Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Neck Circumference and Anthropometric Characteristic Measurements in KNHANES
2.3. MetS Assessment and Biochemical Characteristic Measurements in KNHANES
- (1)
- Abdominal obesity (WC ≥ 85 cm for women and ≥90 cm for men)
- (2)
- High BP (diastolic BP ≥ 85 mmHg or systolic BP ≥ 130 mmHg)
- (3)
- Hyperglycemia (FBG ≥ 100 mg/dL)
- (4)
- Low HDL-C (HDL-C < 50 mg/dL for women and < 40 mg/dL for men)
- (5)
- Hypertriglyceridemia (TG ≥ 150 mg/dL)
2.4. Assessment of Dietary Intake and Dietary Habits in KNHANES
2.5. Assessment of Other Socioeconomic Characteristics
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Study Participants
3.2. Correlation between NC and Risk Factors for MetS
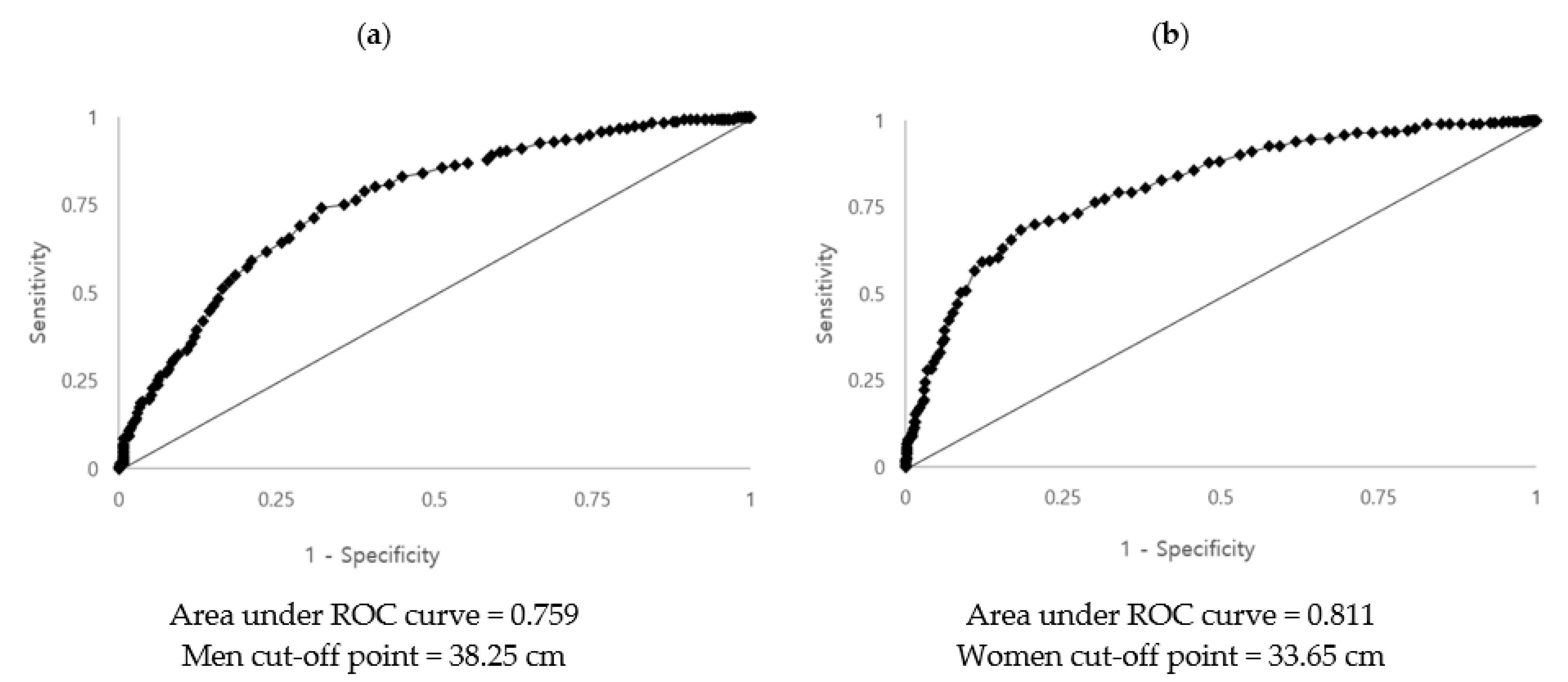
3.3. Determining the Optimal Cut-Off Point of Neck Circumference for Diagnosis of MetS
3.4. Association of the Neck Circumference with MetS and its Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Hara, K.; Matsushita, Y.; Horikoshi, M.; Yoshiike, N.; Yokoyama, T.; Tanaka, H.; Kadowaki, T. A Proposal for the Cutoff Point of Waist Circumference for the Diagnosis of Metabolic Syndrome in the Japanese Population. Diabetes Care 2006, 29, 1123–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korean Society for the Study of Obesity. Available online: http://www.general.kosso.or.kr/html/?pmode=obesityDiagnosis (accessed on 21 April 2021).
