Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Ex Vivo Fecal Incubation Model: In Vitro Broccoli Sprouts Digestion and Ex Vivo Human Fecal Culture
2.2. Human Urine Samples
2.3. Microbial Sequencing
2.4. Isothiocyanate Quantification
2.5. Data Management and Quantification of ASVs
2.6. Diversity Analysis and Visualization
2.7. Beta-Diversity Analysis
2.8. Discriminant Analysis
2.9. Correlation Analysis
2.10. Code Availability
3. Results
3.1. Characteristics of the Pre-Incubation Fecal Slurries
3.2. Incubation with Broccoli Sprout Digest Does Not Alter Gut Microbial Composition
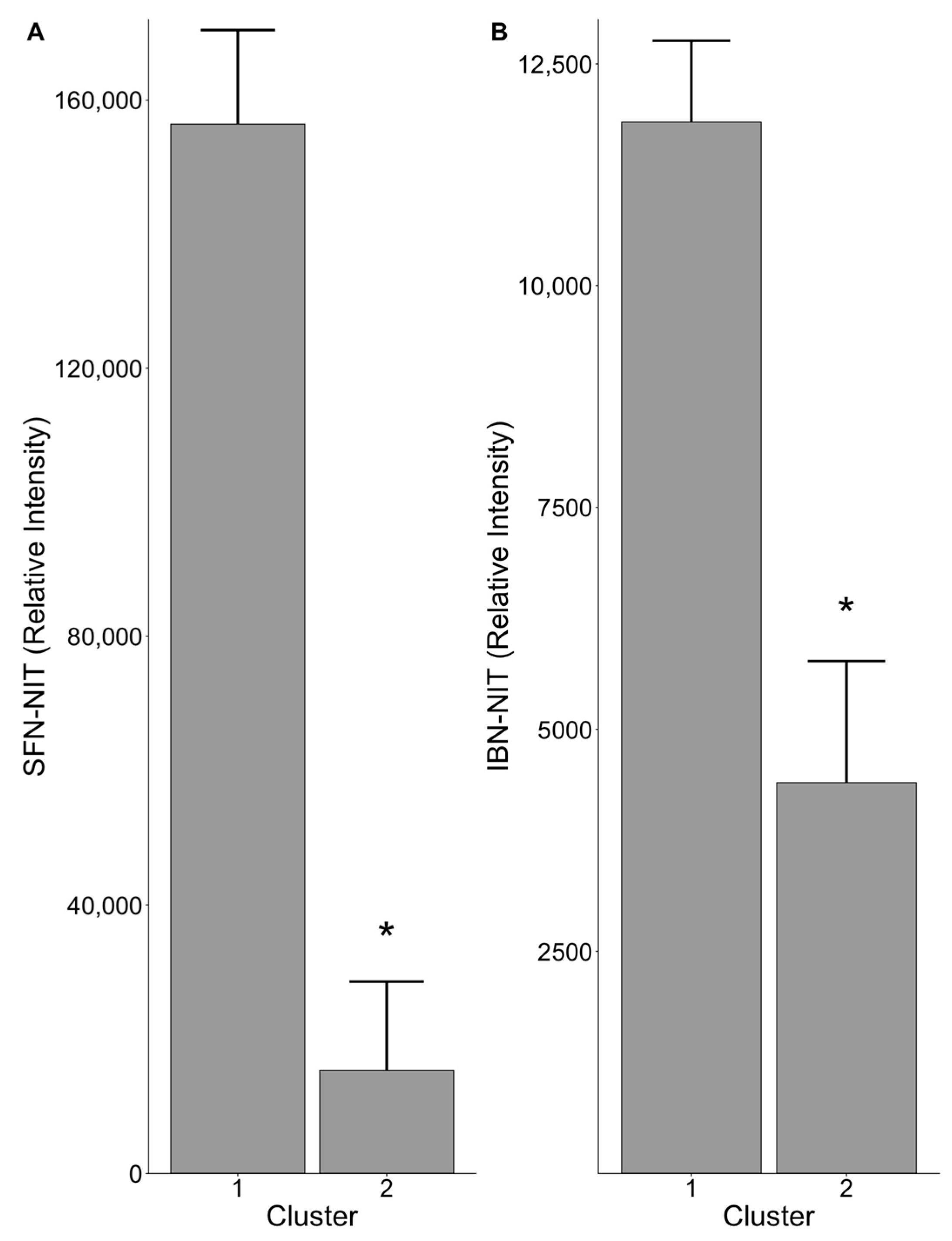
3.3. Gut Microbiome Composition Influences Nitrile Production
3.4. Members of Family Clostridiaceae Are Associated with Nitrile Production
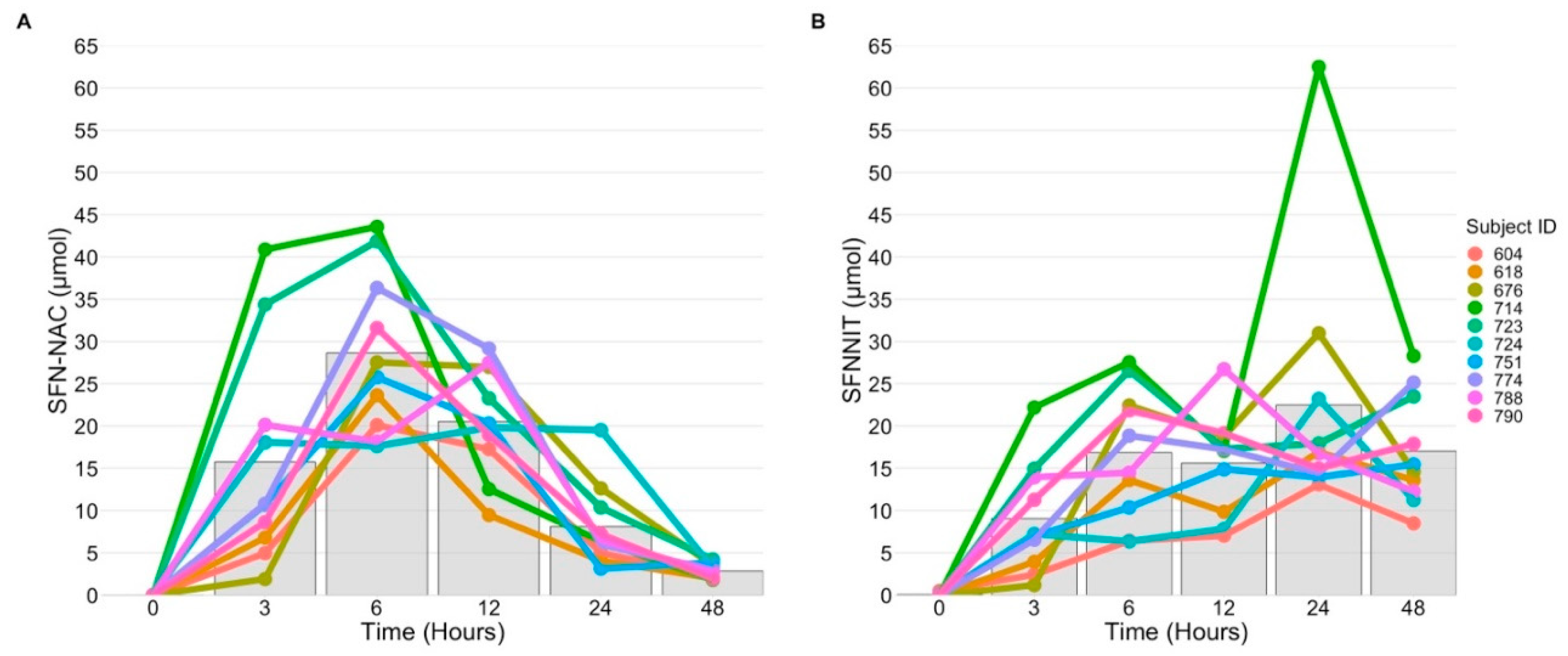
3.5. SFN-NIT Variation Exists in Humans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelino, D.; Jeffery, E. Glucosinolate Hydrolysis and Bioavailability of Resulting Isothiocyanates: Focus on Glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; Schrijver, R.D.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. Italica) Inhibits Formation of the Anticancer Agent Sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Critchley, C.; Pun, S.; Nottingham, S.; O’Hare, T.J. Epithiospecifier Protein Activity in Broccoli: The Link between Terminal Alkenyl Glucosinolates and Sulphoraphane Nitrile. Phytochemistry 2008, 69, 2765–2773. [Google Scholar] [CrossRef]
- Basten, G.P.; Bao, Y.; Williamson, G. Sulforaphane and Its Glutathione Conjugate but Not Sulforaphane Nitrile Induce UDP-Glucuronosyl Transferase (UGT1A1) and Glutathione Transferase (GSTA1) in Cultured Cells. Carcinogenesis 2002, 23, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Azarenko, O.; Jordan, M.A.; Wilson, L. Erucin, the Major Isothiocyanate in Arugula (Eruca Sativa), Inhibits Proliferation of MCF7 Tumor Cells by Suppressing Microtubule Dynamics. PLoS ONE 2014, 9, e100599. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Coldham, N.; Sauer, M.J.; Ioannides, C. Up-Regulation of the CYP1 Family in Rat and Human Liver by the Aliphatic Isothiocyanates Erucin and Sulforaphane. Toxicology 2008, 252, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-H.; Lim, Y.; Kim, S.-J.; Yoo, K.-D.; Yoo, H.-S.; Hong, J.-T.; Lee, M.-Y.; Yun, Y.-P. Sulforaphane Inhibits PDGF-Induced Proliferation of Rat Aortic Vascular Smooth Muscle Cell by up-Regulation of P53 Leading to G1/S Cell Cycle Arrest. Vasc. Pharmacol. 2013, 59, 44–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence Are Accompanied by DNA Hypomethylation and Changes in MicroRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef]
