COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review
Abstract
:1. Introduction
1.1. COVID-19 Disease
1.1.1. Epidemiological Description
1.1.2. Structure of SARS-CoV-2
1.1.3. Transmission and Pathophysiology
1.1.4. Mechanism of Entry and Innate Immunity
1.1.5. Incubation Period
1.1.6. Clinical Symptoms
1.1.7. Adaptive Immunity
1.1.8. Interpersonal Variation of Immunity
1.1.9. Duration of the Immunity
1.1.10. Severity of the Disease
1.1.11. Mortality Rate
1.1.12. COVID-19 Vaccines
1.2. Pregnancy and COVID-19
Vaccine against COVID-19 in Pregnant Women
1.3. Breastfeeding
Composition of Breastmilk
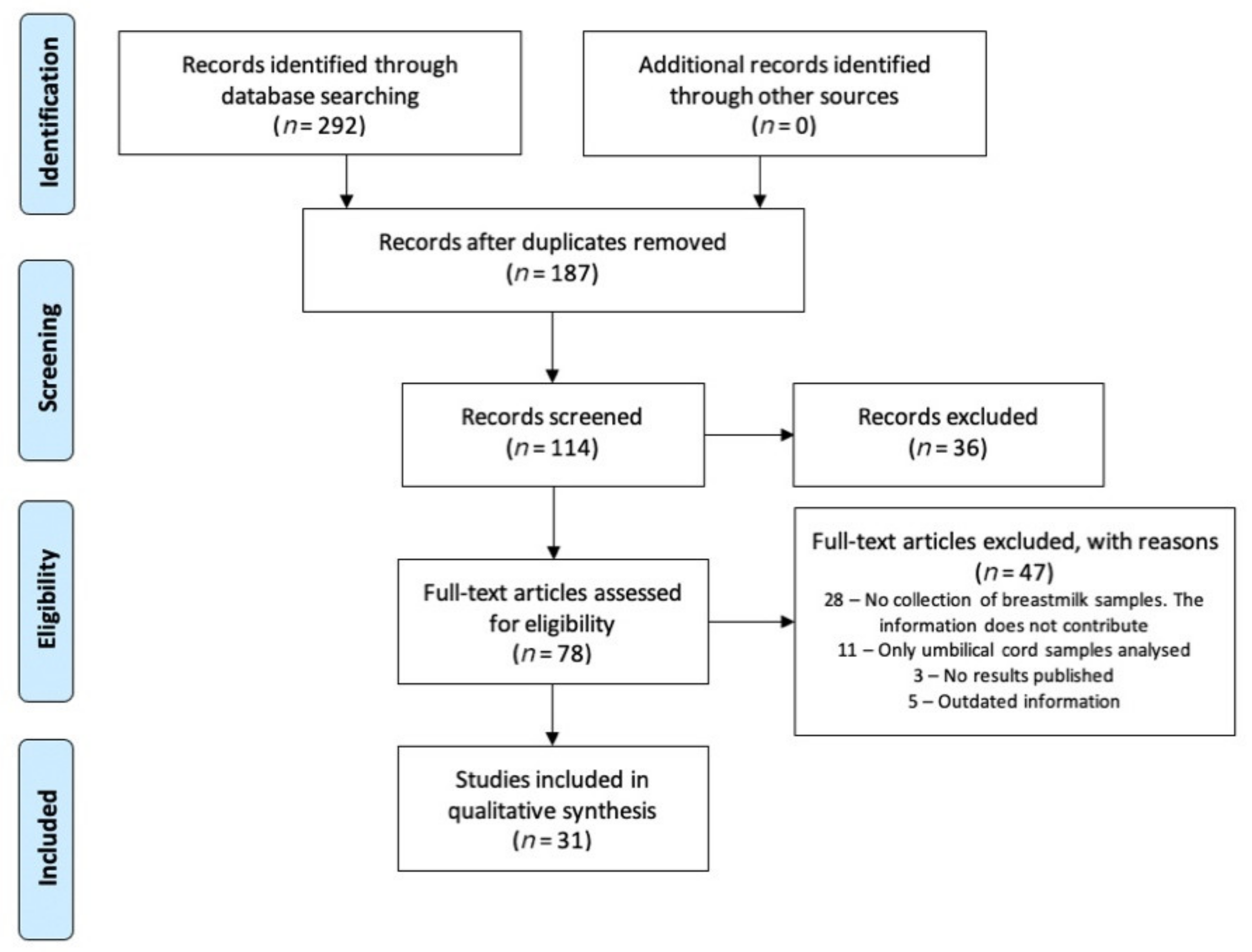
2. Search Methodology
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Sanidad Informe Técnico. Enfermedad por Coronavirus COVID-19. Available online: https://www.aemps.gob.es/; https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/vacunasCovid19.htm (accessed on 12 July 2021).
