–11 | 5–11 |
| Number of females (%) | 60 (17) | 2193 (48) |
| Median (years) | 6.58 | 7.88 |
| IQR (years) | 5.6–8.1 | 7.0–8.8 |
| Diseases | autism spectrum disorder | not evaluated |
| First-level screening test | ||
| What | Total serum IgA tTG IgA tTG IgG EMA IgA | HLA-DQ2/8 total serum IgA tTG IgA (DGP IgG) |
| Where | hospital setting | school |
| Second-level procedures | ||
| What | screening repetition based on prior results | EMA IgA (same frozen serum) |
| Where | hospital setting (city of residence, Italy) | Hospital setting (G. Fracastoro hospital, Verona) |
| Third-level procedures | ||
| What | SIB | SIB |
| Where | hospital setting (city of residence, Italy) | Hospital setting (Ancona and Verona) |
| Classification type for the biopsy | Marsh classification Marsh-Oberhuber classification | Marsh-Oberhuber classification |
| Number of Estimated Subjects Positive for CD Diagnosis | Number of Estimated Subjects Negative for CD Diagnosis | Total Number of Subjects (in the Eligible Study Group) | Overall Estimated Prevalence of CD | Chi-Square Statistic Value | |
|---|---|---|---|---|---|
| Group 1 [Our study] | 8.83 | 396.17 | 405 | 2.18% | n.s. |
| Group 2 [16] | 90.40 | 5614.6 | 5705 | 1.58% | |
| Total | 99.23 | 6010.77 | 6110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi, M.; Santocchi, E.; Brunori, E.; Cosenza, A.; Tancredi, R.; Muratori, F.; Calderoni, S. Prevalence and Clinical Features of Celiac Disease in a Cohort of Italian Children with Autism Spectrum Disorders. Nutrients 2021, 13, 3046. https://doi.org/10.3390/nu13093046
Prosperi M, Santocchi E, Brunori E, Cosenza A, Tancredi R, Muratori F, Calderoni S. Prevalence and Clinical Features of Celiac Disease in a Cohort of Italian Children with Autism Spectrum Disorders. Nutrients. 2021; 13(9):3046. https://doi.org/10.3390/nu13093046
Chicago/Turabian StyleProsperi, Margherita, Elisa Santocchi, Elena Brunori, Angela Cosenza, Raffaella Tancredi, Filippo Muratori, and Sara Calderoni. 2021. "Prevalence and Clinical Features of Celiac Disease in a Cohort of Italian Children with Autism Spectrum Disorders" Nutrients 13, no. 9: 3046. https://doi.org/10.3390/nu13093046
APA StyleProsperi, M., Santocchi, E., Brunori, E., Cosenza, A., Tancredi, R., Muratori, F., & Calderoni, S. (2021). Prevalence and Clinical Features of Celiac Disease in a Cohort of Italian Children with Autism Spectrum Disorders. Nutrients, 13(9), 3046. https://doi.org/10.3390/nu13093046