- Dos Santos, P.W.D.S.; Machado, A.R.T.; De Grandis, R.A.; Ribeiro, D.L.; Tuttis, K.; Morselli, M.; Aissa, A.F.; Pellegrini, M.; Antunes, L.M.G. Transcriptome and DNA Methylation Changes Modulated by Sulforaphane Induce Cell Cycle Arrest, Apoptosis, DNA Damage, and Suppression of Proliferation in Human Liver Cancer Cells. Food Chem. Toxicol. 2020, 136, 111047. [Google Scholar] [CrossRef]
- Clarke, J.D.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Comparison of Isothiocyanate Metabolite Levels and Histone Deacetylase Activity in Human Subjects Consuming Broccoli Sprouts or Broccoli Supplement. J. Agric. Food Chem. 2011, 59, 10955–10963. [Google Scholar] [CrossRef]
- Cao, C.; Wu, H.; Vasilatos, S.N.; Chandran, U.; Qin, Y.; Wan, Y.; Oesterreich, S.; Davidson, N.E.; Huang, Y. HDAC5-LSD1 Axis Regulates Antineoplastic Effect of Natural HDAC Inhibitor Sulforaphane in Human Breast Cancer Cells. Int. J. Cancer 2018, 143, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and Chemopreventive Targets of Sulforaphane in Humans Following Consumption of Broccoli Sprouts or a Myrosinase-Treated Broccoli Sprout Extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Gong, T.-T.; Guo, Q.; Li, X.; Zhang, T.-N.; Liu, F.-H.; He, X.-H.; Lin, B.; Wu, Q.-J. Isothiocyanate Iberin Inhibits Cell Proliferation and Induces Cell Apoptosis in the Progression of Ovarian Cancer by Mediating ROS Accumulation and GPX1 Expression. Biomed. Pharmacother. 2021, 142, 111533. [Google Scholar] [CrossRef]
- Zhang, Y.; Gilmour, A.; Ahn, Y.-H.; de la Vega, L.; Dinkova-Kostova, A.T. The Isothiocyanate Sulforaphane Inhibits MTOR in an NRF2-Independent Manner. Phytomedicine 2021, 86, 153062. [Google Scholar] [CrossRef]