- Pastrian-Soto, G. Bases genéticas y moleculares del COVID-19 (SARS-CoV-2): Mecanismos de patogénesis y de respuesta inmune. Int. J. Odontostomatol. 2020, 14, 331–337. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- COVID-19, Cold, Allergies and the Flu: What Are the Differences? Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/covid-19-cold-flu-and-allergies-differences/art-20503981 (accessed on 14 February 2021).
- WHO. Reguntas y Respuestas Sobre la Enfermedad por Coronavirus (COVID-19). Available online: https://www.who.int/es/emergencies/diseases/novel-coronavirus-2019/advice-for-public/q-a-coronaviruses (accessed on 8 March 2021).
- Solís-García, G.; Gutiérrez-Vélez, A.; Pescador-Chamorro, I.; Zamora-Flores, E.; Vigil-Vázquez, S.; Rodríguez-Corrales, E.; Sánchez-Luna, M. Epidemiology, management and risk of SARS-CoV-2 transmission in a cohort of newborns born to mothers diagnosed with COVID-19 infection. An. Pediatr. 2021, 94, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Montaño Ramírez, L.M.; Flores-Soto, E. COVID-19 y su asociación con los inhibidores de la enzima convertidora de angiotensina y los antagonistas de los receptores para angiotensina II. Rev. Fac. Med. UNAM 2020, 63, 30–34. [Google Scholar] [CrossRef]
- Tikellis, C.; Bernardi, S.; Burns, W.C. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 62–68. [Google Scholar] [CrossRef]
- Garabelli, P.J.; Modrall, J.G.; Penninger, J.M.; Ferrario, C.M.; Chappell, M.C. Distinct roles for angiotensin-converting enzyme 2 and carboxypeptidase A in the processing of angiotensins within the murine heart. Exp. Physiol. 2008, 93, 613–621. [Google Scholar] [CrossRef]
- Stewart, J.A.; Lazartigues, E.; Lucchesi, P.A. The angiotensin converting enzyme 2/Ang-(1-7) axis in the heart: A role for MAS communication? Circ. Res. 2008, 103, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [Green Version]
- González, F.; Maite, A. Bloqueadores del sistema renina-angiotensina: Enemigos o amigos en pacientes con COVID-19. Rev. Venez. Endocrinol. Metab. 2020, 18, pp. 1–3. Available online: https://www.redalyc.org/articulo.oa?id=375563116002 (accessed on 16 July 2021).
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- CDC. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 2 March 2021).
- Fernández-Carrasco, F.J.; Vázquez-Lara, J.M.; González-Mey, U.; Gómez-Salgado, J.; Parrón-Carreño, T.; Rodríguez-Díaz, L. Infección por coronavirus COVID-19 y lactancia materna: Una revisión exploratoria. Rev. Esp. Salud Pública 2020, 94, e202005055. [Google Scholar]
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 15 March 2021).
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Wang, T.T. Immunity after SARS-CoV-2 infections. Nat. Immunol. 2021, 22, 539–540. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Plasencia-Urizarri, T.M.; Aguilera-Rodríguez, R.; Almaguer-Mederos, L.E. Comorbilidades y gravedad clínica de la COVID-19: Revisión sistemática y meta-análisis. Rev. Haban. Cienc. Médicas 2020, 19 (Suppl. 1), e3389. [Google Scholar]
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report 82. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200411-sitrep-82-covid-19.pdf?sfvrsn=74a5d15_2 (accessed on 20 April 2021).
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths from COVID-19. JAMA 2021, 325, 133–134. [Google Scholar] [CrossRef]
- CDC. COVID-19 Hospitalization and Death by Age. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/covid-data/hospitalization-death-by-age.pdf (accessed on 20 April 2021).
- Conlen, M.; Ivory, D.; Yourish, K.; Lai, K.K.R.; Hassan, A.; Calderone, J. One-Third of U.S. Coronavirus Deaths Are Linked to Nursing Homes. Available online: https://www.nytimes.com/interactive/2020/us/coronavirus-nursing-homes.html (accessed on 20 April 2021).
- WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 20 April 2021).
- COVID-19 Vaccine MODERNA. Consejo Interterritorial. Sistema Nacional de Salud. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/Guia_Tecnica_vacuna_Moderna.pdf (accessed on 25 May 2021).
- COVID-19 Vaccine COMIRNATY Pfizer-BioNTech. Consejo Interterritorial. Sistema Nacional de Salud. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/Guia_Tecnica_COMIRNATY.pdf (accessed on 25 May 2021).
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: A systematic review. J. Matern. Fetal Neonatal Med. 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, in press. [Google Scholar] [CrossRef]
- Császár-Nagy, N.; Bókkon, I. Mother-newborn separation at birth in hospitals: A possible risk for neurodevelopmental disorders? Neurosci. Biobehav. Rev. 2018, 84, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Brahm, P.; Valdés, V. The benefits of breastfeeding and associated risks of replacement with baby formulas. Rev. Chil. Pediatr. 2017, 88, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandolfi, E.; Gesualdo, F.; Rizzo, C.; Carloni, E.; Villani, A.; Concato, C.; Linardos, G.; Russo, L.; Ferretti, B.; Campagna, I.; et al. Breastfeeding and Respiratory Infections in the First 6 Months of Life: A Case Control Study. Front. Pediatr. 2019, 7, 152. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Koumbi, L.; Mesiari, C.; Berghea, E.C.; Konstantinou, G.N. Breastfeeding and COVID-19: From Nutrition to Immunity. Front. Immunol. 2021, 12, 946. [Google Scholar] [CrossRef]
- Walker, W.A. Initial intestinal colonization in the human infant and immune homeostasis. Ann. Nutr. Metab. 2013, 63 (Suppl. 2), 8–15. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Joanna Briggs Institute. Critical Appraisal Tools. 2017. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 20 August 2021).
- Marín Gabriel, M.A.; Malalana Martínez, A.M.; Marín Martínez, M.E.; Anel Pedroche, J. Negative Transmission of SARS-CoV-2 to Hand-Expressed Colostrum from SARS-CoV-2-Positive Mothers. Breastfeed. Med. 2020, 15, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Li, W.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020, 14, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N. Engl. J. Med. 2020, 382, e100. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, G.; de Rose, D.U.; Concato, C.; Alario, D.; Olivini, N.; Dotta, A.; Campana, A. Managing COVID-19-Positive Maternal-Infant Dyads: An Italian Experience. Breastfeed. Med. 2020, 15, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, W.; Su, H.; Li, S.; Shereen, M.A.; Lv, Z.; Niu, Z.; Li, D.; Liu, F.; Luo, Z.; et al. Breastfeeding Risk from Detectable Severe Acute Respiratory Syndrome Coronavirus 2 in Breastmilk. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Lowe, B.; Bopp, B. COVID-19 vaginal delivery—A case report. Aust. N. Z. J. Obs. Gynaecol 2020, 60, 465–466. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Cruz-Melguizo, S.; Adrien, M.; Fuentes, L.; Marin, E.; Forti, A.; Perez-Medina, T. Breastfeeding mothers with COVID-19 infection: A case series. Int. Breastfeed. J. 2020, 15, 69. [Google Scholar] [CrossRef]
- Lang, G.J.; Zhao, H. Can SARS-CoV-2-infected women breastfeed after viral clearance? J. Zhejiang Univ. Sci. B 2020, 21, 405–407. [Google Scholar] [CrossRef]
- Bastug, A.; Hanifehnezhad, A.; Tayman, C.; Ozkul, A.; Ozbay, O.; Kazancioglu, S.; Bodur, H. Virolactia in an Asymptomatic Mother with COVID-19. Breastfeed. Med. 2020, 15, 488–491. [Google Scholar] [CrossRef]
- Chu, H.; Li, J.; Yan, J.; Bai, T.; Schnabl, B.; Zou, L.; Yang, L.; Hou, X. Persistent SARS-CoV-2 RNA Positive in Feces but Negative in Breastmilk: A Case Report of COVID-19 in a Breastfeeding Patient. Front. Med. 2020, 7, 562700. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Wu, J.; Xia, M.; Xu, W. Insight from a COVID-19-Infected Lactating Mother with A Healthy Breastfed Infant. Front. Med. Case Rep. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Perrone, S.; Giordano, M.; Meoli, A.; Deolmi, M.; Marinelli, F.; Messina, G.; Lugani, P.; Moretti, S.; Esposito, S. Lack of viral transmission to preterm newborn from a COVID-19 positive breastfeeding mother at 11 days postpartum. J. Med. Virol. 2020, 92, 2346–2347. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Zheng, S.; Chen, X.; Wang, J.; Sheng, X.; Zhou, J.; Cai, H.; Fang, Q.; Yu, F.; et al. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg. Infect. Dis 2020, 26, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Hu, Y.; Li, B.; Xu, J. Breastfed 13 month-old infant of a mother with COVID-19 pneumonia: A case report. Int Breastfeed. J. 2020, 15, 68. [Google Scholar] [CrossRef]