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. The Metabolic Syndrome—from Insulin Resistance to Obesity and Diabetes. Endocrinol. Metab. Clin. N. Am. 2008, 37, 559–579. [Google Scholar] [CrossRef]
- Verweij, L.M.; Terwee, C.B.; Proper, K.I.; Hulshof, C.T.; Van Mechelen, W. Measurement error of waist circumference: Gaps in knowledge. Public Health Nutr. 2013, 16, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, B.; Zhi, X.; He, J.; Ma, P.; Yu, L.; Zheng, Q.; Sun, G. Neck circumference associated with arterial blood pressures and hypertension: A cross-sectional community-based study in northern Han Chinese. Sci. Rep. 2017, 7, 2620. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association with metabolic risk factors in the Framingham heart study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Siöström, C.D.; Lissner, L.; Siostrom, L. Relationships Between Changes in Body Composition and Changes in Cardiovascular Risk Factors: The SOS Intervention Study. Obes. Res. 1997, 5, 519–530. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mokan, M.; Simoneau, J.A.; Mandarino, L.J. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J. Clin. Investig. 1993, 92, 91–98. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Alfarsi, S.; Adams, P.W.; Wynn, V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 1976, 12, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.R.; Pencina, M.J.; D’Agostino, R.B.; Meigs, J.B.; Vasan, R.S.; Fox, C.S. Neck Circumference and the Development of Cardiovascular Disease Risk Factors in the Framingham Heart Study. Diabetes Care 2013, 36, e3. [Google Scholar] [CrossRef] [PubMed]
- Khalangot, M.; Gurianov, V.; Okhrimenko, N.; Luzanchuk, I.; Kravchenko, V. Neck circumference as a risk factor of screen-detected diabetes mellitus: Community-based study. Diabetol. Metab. Syndr. 2016, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Sun, N.; Li, X.; Zheng, Q.; Li, L.; Gu, C.; Feng, B. Neck circumference is a valuable tool for identifying metabolic syndrome and obesity in Chinese elder subjects: A community-based study. Diabetes/Metab. Res. Rev. 2014, 30, 69–76. [Google Scholar] [CrossRef]
- Ahbab, S.; Ataoglu, H.E.; Tuna, M.; Karasulu, L.; Çetin, F.; Temiz, L.; Yenigün, M. Neck circumference. metabolic syndrome and obstructive sleep apnea syndrome; Evaluation of possible linkage. Med. Sci. Monit. 2013, 19, 111–117. [Google Scholar] [CrossRef]
- Fu, W.; Zou, L.; Yin, X.; Wu, J.; Zhang, S.; Mao, J.; Cao, S.; Li, W.; Gan, Y.; Yan, S.; et al. Association between neck circumference and cardiometabolic disease in Chinese adults: A community-based cross-sectional study. BMJ Open 2019, 9, e026253. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Ge, H.; Zhu, M.-F.; Wang, L.-J.; Chen, L.; Tan, Y.-Z.; Chen, Y.-M.; Zhu, H.-L. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc. Diabetol. 2013, 12, 76. [Google Scholar] [CrossRef]