- Rutz, J.; Juengel, E.; Euler, S.; Maxeiner, S.; Justin, S.; Roos, F.; Chun, F.K.-H.; Blaheta, R.A. Chronic Sulforaphane Application Does Not Induce Resistance in Renal Cell Carcinoma Cells. Anticancer Res. 2018, 38, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. (Phila) 2015, 8, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Atkinson, S.J.; Akbareian, S.E.; Zhou, Z.; Munsterberg, A.; Robinson, S.D.; Bao, Y. Sulforaphane Exerts Anti-Angiogenesis Effects against Hepatocellular Carcinoma through Inhibition of STAT3/HIF-1α/VEGF Signalling. Sci. Rep. 2017, 7, 12651. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Jeffery, E.H. Comparison of the Bioactivity of Two Glucoraphanin Hydrolysis Products Found in Broccoli, Sulforaphane and Sulforaphane Nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef]
- Keck, A.-S.; Staack, R.; Jeffery, E.H. The Cruciferous Nitrile Crambene Has Bioactivity Similar to Sulforaphane When Administered to Fischer 344 Rats but Is Far Less Potent in Cell Culture. Nutr. Cancer 2002, 42, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Boadas-Vaello, P.; Jover, E.; Saldaña-Ruíz, S.; Soler-Martín, C.; Chabbert, C.; Bayona, J.M.; Llorens, J. Allylnitrile Metabolism by CYP2E1 and Other CYPs Leads to Distinct Lethal and Vestibulotoxic Effects in the Mouse. Toxicol. Sci. 2009, 107, 461–472. [Google Scholar] [CrossRef]
- Tanii, H.; Higashi, T.; Nishimura, F.; Higuchi, Y.; Saijoh, K. Effects of Cruciferous Allyl Nitrile on Phase 2 Antioxidant and Detoxification Enzymes. Med. Sci. Monit. 2008, 14, BR189–BR192. [Google Scholar] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast Cancer Risk in Premenopausal Women Is Inversely Associated with Consumption of Broccoli, a Source of Isothiocyanates, but Is Not Modified by GST Genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Lam, T.K.; Gallicchio, L.; Boyd, K.; Shiels, M.; Hammond, E.; Tao, X.G.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous Vegetable Consumption and Lung Cancer Risk: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Epplein, M.; Wilkens, L.R.; Tiirikainen, M.; Dyba, M.; Chung, F.-L.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Marchand, L.L. Urinary Isothiocyanates; Glutathione S-Transferase M1, T1, and P1 Polymorphisms; and Risk of Colorectal Cancer: The Multiethnic Cohort Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 314–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, H.J.; Probst-Hensch, N.M.; Louie, A.D.; Kau, I.H.; Witte, J.S.; Ingles, S.A.; Frankl, H.D.; Lee, E.R.; Haile, R.W. Glutathione Transferase Null Genotype, Broccoli, and Lower Prevalence of Colorectal Adenomas. Cancer Epidemiol. Biomark. Prev. 1998, 7, 647–652. [Google Scholar]
