Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karonova, T.L.; Vashukova, M.A.; Gusev, D.A.; Golovatuk, K.A.; Grineva, E.N. Vitamin D deficiency as a factor for immunity stimulation and lower risk of acute respiratory infections and COVID-19. Arter. Hypertens. 2020, 26, 295–303. [Google Scholar] [CrossRef]
- Zemb, P.; Bergman, P.; Camargo, C.A., Jr.; Cavalier, E.; Cormier, C.; Courbebaisse, M.; Hollis, B.; Joulia, F.; Minisola, S.; Pilz, S.; et al. Vitamin D deficiency and COVID-19 pandemic. Glob. Antimicrob. Resist. 2020, 22, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef] [Green Version]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch. Intern. Med. 2009, 169, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar] [CrossRef] [Green Version]
- White, J.H. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef] [Green Version]
- Laaksi, I. Vitamin D and respiratory infection in adults. Proc. Nutr. Soc. 2012, 71, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Campbell, P.A.; Wu-Young, M.; Lee, R.C. Rapid response to Elisabeth Mahase E: Covid-19: What treatments are being investigated? BMJ 2020, 368, m1252. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.M.; Adams, J.S.; Kermani-Arab, V.; Bakke, A.C.; Sakai, R.; Jordan, S.C. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985, 134, 3032–3035. [Google Scholar]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutolo, M.; Paolino, S.; Smith, V. Evidences for a protective role of vitamin D in COVID-19. RMD Open 2020, 6, e001454. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Xu, J. Effect of Vitamin D on ACE2 and Vitamin D receptor expression in rats with LPS-induced acute lung injury. Chin. J. Emerg. Med. 2016, 25, 1284–1289. [Google Scholar] [CrossRef]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Vasarhelyi, B.; Satori, A.; Olajos, F.; Szabo, A.; Beko, G. Low vitamin D levels among patients at Semmelweis University: Retrospective analysis during a one-year period. Orv. Hetil. 2011, 152, 1272–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhonghua, L.Z.B.X.Z.Z. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. CMA.J. 2020, 41, 145–151. [Google Scholar] [CrossRef]
- White, C.; Nafilyan, V. Coronavirus (COVID-19) Related Deaths by Ethnic Group, England and Wales: 2 March 2020 to 10 April 2020; Office for National Statistics: Newport, UK, 2020.