- Espósito, R.C.; de Medeiros, P.J.; de Souza Silva, F.; Oliveira, A.G.; Aragão, C.F.S.; Rocha, H.A.O.; Moreira, S.A.; de Farias Sales, V.S. Prevalence of the metabolic syndrome according to different criteria in the male population during the Blue November Campaign in Natal, RN, Northeastern Brazil. Diabetes Metab. Syndr. Obes. 2018, 11, 401–408. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the Metabolic Syndrome Among US Adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- dos Santos, H.C.M.; de Orange, L.G.; de Lima, C.R.; de Azevedo, M.M.S.; Dourado, K.F.; de Andrade, S.P. Metabolic syndrome and other risk factors for cardiovascular disease in an obese population. Rev. Bras. Cardiol. 2013, 26, 442–449. [Google Scholar]
- Kweon, S.; Kim, Y.; Jang, M.-J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P. Metabolic Syndrome—Role of Dietary Fat Type and Quantity. Nutrients 2019, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.J.; Schweitzer, A.; Hoffman, H.J.; Onyewu, C.; Hurtado, M.E.; Hoffman, E.P.; Klein, C.J. Neck and Waist Circumference Biomarkers of Cardiovascular Risk in a Cohort of Predominantly African-American College Students: A Preliminary Study. J. Acad. Nutr. Diet. 2014, 114, 107–116. [Google Scholar] [CrossRef]
- Laohabut, I.; Udol, K.; Phisalprapa, P.; Srivanichakorn, W.; Chaisathaphol, T.; Washirasaksiri, C.; Sitasuwan, T.; Chouriyagune, C.; Auesomwang, C. Neck circumference as a predictor of metabolic syndrome: A cross-sectional study. Prim. Care Diabetes 2020, 14, 265–273. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Peng, L.; Li, J.; Gao, Y.; Yan, B.; Fang, B.; Wang, G. The association of neck circumference with incident congestive heart failure and coronary heart disease mortality in a community-based population with or without sleep-disordered breathing. BMC Cardiovasc. Disord. 2018, 18, 108. [Google Scholar] [CrossRef]
- Chen, J.-M.; Li, Q.-W.; Jiang, G.-X.; Zeng, S.-J.; Shen, J.; Sun, J.; Wu, D.-H.; Cheng, Q. Association of neck circumference and cognitive impairment among Chinese elderly. Brain Behav. 2018, 8, e00937. [Google Scholar] [CrossRef]
- Limpawattana, P.; Manjavong, M.; Sopapong, R. Can Neck Circumference Predict Metabolic Syndrome? An Experience from A University Community. Endocr. Pract. 2016, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Stabe, C.; Vasques, A.C.J.; Lima, M.M.O.; Tambascia, M.A.; Pareja, J.C.; Yamanaka, A.; Geloneze, B. Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: Results from the Brazilian Metabolic Syndrome Study. Clin. Endocrinol. 2013, 78, 874–881. [Google Scholar] [CrossRef]
- Hingorjo, M.R.; Zehra, S.; Imran, E.; Qureshi, M.A. Neck circumference: A supplemental tool for the diagnosis of metabolic syndrome. J. Pak. Med. Assoc. 2016, 66, 1221–1226. [Google Scholar]