- Moy, K.A.; Yuan, J.-M.; Chung, F.-L.; Van Den Berg, D.; Wang, R.; Gao, Y.-T.; Yu, M.C. Urinary Total Isothiocyanates and Colorectal Cancer: A Prospective Study of Men in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Kampman, E.; Samowitz, W.; Caan, B.J.; Potter, J.D. Interplay between Dietary Inducers of GST and the GSTM-1 Genotype in Colon Cancer. Int. J. Cancer 2000, 87, 728–733. [Google Scholar] [CrossRef]
- Seow, A.; Vainio, H.; Yu, M.C. Effect of Glutathione-S-Transferase Polymorphisms on the Cancer Preventive Potential of Isothiocyanates: An Epidemiological Perspective. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 592, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Seow, A.; Yuan, J.-M.; Sun, C.-L.; Van Den Berg, D.; Lee, H.-P.; Yu, M.C. Dietary Isothiocyanates, Glutathione S -Transferase Polymorphisms and Colorectal Cancer Risk in the Singapore Chinese Health Study. Carcinogenesis 2002, 23, 2055–2061. [Google Scholar] [CrossRef]
- Turner, F.; Smith, G.; Sachse, C.; Lightfoot, T.; Garner, R.C.; Wolf, C.R.; Forman, D.; Bishop, D.T.; Barrett, J.H. Vegetable, Fruit and Meat Consumption and Potential Risk Modifying Genes in Relation to Colorectal Cancer. Int. J. Cancer 2004, 112, 259–264. [Google Scholar] [CrossRef]
- Tijhuis, M.J.; Wark, P.A.; Aarts, J.M.M.J.G.; Visker, M.H.P.W.; Nagengast, F.M.; Kok, F.J.; Kampman, E. GSTP1 and GSTA1 Polymorphisms Interact with Cruciferous Vegetable Intake in Colorectal Adenoma Risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gao, Y.-T.; Shu, X.-O.; Cai, Q.; Li, G.-L.; Li, H.-L.; Ji, B.-T.; Rothman, N.; Dyba, M.; Xiang, Y.-B.; et al. Isothiocyanate Exposure, Glutathione S-Transferase Polymorphisms, and Colorectal Cancer Risk1234. Am. J. Clin. Nutr. 2010, 91, 704–711. [Google Scholar] [CrossRef]
- Brauer, H.A.; Libby, T.E.; Mitchell, B.L.; Li, L.; Chen, C.; Randolph, T.W.; Yasui, Y.Y.; Lampe, J.W.; Lampe, P.D. Cruciferous Vegetable Supplementation in a Controlled Diet Study Alters the Serum Peptidome in a GSTM1-Genotype Dependent Manner. Nutr. J. 2011, 10, 11. [Google Scholar] [CrossRef]
- Navarro, S.L.; Chang, J.-L.; Peterson, S.; Chen, C.; King, I.B.; Schwarz, Y.; Li, S.S.; Li, L.; Potter, J.D.; Lampe, J.W. Modulation of Human Serum Glutathione S-Transferase-A1/2 Concentration by Cruciferous Vegetables in a Controlled Feeding Study Is Influenced by GSTM1 and GSTT1 Genotypes. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2974–2978. [Google Scholar] [CrossRef]