- Hope-Simpson, R.E. The role of season in the epidemiology of influenza. J. Hyg. 1981, 86, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Lavie, C.J.; Sanchis-Gomar, F.; Henry, B.M.; Lippi, G. COVID-19 and obesity: Links and risks. Expert Rev. Endocrinol. Metab. 2020, 15, 215–216. [Google Scholar] [CrossRef]
- Hussain, A.; Bhowmik, B.; do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 2020, 162, 108142. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; Franch, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolo, A.; Lucchini, R.; Keller, F.; Cantu, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Karonova, T.; Andreeva, A.; Nikitina, I.; Belyaeva, O.; Mokhova, E.; Galkina, O.; Vasilyeva, E.; Grineva, E. Prevalence of Vitamin D deficiency in the North-West region of Russia: A cross-sectional study. J. Steroid Biochem. Mol. Biol. 2016, 164, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Pigarova, E.A.; Rozhinskaya, L.Y.; Belaya, J.E.; Dzeranova, L.K.; Karonova, T.L.; Ilyin, A.V.; Melnichenko, G.A.; Dedov, I.I. Russian Association of endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults. Probl. Endocrinol. 2016, 62, 60–84. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1045–1062. [Google Scholar] [CrossRef]
- Infante, M.; Buoso, A.; Pieri, M.; Lupisella, S.; Nuccetelli, M.; Bernardini, S.; Fabbri, A.; Iannetta, M.; Andreoni, M.; Colizzi, V.; et al. Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: An italian retrospective study. J. Am. Coll. Nutr. 2021, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.W.J.; Zheng, S. Obesity and COVID-19: The clash of two pandemics. Obes. Res. Clin. Pract. 2020, 14, 380–382. [Google Scholar] [CrossRef]
- Carter, S.J.; Baranauskas, M.N.; Fly, A.D. Considerations for obesity, vitamin D, and physical activity amid the COVID-19 pandemic. Obesity 2020, 28, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Ghasemian, R.; Shamshirian, A.; Heydari, K.; Malekan, M.; Alizadeh-Navaei, R.; Ebrahimzadeh, M.A.; Ebrahimi Warkiani, M.; Jafarpour, H.; Razavi Bazaz, S.; Rezaei Shahmirzadi, A.; et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 29, e14675. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Zneng, F.; Chen, R.; Yao, R.; Huang, Y.; Tan, X.; Liu, J.; Li, N.; Xie, Y. Dynamic changes in the immune response correlate with disease severity and outcomes during infection with SARS-CoV-2. Infect. Dis. Ther. 2021, 10, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Kwon, N.J.; Choi, S.J.; Kang, C.K.; Choe, P.G.; Kim, J.Y.; Yun, J.; Lee, G.W.; Seong, M.W.; Kim, N.J.; et al. Virus Isolation from the First Patient with SARS-CoV-2 in Korea. J. Korean Med. Sci. 2020, 35, e84. [Google Scholar] [CrossRef]