- Patry-Parisien, J.; Shields, M.; Bryan, S. Comparison of waist circumference using the World Health Organization and National Institutes of Health protocols. Health Rep. 2012, 23, 53–60. [Google Scholar] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Anothaisintawee, T.; Sansanayudh, N.; Thamakaison, S.; Lertrattananon, D.; Thakkinstian, A. Neck Circumference as an Anthropometric Indicator of Central Obesity in Patients with Prediabetes: A Cross-Sectional Study. BioMed Res. Int. 2019, 2019, 4808541. [Google Scholar] [CrossRef]
- Huang, B.-X.; Zhu, M.-F.; Wu, T.; Zhou, J.-Y.; Liu, Y.; Chen, X.-L.; Zhou, R.-F.; Wang, L.-J.; Chen, Y.-M.; Zhu, H.-L. Neck Circumference, along with Other Anthropometric Indices, Has an Independent and Additional Contribution in Predicting Fatty Liver Disease. PLoS ONE 2015, 10, e0118071. [Google Scholar] [CrossRef]
- Li, H.-X.; Zhang, F.; Zhao, D.; Xin, Z.; Guo, S.-Q.; Wang, S.-M.; Zhang, J.-J.; Wang, J.; Li, Y.; Yang, G.-R.; et al. Neck circumference as a measure of neck fat and abdominal visceral fat in Chinese adults. BMC Public Health 2014, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arbelaez, D.; Camacho, P.A.; Cohen, D.D.; Saavedra-Cortes, S.; Lopez-Lopez, C.; Lopez-Jaramillo, P. Neck circumference as a predictor of metabolic syndrome, insulin resistance and low-grade systemic inflammation in children: The ACFIES study. BMC Pediatr. 2016, 16, 31. [Google Scholar] [CrossRef]
- Selvan, C.; Dutta, D.; Thukral, A.; Nargis, T.; Kumar, M.; Mukhopadhyay, S.; Chowdhury, S. Neck height ratio is an important predictor of metabolic syndrome among Asian Indians. Indian J. Endocrinol. Metab. 2016, 20, 831. [Google Scholar] [CrossRef]
| Variables | Total (n = 2234) | Men (n = 974) | Women (n = 1260) | p |
|---|---|---|---|---|
| Energy (kcal/day) | 1950.9 ± 19.5 | 2262.3 ± 30.2 | 1639.5 ± 5 | <0.001 |
| Carbohydrate (g/1000 kcal) | 152.3 ± 0.8 | 147.5 ± 1.3 | 157.0 ± 0.9 | <0.001 |
| Fat (g/1000 kcal) | 22.5 ± 0.3 | 21.7 ± 0.3 | 23.3 ± 0.3 | <0.001 |
| Protein (g/1000 kcal) | 36.8 ± 0.3 | 36.3 ± 0.4 | 37.2 ± 0.4 | 0.050 |
| Water (g/1000 kcal) | 566.6 ± 7.4 | 518.2 ± 9.7 | 615.0 ± 9.6 | <0.001 |
| Sugar (g/1000 kcal) | 32.4 ± 0.4 | 28.5 ± 0.6 | 36.4 ± 0.7 | <0.001 |
| Calcium (mg/1000 kcal) | 283.2 ± 3.5 | 261.5 ± 4.2 | 304.9 ± 5.5 | <0.001 |
| Phosphorus (mg/1000 kcal) | 562.3 ± 3.7 | 540.3 ± 5.1 | 584.3 ± 4.6 | <0.001 |
| Sodium (mg/1000 kcal) | 1852.9 ± 21.6 | 1913.1 ± 28.7 | 1792.7 ± 27.4 | 0.001 |
| Potassium (mg/1000 kcal) | 1554.9 ± 11.8 | 1448.5 ± 16.0 | 1661.3 ± 15.1 | <0.001 |
| Vitamin C (mg/1000 kcal) | 38.3 ± 1.0 | 31.1 ± 1.2 | 45.5 ± 1.4 | <0.001 |
| Cereals (g/day) | 252.2 ± 2.2 | 287.3 ± 3.2 | 217.1 ± 2.4 | 0.199 |