- Navarro, S.L.; Schwarz, Y.; Song, X.; Wang, C.-Y.; Chen, C.; Trudo, S.P.; Kristal, A.R.; Kratz, M.; Eaton, D.L.; Lampe, J.W. Cruciferous Vegetables Have Variable Effects on Biomarkers of Systemic Inflammation in a Randomized Controlled Trial in Healthy Young Adults12. J. Nutr. 2014, 144, 1850–1857. [Google Scholar] [CrossRef]
- Peterson, S.; Schwarz, Y.; Li, S.S.; Li, L.; King, I.B.; Chen, C.; Eaton, D.L.; Potter, J.D.; Lampe, J.W. CYP1A2, GSTM1, and GSTT1 Polymorphisms and Diet Effects on CYP1A2 Activity in a Crossover Feeding Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3118–3125. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Williams, D.E.; Dashwood, R.H.; Stevens, J.F.; Ho, E. Metabolism and Tissue Distribution of Sulforaphane in Nrf2 Knockout and Wild-Type Mice. Pharm. Res. 2011, 28, 3171–3179. [Google Scholar] [CrossRef]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.A.; Rossiter, J.T.; Mithen, R.F.; Kroon, P.A. Isothiocyanate Concentrations and Interconversion of Sulforaphane to Erucin in Human Subjects after Consumption of Commercial Frozen Broccoli Compared to Fresh Broccoli. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef]
- Keck, A.-S.; Finley, J.W. Cruciferous Vegetables: Cancer Protective Mechanisms of Glucosinolate Hydrolysis Products and Selenium. Integr. Cancer Ther. 2004, 3, 5–12. [Google Scholar] [CrossRef]
- Getahun, S.M.; Chung, F.-L. Conversion of Glucosinolates to Isothiocyanates in Humans after Ingestion of Cooked Watercress. Cancer Epidemiol. Biomark. Prev. 1999, 8, 447–451. [Google Scholar]
- Li, F.; Hullar, M.A.J.; Schwarz, Y.; Lampe, J.W. Human Gut Bacterial Communities Are Altered by Addition of Cruciferous Vegetables to a Controlled Fruit- and Vegetable-Free Diet. J. Nutr. 2009, 139, 1685–1691. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of Glucoraphanin Metabolism in Vivo and Ex Vivo by Human Gut Bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic Acid Bacteria Convert Glucosinolates to Nitriles Efficiently Yet Differently from Enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The Metabolism of Methylsulfinylalkyl- and Methylthioalkyl-Glucosinolates by a Selection of Human Gut Bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Albaser, A.A.; Nueno-Palop, C.; Bennett, M.H.; Narbad, A.; Rossiter, J.T. Glucosinolate and Desulfo-Glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr. Microbiol. 