- Tam, P.C.K.; Ly, K.M.; Kernich, M.L.; Spurrier, N.; Lawrence, D.; Gordon, D.L.; Tucker, E.C. Detectable Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Human Breast Milk of a Mildly Symptomatic Patient With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 72, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.K.; Kumble, A.; Varghese, A.; Sherigar, B.; Reddy, R. A case report of neonatal coronavirus disease-19 infection in South India. Indian J. Case Rep. 2020, 6, 461–463. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, 192, E647–E650. [Google Scholar] [CrossRef]
- Hinojosa-Velasco, A.; de Oca, P.V.B.; García-Sosa, L.E.; Mendoza-Durán, J.G.; Pérez-Méndez, M.J.; Dávila-González, E.; Ramírez-Hernández, D.G.; García-Mena, J.; Zárate-Segura, P.; Reyes-Ruiz, J.M.; et al. A case report of newborn infant with severe COVID-19 in Mexico: Detection of SARS-CoV-2 in human breast milk and stool. Int. J. Infect. Dis. 2020, 100, 21–24. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Chen, L.; Liu, W.; Cao, Y.; Zhang, J.; Feng, L. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin. Infect. Dis. 2020, 71, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Lei, D.; Fang, C.; Li, C.; Wang, M.; Liu, Y.; Bao, Y.; Sun, Y.; Huang, J.; Guo, Y.; et al. Perinatal Transmission of 2019 Coronavirus Disease-Associated Severe Acute Respiratory Syndrome Coronavirus 2: Should We Worry? Clin. Infect. Dis. 2021, 72, 862–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhalik, M.; Dash, S.; El-Atawi, K.; Mahfouz, R.; Ramzy, A.; Dsouza, D.; Debek, K.; Varughese, S.; Augustine, N. Clinical profile of neonates delivered from mothers with confirmed COVID-19 infection: An experience from a Tertiary Perinatal Care Center in Dubai, UAE. J. Pediatr. Neonatal Care 2020, 10, 142–146. [Google Scholar] [CrossRef]
- Ronchi, A.; Pietrasanta, C.; Zavattoni, M.; Saruggia, M.; Schena, F.; Sinelli, M.T.; Agosti, M.; Tzialla, C.; Varsalone, F.F.; Testa, L.; et al. Evaluation of Rooming-in Practice for Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Italy. JAMA Pediatr. 2021, 175, 260–266. [Google Scholar] [CrossRef]
- Patil, U.P.; Maru, S.; Krishnan, P.; Carroll-Bennett, R.; Sanchez, J.; Noble, L.; Wasserman, R. Newborns of COVID-19 mothers: Short-term outcomes of colocating and breastfeeding from the pandemic’s epicenter. J. Perinatol. 2020, 40, 1455–1458. [Google Scholar] [CrossRef]
- Bertino, E.; Moro, G.E.; De Renzi, G.; Viberti, G.; Cavallo, R.; Coscia, A.; Rubino, C.; Tonetto, P.; Sottemano, S.; Campagnoli, M.F.; et al. Detection of SARS-CoV-2 in Milk From COVID-19 Positive Mothers and Follow-Up of Their Infants. Front. Pediatr. 2020, 8, 597699. [Google Scholar] [CrossRef]
- Le, H.T.; Nguyen, L.V.; Tran, D.M.; Do, H.T.; Tran, H.T.; Le, Y.T.; Phan, P.H. The first infant case of COVID-19 acquired from a secondary transmission in Vietnam. Lancet Child. Adolesc. Health 2020, 4, 405–406. [Google Scholar] [CrossRef]
- Gao, X.; Wang, S.; Zeng, W.; Chen, S.; Wu, J.; Lin, X.; Liu, Y.; Sun, Z.; Feng, L. Clinical and immunologic features among COVID-19-affected mother-infant pairs: Antibodies to SARS-CoV-2 detected in breast milk. New Microbes New Infect. 2020, 37, 100752. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Belfort, M.B.; Pace, C.D.W.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio 2021, 12, e03192-20. [Google Scholar] [CrossRef]
- Dong, Y.; Chi, X.; Hai, H.; Sun, L.; Zhang, M.; Xie, W.F.; Chen, W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg. Microbes Infect. 2020, 9, 1467–1469. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk after COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef]