| Potatoes and starches (g/day) | 71.2 ± 2.6 | 65.9 ± 3.5 | 76.5 ± 3.5 | <0.001 |
| Sugars (g/day) | 12.3 ± 0.4 | 12.8 ± 0.5 | 11.9 ± 0.5 | <0.001 |
| Legumes (g/day) | 54.0 ± 1.7 | 56.4 ± 2.4 | 51.6 ± 2.3 | <0.001 |
| Vegetables (g/day) | 292.8 ± 4.2 | 329.8 ± 6.3 | 255.8 ± 4.2 | 0.567 |
| Mushrooms (g/day) | 17.7 ± 0.9 | 17.6 ± 1.2 | 17.8 ± 1.1 | <0.001 |
| Fruits (g/day) | 225.3 ± 6.4 | 217.4 ± 6.4 | 233.3 ± 10.1 | 0.640 |
| Vegetable oils (g/day) | 6.9 ± 0.2 | 8.0 ± 0.2 | 5.8 ± 0.2 | <0.001 |
| Meat (g/day) | 171.3 ± 3.5 | 212.2 ± 5.6 | 130.3 ± 3.5 | <0.001 |
| Eggs (g/day) | 54.1 ± 1.1 | 58.0 ± 1.5 | 50.2 ± 1.3 | <0.001 |
| Seafoods (g/day) | 126.6 ± 3.0 | 141.7 ± 4.1 | 130.3 ± 3.5 | <0.001 |
| Beverages (g/day) | 163.3 ± 5.4 | 181.6 ± 7.4 | 144.9 ± 5.5 | <0.001 |
| Alcoholic beverages (g/day) | 387.2 ± 18.9 | 530.6 ± 29.3 | 243.9 ± 20.7 | <0.001 |
| Variables | Total (n = 2234) | Men (n = 974) | Women (n = 1260) | p |
|---|---|---|---|---|
| Age groups, n (%) | 0.157 | |||
| 40–49 years | 872(39.0) | 381(39.1) | 491(39.0) | |
| 50–59 years | 881(39.4) | 372(38.2) | 509(40.4) | |
| 60–64 years | 481(21.5) | 221(22.7) | 260(20.6) | |
| History of diseases (yes), n (%) | ||||
| Hypertension | 455(20.4) | 238(24.4) | 217(17.2) | 0.010 |
| Stroke | 29(1.3) | 19(2.0) | 10(0.8) | 0.022 |
| Cardiovascular disease | 38(1.7) | 30(3.1) | 8(0.6) | 0.001 |
| Diabetes | 175(7.8) | 99(10.2) | 76(6.0) | 0.012 |
| Cancer | 65(2.9) | 23(2.4) | 42(3.3) | 0.012 |
| Depression | 109(4.9) | 27(2.8) | 82(6.5) | <0.001 |
| Obstructive sleep apnea (yes), n (%) | 15(0.7) | 13(1.3) | 2(0.2) | <0.001 |
| Smoking status, n (%) | <0.001 | |||
| Current | 411(18.5) | 353(36.7) | 58(4.6) | |
| Former | 536(24.2) | 442(45.9) | 94(7.5) | |
| Never | 1269(57.3) | 168(17.4) | 1101(87.9) | |
| Drinking use (yes), n (%) | 2045(92.3) | 932(96.8) | 1113(88.8) | <0.001 |
| Grip strength (right hand, kg) | 30.6 ± 0.2 | 38.8 ± 0.3 | 22.5 ± 0.2 | <0.001 |
| WC (cm) | 84.4 ± 0.2 | 88.2 ± 0.3 | 80.7 ± 0.3 | <0.001 |
| NC (cm) | 35.5 ± 0.1 | 38.3 ± 0.1 | 32.7 ± 0.1 | <0.001 |
| BMI (kg/m2) | 24.1 ± 0.1 | 24.7 ± 0.1 | 23.5 ± 0.1 | <0.001 |
| SBP (mmHg) | 118.3 ± 0.4 | 120.3 ± 0.5 | 116.3 ± 0.6 | <0.001 |
| DBP (mmHg) | 78.2 ± 0.3 | 80.4 ± 0.4 | 76.0 ± 0.3 | <0.001 |
| Fasting blood glucose (mg/dL) | 102.5 ± 0.7 | 106.31.0 | 98.7 ± 0.7 | <0.001 |
| HbA1c (%) | 5.8 ± 0.0 | 5.9 ± 0.0 | 5.8 ± 0.0 | <0.001 |
| Total-C (mg/dL) | 200.6 ± 0.9 | 198.1 ± 1.2 | 203.2 ± 1.3 | 0.002 |
| HDL-C (mg/dL) | 53.0 ± 0.4 | 48.6 ± 0.4 | 57.4 ± 0.5 | <0.001 |
| TG (mg/dL) | 145.6 ± 3.1 | 177.4 ± 5.2 | 113.8 ± 2.5 | <0.001 |
| LDL-C (mg/dL) | 120.2 ± 2.2 | 113.2 ± 2.6 | 127.3 ± 3.4 | <0.001 |
| AST (IU/L) | 25.2 ± 0.3 | 27.6 ± 0.6 | 22.7 ± 0.3 | <0.001 |
| ALT (IU/L) | 24.9 ± 0.6 | 30.0 ± 1.1 | 19.8 ± 0.5 | <0.001 |
| MetS | <0.001 | |||
| No | 1591(71.2) | 610(62.6) | 981(77.9) | |