2016, 73, 442–451. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S.; Udomwong, P.; Saengha, W.; Gregori, M. Formation of Sulforaphane and Iberin Products from Thai Cabbage Fermented by Myrosinase-Positive Bacteria. Molecules 2018, 23, 955. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli Consumption Affects the Human Gastrointestinal Microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Tapp, H.S.; Saha, S.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Consumption of a Diet Rich in Brassica Vegetables Is Associated with a Reduced Abundance of Sulphate-reducing Bacteria: A Randomised Crossover Study. Mol. Nutr. Food Res. 2017, 61, 1600992. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Boccellino, M.; Barone, B.; Zazzo, E.D.; Sciarra, A.; Galasso, G.; Settembre, G.; Quagliuolo, L.; Imbimbo, C.; Boffo, S.; et al. The Crosstalk between Prostate Cancer and Microbiota Inflammation: Nutraceutical Products Are Useful to Balance This Interplay? Nutrients 2020, 12, 2648. [Google Scholar] [CrossRef] [PubMed]
- Center for Food Safety and Applied Nutrition Guidance for Industry. Compliance with and Recommendations for Implementation of the Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption for Sprout Operations; Center for Food Safety and Applied Nutrition Guidance for Industry: College Park, MD, USA, 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-compliance-and-recommendations-implementation-standards-growing-harvesting (accessed on 20 August 2021).
- Yang, Y.; Meier, F.; Lo, J.A.; Yuan, W.; Sze, V.L.P.; Chung, H.-J.; Yuk, H.-G. Overview of Recent Events in the Microbiological Safety of Sprouts and New Intervention Technologies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 265–280. [Google Scholar] [CrossRef]
- Fahey, J.W.; Ourisson, P.J.; Degnan, F.H. Pathogen Detection, Testing, and Control in Fresh Broccoli Sprouts. Nutr. J. 2006, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Henson, W.Y. U.S. EPA, Pesticides, Label, Ecr Calcium Hypochlorite T, 3/17/2011. 2011. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/086460-00004-20110317.pdf (accessed on 20 August 2021).