- Juncker, H.G.; Romijn, M.; Loth, V.N.; Ruhé, E.J.M.; Bakker, S.; Kleinendorst, S.; de Groot, C.J.M.; Pajkrt, D.; Korosi, A.; van Goudoever, J.B.; et al. Antibodies against SARS-CoV-2 in Human Milk: Milk Conversion Rates in the Netherlands. J. Hum. Lact. 2021. [Google Scholar] [CrossRef]
- Duncombe, C.J.; McCulloch, D.J.; Shuey, K.D.; Logue, J.K.; Franko, N.M.; Wolf, C.R.; Frivold, C.J.; Chu, H.Y. Dynamics of breast milk antibody titer in the six months following SARS-CoV-2 infection. J. Clin. Virol. 2021, 142, 104916. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Prahl, M.; Cassidy, A.G.; Gay, C.; Wu, A.H.B.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; Basilio, E.; Warrier, L.; et al. COVID-19 mRNA Vaccination in Lactation: Assessment of adverse effects and transfer of anti-SARS-CoV2 antibodies from mother to child. medRxiv 2021. [Google Scholar] [CrossRef]
- Jakuszko, K.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Rukasz, D.; Kłak, R.; Królak-Olejnik, B.; Krajewska, M. Immune Response to Vaccination against COVID-19 in Breastfeeding Health Workers. Vaccines 2021, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Terracciano, D.; Cennamo, M.; Aiello, F.; La Civita, E.; Esposito, G.; Gargiulo, V.; Maruotti, G.M.; Portella, G.; Sarno, L. COVID-19 Vaccine mRNABNT162b2 Elicits Human Antibody Response in Milk of Breastfeeding Women. Vaccines 2021, 9, 785. [Google Scholar] [CrossRef]
- Low, J.M.; Low, Y.W.; Zhong, Y.; Lee, C.Y.C.; Chan, M.; Ng, N.B.H.; Amin, Z.; Ng, Y.P.M. Titres and neutralising capacity of SARS-CoV-2-specific antibodies in human milk: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2021, F1–F7. [Google Scholar] [CrossRef]
| Sign or Symptom | COVID-19 | Cold | Allergies | Flu |
|---|---|---|---|---|
| Cough | Generally (dry cough) | Generally | Sometimes | Generally |
| Muscle pain | Generally | Sometimes | Never | Generally |
| Fatigue | Generally | Sometimes | Sometimes | Generally |
| Throat pain | Generally | Generally | Rarely | Generally |
| Blocked or runny nose | Generally | Generally | Generally | Generally |
| Fever | Generally | Sometimes | Never | Generally—not always |
| Loss of taste/smell | Generally (initially, often without a blocked nose) | Sometimes (especially with a blocked nose) | Sometimes | Rarely |
| Shortness of breath/trouble breathing | Generally | Generally | ||
| Diarrhoea | Sometimes | Never | Never | Sometimes (more common in children) |
| Nausea or vomiting | Sometimes | Never | Never | Sometimes (more common in children) |
| Conjunctivitis | Sometimes | Sometimes | ||
| Sneezing | Rarely | Sometimes | Generally | |
| Itchy nose, eyes, mouth, or ears | Never | Generally |
| Ref. | Year | Type of Study | Sample | Results | Conclusions |
|---|---|---|---|---|---|
| [54] | 2020 | Observational, prospective study | 7 | Of the seven breastmilk samples collected from infected mothers, all were negative for SARS-CoV-2 by RT-PCR. | Breastmilk was not a source of SARS-CoV-2 transmission. Expressing breastmilk manually, when direct breastfeeding is not possible, appears to be a safe way to feed newborns of mothers who are infected with COVID-19. |
| [55] | 2020 | Observational, prospective study | 10 | Of the ten breastmilk samples collected from infected mothers, all were negative for SARS-CoV-2 by RT-PCR. | Breastmilk was not a source of SARS-CoV-2 transmission. The most important strategies in preventing neonatal SARS-CoV-2 infection are to prevent maternal infection and reduce the possibility of neonatal exposure to the virus. |
| [56] | 2020 | Observational study | 6 | Of the six breastmilk samples collected from infected mothers, all were negative for SARS-CoV-2 by RT-PCR. | SARS-CoV-2 was not found in breastmilk. |
| [57] | 2020 | A case report | 2 | Of the two breastmilk samples collected from infected mothers, all were negative for SARS-CoV-2 by RT-PCR. | Health workers must protect, motivate and encourage breastfeeding. The most likely mother-child transmission is through respiratory droplets. |
| [58] | 2020 | A case report | 2 | Of the 11 breastmilk samples that were collected from two infected mothers (four samples from Mother 1, seven samples from Mother 2), the four samples from Mother 1 were negative and the first four samples from Mother 2 were positive while the last three were negative for SARS-CoV-2 by RT-PCR. | Whether the neonate was infected through breastmilk or other modes of transmission is unknown. Further studies are needed. |