| Yes | 643(28.8) | 364(37.4) | 279(22.1) |
| Neck Circumference | ||||||
|---|---|---|---|---|---|---|
| Men (n = 974) | Women (n = 1260) | Total (n = 2234) | ||||
| r | p | r | p | r | p | |
| Age | −0.079 | 0.014 | 0.132 | <0.001 | 0.031 | 0.147 |
| BMI | 0.809 | <0.001 | 0.770 | <0.001 | 0.597 | <0.001 |
| WC | 0.767 | <0.001 | 0.766 | <0.001 | 0.731 | <0.001 |
| SBP | 0.131 | <0.001 | 0.165 | <0.001 | 0.197 | <0.001 |
| DBP | 0.186 | <0.001 | 0.134 | <0.001 | 0.270 | <0.001 |
| FBG | 0.187 | <0.001 | 0.340 | <0.001 | 0.275 | <0.001 |
| HbA1c | 0.188 | <0.001 | 0.353 | <0.001 | 0.231 | <0.001 |
| Total-C | 0.053 | 0.097 | −0.001 | 0.981 | −0.045 | 0.033 |
| HDL-C | −0.280 | <0.001 | −0.296 | <0.001 | −0.401 | <0.001 |
| TG | 0.199 | <0.001 | 0.317 | <0.001 | 0.343 | <0.001 |
| LDL-C | 0.041 | 0.523 | 0.055 | 0.534 | −0.096 | 0.062 |
| Study Variables | Total | Men * | Women * |
|---|---|---|---|
| Crude OR (95% CI) p | Crude OR (95% CI) p | Crude OR (95% CI) p | |
| Increased WC (Abdominal obesity) | 1.182(1.157–1.208) <0.001 | 1.248(1.205–1.293) <0.001 | 1.276(1.234–1.319) <0.001 |
| High BP | 1.058(1.038–1.079) <0.001 | 1.041(1.013–1.070) 0.004 | 1.019(0.987–1.007) 0.249 |
| Hyperglycemia | 1.012(1.003–1.021) 0.009 | 1.002(0.994–1.011) 0.606 | 1.009(0.998–1.020) 0.099 |
| Low HDL-C | 0.969(0.957–0.981) <0.001 | 0.989(0.970–1.009) 0.269 | 0.988(0.969–1.006) 0.196 |
| Hypertriglyceridemia | 1.002(1.000–1.004) 0.012 | 1.001(1.000–1.002) 0.157 | 1.002(1.000–1.004) 0.042 |
| Total | Men * | Women * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Model 1 | 6.468 | 4.993–8.380 | <0.001 | 6.902 | 4.410–8.416 | <0.001 | 12.143 | 8.533–17.280 | <0.001 |
| Model 2 | 6.513 | 5.041–8.415 | <0.001 | 6.223 | 4.477–8.651 | <0.001 | 7.783 | 5.775–10.490 | <0.001 |
| Model 3 | 5.830 | 4.702–7.922 | <0.001 | 5.830 | 4.153–8.183 | <0.001 | 11.538 | 7.971–16.701 | <0.001 |
| Model 4 | 2.853 | 2.089–3.896 | <0.001 | 1.899 | 1.239–2.910 | 0.003 | 4.515 | 2.982–6.836 | <0.001 |
| Model 5 | 1.807 | 1.272–2.569 | <0.001 | 2.014 | 1.348–3.008 | 0.010 | 3.650 | 2.382–5.594 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-Y.; Moon, H.-R.; Yun, J.-M. Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study. Nutrients 2021, 13, 3029. https://doi.org/10.3390/nu13093029
Kim K-Y, Moon H-R, Yun J-M. Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study. Nutrients. 2021; 13(9):3029. https://doi.org/10.3390/nu13093029
Chicago/Turabian StyleKim, Kyoung-Yun, Ha-Rin Moon, and Jung-Mi Yun. 2021. "Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study" Nutrients 13, no. 9: 3029. https://doi.org/10.3390/nu13093029
APA StyleKim, K.-Y., Moon, H.-R., & Yun, J.-M. (2021). Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study. Nutrients, 13(9), 3029. https://doi.org/10.3390/nu13093029