- Aura, A.-M.; Härkönen, H.; Fabritius, M.; Poutanen, K. Development of AnIn VitroEnzymic Digestion Method for Removal of Starch and Protein and Assessment of Its Performance Using Rye AndWheat Breads. J. Cereal Sci. 1999, 29, 139–152. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in Vitro Method to Simulate Phenolic Compound Release from the Food Matrix in the Gastrointestinal Tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Sarvan, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane Formation and Bioaccessibility Are More Affected by Steaming Time than Meal Composition during in Vitro Digestion of Broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef]
- Rychlik, J.; Olejnik, A.; Olkowicz, M.; Kowalska, K.; Juzwa, W.; Myszka, K.; Dembczyński, R.; Moyer, M.P.; Grajek, W. Antioxidant Capacity of Broccoli Sprouts Subjected to Gastrointestinal Digestion. J. Sci. Food Agric. 2015, 95, 1892–1902. [Google Scholar] [CrossRef]
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial Communities and Metabolic Activity of Faecal Cultures from Equol Producer and Non-Producer Menopausal Women under Treatment with Soy Isoflavones. BMC Microbiol. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Illumina 16S Sample Preparation Guide. Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 20 August 2021).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Axton, E.R.; Beaver, L.M.; St Mary, L.; Truong, L.; Logan, C.R.; Spagnoli, S.; Prater, M.C.; Keller, R.M.; Garcia-Jaramillo, M.; Ehrlicher, S.E.; et al. Treatment with Nitrate, but Not Nitrite, Lowers the Oxygen Cost of Exercise and Decreases Glycolytic Intermediates While Increasing Fatty Acid Metabolites in Exercised Zebrafish. J. Nutr. 2019, 149, 2120–2132. [Google Scholar] [CrossRef]
- Housley, L.; Magana, A.A.; Hsu, A.; Beaver, L.M.; Wong, C.P.; Stevens, J.F.; Choi, J.; Jiang, Y.; Bella, D.; Williams, D.E.; et al. Untargeted Metabolomic Screen Reveals Changes in Human Plasma Metabolite Profiles Following Consumption of Fresh Broccoli Sprouts. Mol. Nutr. Food Res. 2018, 62, 1700665. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan. (accessed on 20 August 2021).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Wong, M.A. A K-Means Clustering Algorithm. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and Metabolism of Isothiocyanates Formed from Broccoli Glucosinolates: Effects of BMI and Daily Consumption in a Randomised Clinical Trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef]
- Kensler, T.W.; Chen, J.-G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.-B.; Wu, Y.; et al. Effects of Glucosinolate-Rich Broccoli Sprouts on Urinary Levels of Aflatoxin-DNA Adducts and Phenanthrene Tetraols in a Randomized Clinical Trial in He Zuo Township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Ng, D.; Carmella, S.G.; Chen, M.; Jacobson, L.P.; Muñoz, A.; Egner, P.A.; Chen, J.G.; Qian, G.S.; Chen, T.Y.; et al. Modulation of the Metabolism of Airborne Pollutants by Glucoraphanin-Rich and Sulforaphane-Rich Broccoli Sprout Beverages in Qidong, China. Carcinogenesis 2012, 33, 101–107. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.-G.; Zarth, A.T.; Ng, D.K.; Wang, J.-B.; Kensler, K.H.; Jacobson, L.P.; Muñoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and Sustainable Detoxication of Airborne Pollutants by Broccoli Sprout Beverage: Results of a Randomized Clinical Trial in China. Cancer Prev. Res. (Phila) 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Luang-In, V.; Albaser, A.A.; Rossiter, J.T. Characterization of a Recombinant β-Glucosidase of GH3 Family from Glucosinolate-Metabolizing Human Gut Bacterium Enterococcus Casseliflavus CP1 for Nitrile Production. Songklanakarin J. Sci. Technol. (SJST) 2020, 42, 549–556. [Google Scholar] [CrossRef]