| [59] | 2020 | A case report | 5 | Of the 11 breastmilk samples that were collected from five infected mothers (one sample from Mother 1, two samples from Mother 2, two samples from Mother 3, two samples from Mother 4, one sample from Mother 5), only two samples from Mother 3 were positive for SARS-CoV-2 by RT-PCR. | Conclusions are limited due to the small sample size. |
| [60] | 2020 | A case report | 1 | The neonate was fed breastmilk in the mother’s room and did not become infected. | Encourage breastfeeding and safe room sharing. |
| [61] | 2020 | A retrospective case series | 22 | The neonates were fed breastmilk (20/22) and infant formula (2/22). Nine of the 11 symptomatic mothers were isolated. No infants were infected. | Breastfeed with precautions, after maternal isolation: donor human breastmilk or infant formula until breastfeeding is resumed. |
| [62] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. The neonate was fed breastmilk after isolation from the mother. | Breastfeeding after isolation and negative test. Feed the neonate pumped breastmilk during isolation. |
| [63] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was positive for SARS-CoV-2 by RT-PCR. Breastfeeding was discontinued after detection of the virus in breastmilk. | Decision of whether to breastfeed should be taken by parents and doctor. |
| [64] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. The neonate was breastfed breastmilk pumped from the mother and separated from their mother. The neonate was not infected. | SARS-CoV-2 is rarely transmitted through breastmilk. In addition, there may be an induction of passive immunity from IgG. |
| [65] | 2020 | A case report | 1 | Breastfeeding was discontinued after the mother’s diagnosis and the infant was separated. The neonate was not infected. | Temporarily stop breastfeeding. |
| [66] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. The neonate was fed pumped breastmilk directly after isolation from the mother. | Breastfeed cautiously when virus is not found in breastmilk. |
| [67] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. | Transmission of the infection from mother to child is very unlikely. |
| [68] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. The neonate was breastfed. | Breastfeeding is safe. |
| [69] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was positive for SARS-CoV-2 by RT-PCR. Direct breastfeeding was stopped and pumped breastmilk was given when it was confirmed that the infant had been infected with COVID-19. | Breastfeeding should be continued in infected nursing babies. There were no adverse effects. Finding SARS-CoV-2 RNA in the breastmilk sample does not indicate a viable virus. |
| [70] | 2020 | A case report | 1 | Infant formula was given to the neonate and direct breastfeeding was resumed after the baby tested positive. | Encourage breastfeeding and safe room sharing when both mother and infant are infected. |
| [71] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was positive for SARS-CoV-2 by RT-PCR. The neonate was breastfed as they were also infected. | More studies are needed to identify transmission routes. |
| [72] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was positive for SARS-CoV-2 by RT-PCR. The neonate was fed infant formula. | Breastfeeding can have a protective effect on the infant. |
| [73] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. The neonate was breastfed pumped breastmilk from the mother and separated from their mother. | Do not breastfeed when both mother and infant are infected. |
| [74] | 2020 | A case series | 2 | The two breastmilk samples collected from infected mothers were negative for SARS-CoV-2 by RT-PCR. The neonates were fed infant formula. One of the two neonates was separated from their mother. | There is a low risk of vertical transmission through breastmilk. Furthermore, it plays a potentially protective role for passive antibodies. |