- Sulli, A.; Gotelli, E.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Grosso, M.; Ferone, D.; Smith, V.; Cutolo, M. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 2021, 13, 717. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; Shakir, I.; Shams, H.; Grant, W.B.; Alkattan, K. Lung-centric inflammation of COVID-19: Potential modulation by vitamin D. Nutrients 2021, 13, 2216. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef] [Green Version]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeno, J.M.; Fernandez-Delgado, R.; Fett, C.; Castano-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-kappaB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Brown, L.A.S.; Hao, L.; Hebbar, G.; Lee, M.J.; Liu, S.; Ziegler, T.R.; et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
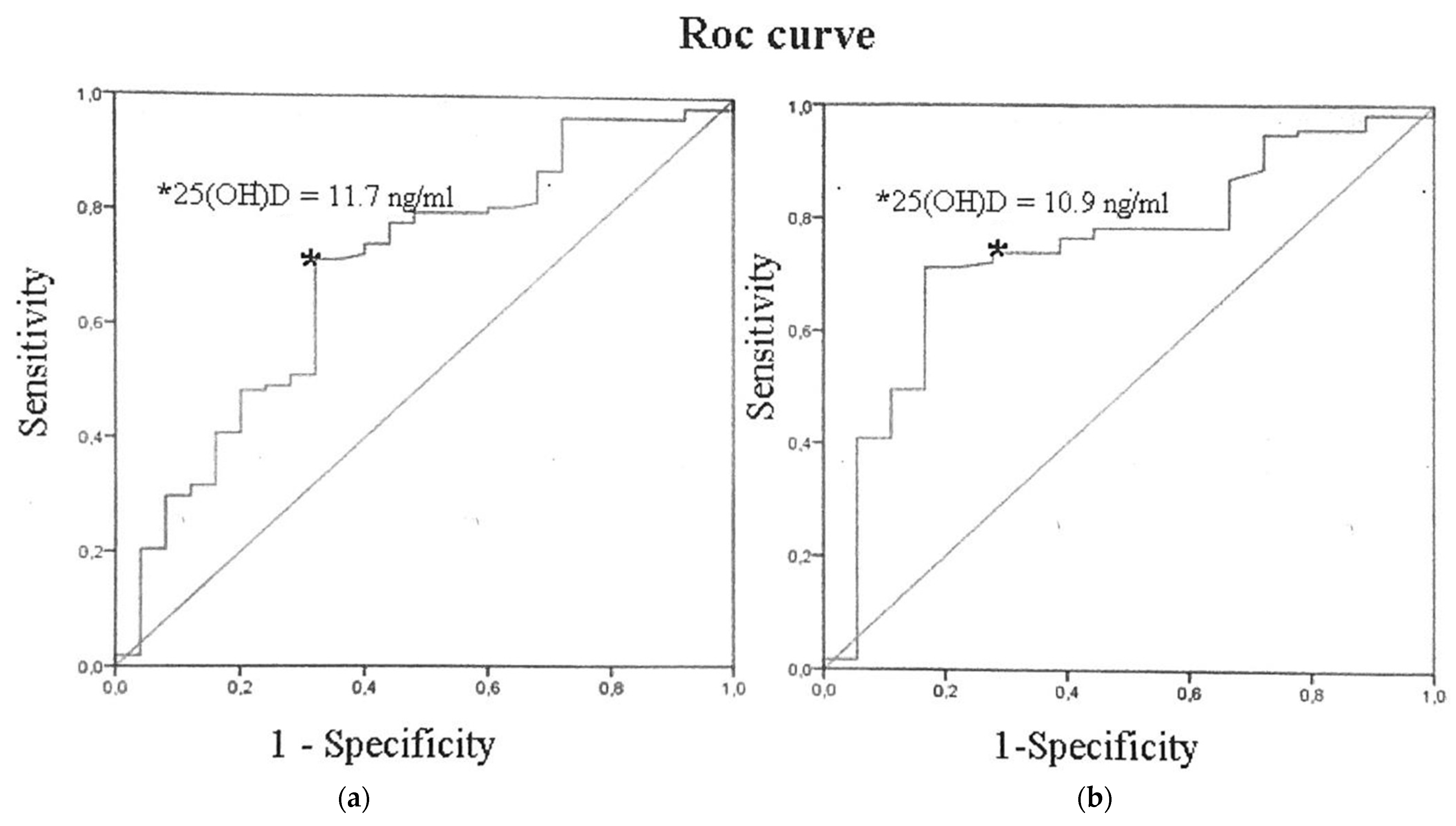
| Parameter | Severe Course n = 25 | Moderate Course n = 108 | p |
|---|---|---|---|
| Age, y, M ± SD | 57 ± 3 | 51 ± 1 | 0.02 |
| Sex, m/f, n (%) | 15(60)/10(40) | 61(57)/47(44) | 0.75 |
| Obesity, n (%) | 16 (64) | 23 (21) | 0.00 |
| AH, n (%) | 15 (60) | 46 (43) | 0.12 |
| CAD, n (%) | 11 (44) | 25 (23) | 0.04 |
| DM, n (%) | 8 (32) | 18 (17) | 0.00 |
| Death, n (%) | 15 (60) | 3 (3) | 0.00 |
| Volume of lung tissue lesions (CT), n (%) | 0.00 | ||
| 0 | 0 | 7 (7) | |
| 1 | 1 (4) | 19 (18) | |
| 2 | 5 (20) | 41 (38) | |
| 3 | 5 (20) | 32 (30) | |
| 4 | 14 (56) | 9 (8) | |
| 25(OH)D, ng/mL, Me [25; 75] | 9.7 [6.0; 14.9] | 14.6 [10.6; 24.4] | 0.00 |
| Vitamin D status, n (%) | 0.003 | ||
| Normal | 1 (4) | 11 (10) | |
| Insufficiency | 3 (12) | 28 (26) | |
| Mild deficiency | 8 (32) | 45 (42) | |
| Severe deficiency | 13 (52) | 24 (22) | |
| Bed days, M ± SD | 21.0 ± 2.5 | 17.0 ± 0.9 | 0.16 |
| Glucose max, mmol/L, Me [25%; 75%] | 10.3 [8.4; 18.4] | 6.15, 0; 9.7 | 0.00 |
| CRP baseline, mg/L, Me [25%; 75%] | 64.7 [36.4; 200.0] | 34.7 [15.9; 89.6] | 0.01 |
| CRP max, mg/L, Me [25%; 75%] | 265.1 [182.2; 322.0] | 60.0 [21.4; 137.3] | 0.00 |
| IL-6 baseline, pg/mL, Me [25%; 75%] | 22.0 [10.8; 75.0] | 7.8 [2.4; 20.7] | 0.001 |
| IL-6 max, pg/mL, Me [25%; 75%] | 36.4 [20.1; 282.0] | 10.4 [2.8; 25.1] | 0.00 |
| Ferritin baseline, μg/L, Me [25%; 75%] | 895.4 [317.1; 1581.7] | 357.3 [172.2; 811.3] | 0.01 |