- Lai, R.-H.; Miller, M.J.; Jeffery, E. Glucoraphanin Hydrolysis by Microbiota in the Rat Cecum Results in Sulforaphane Absorption. Food Funct. 2010, 1, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; MacLeod, A.J. The Effects of PH on Glucosinolate Degradation by a Thioglucoside Glucohydrolase Preparation. Phytochemistry 1980, 19, 2547–2551. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Sørensen, H. Initial and Final Products, Nitriles, and Ascorbigens Produced in Myrosinase-Catalyzed Hydrolysis of Indole Glucosinolates. J. Agric. Food Chem. 1998, 46, 1563–1571. [Google Scholar] [CrossRef]
- Wathelet, J.-P.; Iori, R.; Leoni, O.; Rollin, P.; Mabon, N.; Marlier, M.; Palmieri, S. A Recombinant β-O-Glucosidase from Caldocellum Saccharolyticum to Hydrolyse Desulfo-Glucosinolates. Biotechnol. Lett. 2001, 23, 443–446. [Google Scholar] [CrossRef]
- Lu, M.; Hashimoto, K.; Uda, Y. Rat Intestinal Microbiota Digest Desulfosinigrin to Form Allyl Cyanide and 1-Cyano-2,3-Epithiopropane. Food Res. Int. 2011, 44, 1023–1028. [Google Scholar] [CrossRef]
- Berteau, O.; Guillot, A.; Benjdia, A.; Rabot, S. A New Type of Bacterial Sulfatase Reveals a Novel Maturation Pathway in Prokaryotes*. J. Biol. Chem. 2006, 281, 22464–22470. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Zhu, Y.; Mupunga, J.; Zou, L.; Liu, C.; Liu, S.; Mao, J. Broccoli Ingestion Increases the Glucosinolate Hydrolysis Activity of Microbiota in the Mouse Gut. Int. J. Food Sci. Nutr. 2019, 70, 585–594. [Google Scholar] [CrossRef]
- Bheemreddy, R.M.; Jeffery, E.H. The Metabolic Fate of Purified Glucoraphanin in F344 Rats. J. Agric. Food Chem. 2007, 55, 2861–2866. [Google Scholar] [CrossRef]


| ASV | Term * | Adjusted p-Value | Log2 Fold Change § | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|---|---|---|
| ASV1 | NC | 1.95 × 10−207 | −8.03 | Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Escherichia/Shigella |
| ASV1 | Broc | 1.13 × 10−281 | −10.85 | Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Escherichia/Shigella |
| ASV49 | NC | 2.47 × 10−21 | −1.51 | Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Melissococcus |
| ASV49 | Broc | 3.60 × 10−03 | 2.02 | Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Melissococcus |
| ASV556 | Broc | 7.54 × 10−46 | 3.05 | Firmicutes | Clostridia | Clostridiales | Clostridiaceae_1 | f_Clostridiaceae_1_ASV556 |
| ASV683 | Broc | 5.77 × 10−38 | 2.95 | Firmicutes | Clostridia | Clostridiales | Clostridiaceae_1 | f_Clostridiaceae_1_ASV683 |
| ASV872 | Broc | 8.84 × 10−28 | 4.26 | Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Collinsella |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouranis, J.A.; Beaver, L.M.; Choi, J.; Wong, C.P.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; Ho, E. Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts. Nutrients 2021, 13, 3013. https://doi.org/10.3390/nu13093013
Bouranis JA, Beaver LM, Choi J, Wong CP, Jiang D, Sharpton TJ, Stevens JF, Ho E. Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts. Nutrients. 2021; 13(9):3013. https://doi.org/10.3390/nu13093013
Chicago/Turabian StyleBouranis, John A., Laura M. Beaver, Jaewoo Choi, Carmen P. Wong, Duo Jiang, Thomas J. Sharpton, Jan F. Stevens, and Emily Ho. 2021. "Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts" Nutrients 13, no. 9: 3013. https://doi.org/10.3390/nu13093013
APA StyleBouranis, J. A., Beaver, L. M., Choi, J., Wong, C. P., Jiang, D., Sharpton, T. J., Stevens, J. F., & Ho, E. (2021). Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts. Nutrients, 13(9), 3013. https://doi.org/10.3390/nu13093013