| Ferritin max, μg/L, Me [25%; 75%] | 1347.6 [835.6; 2197.1] | 496.1 [257.4; 1057.4] | 0.00 |
| Parameter | Deficiency n =90 | Insufficiency n = 31 | Normal n = 12 | p | |
|---|---|---|---|---|---|
| Severe Deficiency n =37 | Mild Deficiency n = 53 | ||||
| Age, y, M ± SD | 52 ± 3 | 53 ± 2 | 49 ± 2 | 51 ± 3 | 0.39 * 0.74 |
| Sex, m/f, n (%) | 27 (73)/ 10 (27) | 26 (49)/ 27 (51) | 14 (45)/ 17 (55) | 9 (75)/ 3 (25) | 0.17 * 0.02 |
| Obesity, n (%) | 13 (35) | 19 (36) | 6 (19) | 1 (8) | 0.06 * 0.36 |
| AH, n (%) | 19 (51) | 28 (53) | 11 (36) | 3 (25) | 0.09 * 0.43 |
| CAD, n (%) | 15 (41) | 14 (26) | 6 (19) | 1 (8) | 0.12 * 0.03 |
| DM, n (%) | 7 (19) | 14 (26) | 5 (16) | 0 | 0.00 * 0.00 |
| Severe course, n (%) | 13 (35) | 8 (15) | 3 (10) | 1 (8) | 0.15 * 0.003 |
| Parameter | Death n = 18 | Discharged n = 115 | p |
|---|---|---|---|
| Age, y, M ± SD | 62 ± 3 | 50 ± 1 | 0.00 |
| Sex, m/f, n (%) | 10 (56)/ 8 (44) | 66 (57)/ 49 (43) | 0.88 |
| Obesity, n (%) | 12 (67) | 27 (24) | 0.00 |
| AH, n (%) | 14 (78) | 47 (41) | 0.004 |
| CAD, n (%) | 11 (61) | 25 (22) | 0.00 |
| DM, n (%) | 6 (33) | 20 (17) | 0.00 |
| Severe course, n (%) | 15 (83) | 10 (9) | 0.00 |
| Volume of lung tissue lesions (CT), n (%) | 0.00 | ||
| 0 | 0 | 7 (6) | |
| 1 | 0 | 20 (17) | |
| 2 | 4 (22) | 42 (37) | |
| 3 | 3 (17) | 34 (30) | |
| 4 | 11 (61) | 12 (10) | |
| 25(OH)D, ng/mL, Me [25%; 75%] | 9.6 [6.0; 11.5] | 14.8 [10.1; 24.3] | 0.001 |
| Vitamin D status, n (%) | |||
| Normal, n (%) | 1 (6) | 11 (10) | 0.02 |
| Insufficiency, n (%) | 0 | 31 (27) | |
| Mild deficiency, n (%) | 7 (39) | 46 (40) | |
| Severe deficiency, n (%) | 10 (55) | 27 (24) | 0.005 |
| Bed days, M ± SD | 16 ± 3 | 18 ± 1 | 0.27 |
| Glucose baseline, mmol/L | 7.0 [6.1; 10.0] | 5.9 [5.0; 7.5] | 0.03 |
| Glucose max, mmol/L | 10.8 [7.7; 18.4] | 6.3 [5.0; 10.0] | 0.00 |
| CRP baseline, mg/L | 74.6 [36.4; 168.2] | 35.5 [16.3; 90.0] | 0.02 |
| CRP max, mg/L | 255.5 [182.2; 308.0] | 67.4 [22.2; 140.1] | 0.00 |
| IL-6 baseline, pg/mL | 37.4 [10.8; 87.6] | 8.3 [2.4; 20.7] | 0.00 |
| IL-6 max, pg/mL | 37.7 [12.7; 453.5] | 11.8 [2.9; 27.6] | 0.001 |
| Ferritin baseline, μg/L, n = 131 | 965.0 [680.1; 1581.7] | 366.5 [172.2; 895.4] | 0.005 |
| Ferritin max, μg/L | 1699.1 [1119.5; 2197.1] | 536.1 [260.0; 1051.0] | 0.00 |
| Predictor | Unadjusted | Model 1 Adjusted | Model 2 Adjusted | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.03 (1.01–1.07) | 0.04 | 1.04 (1.01–1.07) | 0.04 | 1.03 (0.99–1.08) | 0.17 |
| Male | 1.16 (0.48–2.80) | 0.75 | 0.77 (0.20–2.02) | 0.59 | 0.85 (0.32–2.29) | 0.75 |
| Obesity | 6.57 (2.57–16.78) | 0.000 | ||||
| AH | 2.02 (0.83–4.91) | 0.12 | 0.98 (0.28–3.43) | 0.98 | ||
| CAD | 2.61 (1.05–6.46) | 0.04 | 1.18 (0.34–4.08) | 0.79 | ||
| DM | 2.35 (0.88–6.28) | 0.08 | 2.25 (0.77–6.57) | 0.14 | ||
| 25(OH)D < 20 ng/mL | 2.97 (0.95–9.27) | 0.06 | 2.72 (0.86–8.59] | 0.09 | 2.48 (0.77–7.99) | 0.13 |
| 25(OH)D < 10 ng/mL | 3.79 (1.53–9.39) | 0.004 | 4.09 (1.58–10.67) | 0.004 | 4.17 (1.54–11.27) | 0.005 |
| Predictor | Unadjusted | Model 1 Adjusted | Model 2 Adjusted | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.07 (1.03–1.13) | 0.001 | 1.09 (1.03–1.16) | 0.002 | 1.07 (1.01–1.15) | 0.03 |
| Male | 0.93 (0.34–2.52) | 0.88 | 0.43 (0.13–1.41) | 0.16 | 0.51 (0.15–1.69) | 0.27 |
| Obesity | 6.52 (2.23–19.02) | 0.001 | ||||
| AH | 5.06 (1.57–16.34) | 0.07 | 1.59 (0.35–5.54) | 0.56 | ||
| CAD | 5.66 (1.99–16.10) | 0.001 | 1.40 (0.35–5.54) | 0.63 | ||
| DM | 2.38 (0.79–7.08) | 0.12 | 1.98 (0.57–6.92) | 0.28 | ||
| 25(OH)D < 20 ng/mL | 9.78 (1.26–76.14) | 0.03 | 8.60 (1.07–69.12) | 0.05 | 7.87 (0.96–64.43) | 0.06 |
| 25(OH)D < 10 ng/mL | 4.07 (1.46–11.35) | 0.007 | 5.68 (1.74–18.52) | 0.004 | 5.79 (1.66–20.22) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karonova, T.L.; Andreeva, A.T.; Golovatuk, K.A.; Bykova, E.S.; Simanenkova, A.V.; Vashukova, M.A.; Grant, W.B.; Shlyakhto, E.V. Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19. Nutrients 2021, 13, 3021. https://doi.org/10.3390/nu13093021
Karonova TL, Andreeva AT, Golovatuk KA, Bykova ES, Simanenkova AV, Vashukova MA, Grant WB, Shlyakhto EV. Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19. Nutrients. 2021; 13(9):3021. https://doi.org/10.3390/nu13093021
Chicago/Turabian StyleKaronova, Tatiana L., Alena T. Andreeva, Ksenia A. Golovatuk, Ekaterina S. Bykova, Anna V. Simanenkova, Maria A. Vashukova, William B. Grant, and Evgeny V. Shlyakhto. 2021. "Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19" Nutrients 13, no. 9: 3021. https://doi.org/10.3390/nu13093021
APA StyleKaronova, T. L., Andreeva, A. T., Golovatuk, K. A., Bykova, E. S., Simanenkova, A. V., Vashukova, M. A., Grant, W. B., & Shlyakhto, E. V. (2021). Low 25(OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19. Nutrients, 13(9), 3021. https://doi.org/10.3390/nu13093021