| [75] | 2020 | Observational, retrospective study | 36 | Thirty-two of the 36 neonates were breastfed, both directly and pumped. Nine of the 36 neonates were separated from their mother. Thirty-four of 36 neonates tested negative for SARS-CoV-2 and two tested positive. | Support of direct breastfeeding or fed with pumped breastmilk with precautions. |
| [76] | 2020 | Prospective, multicentre study | 61 | Forty-five of 62 neonates were directly breastfed, 13 were directly breastfed and fed infant formula, three were fed infant formula, and one was directly breastfed and fed pumped breastmilk. Eleven of 62 neonates were separated from their mother. No neonate was positive for SARS-CoV-2 at birth and two from 62 were positive at seven and 20 days of life, respectively. | Encourage breastfeeding and room sharing with good conditions when mothers are infected. |
| [77] | 2020 | Transversal, retrospective study | 45 | Thirty-one of 33 neonates were breastfed. Thirty-three of 45 neonates were not separated from the mother. Forty-two of 45 neonates tested negative for SARS-CoV-2. | Encourage breastfeeding and appropriate safe room sharing. |
| [78] | 2020 | Collaborative, observational, prospective study | 14 | Of the 14 breastmilk samples collected from 14 infected mothers, 13 were negative for SARS-CoV-2 by RT-PCR. Eleven of 12 neonates were exclusively breastfed. Neonates were not separated from mothers. Four of 12 neonates tested positive for SARS-CoV-2. | Encourage breastfeeding or feeding with pumped breastmilk (when in isolation) regardless of results with appropriate precautions. |
| [79] | 2020 | A case report | One (not infected) | The neonate tested positive for SARS-CoV-2. They were exclusively breastfed and not separated from the mother | More studies are needed to identify transmission routes. |
| Ref. | Year | Type of Study | Sample | Results | Conclusions |
|---|---|---|---|---|---|
| [45] | 2021 | Prospective cohort group study | 131 (31 lactating women) | IgA, IgM and IgG antibodies were found in the 31 breastmilk samples from vaccinated mothers. The second dose of the vaccine produced an increase in SARS-CoV-2-specific IgG, but not in IgA. Immune transfer to neonates was observed through breastmilk. | COVID-19 mRNA vaccines generated robust humoral immunity in lactating women. Immune transfer to neonates occurred through the placenta and breastmilk. |
| [80] | 2020 | Ambispective, observational clinical analysis | 4 | Three breastmilk samples tested positive for SARS-CoV-2 IgM or IgG. Three neonates tested positive for IgG. One neonate tested positive for IgM within 24 h after birth. | Breastfeeding has a low risk of transmitting SARS-CoV-2. Mothers should continue to breastfeed, but take precautions. Babies may benefit from direct acquisition of SARS-CoV-2 antibodies |
| [81] | 2021 | Prospective longitudinal study | 18 | Of the 18 breastmilk samples collected from the 18 infected mothers, all tested negative for SARS-CoV-2 by RT-PCR. Breastmilk contained anti-SARS-CoV-2 IgA and IgG that neutralised the activity of SARS-CoV-2. | Breastmilk produced by infected mothers is a beneficial source of anti-SARS-CoV-2 IgA and IgG and is capable of neutralising the activity of the virus. These results support recommendations to continue breastfeeding during mild to moderate illness. |
| [82] | 2020 | A case report | 1 | The breastmilk sample collected from the infected mother was negative for SARS-CoV-2 by RT-PCR. IgA and IgG antibodies were found in the sample. | IgG and IgA in breastmilk can provide immune protection. |
| [83] | 2021 | Prospective cohort group study | 84 (504 breastmilk samples) | IgA antibodies were found in 86.1% of the samples and IgG in 97% of the samples. These antibodies showed neutralising effects of the virus. | Robust secretion of SARS-CoV-2 specific IgA and IgG was found in breastmilk after maternal vaccination with virus neutralisation, suggesting a potential protective effect against infection in the infant. |
| Were Patient’s Demographic Characteristics Clearly Described? | Was the Patient’s History Clearly Described and Presented as a Timeline? | Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | Were Diagnostic Tests or Methods and the Results Clearly Described? | Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | Was the Post-Intervention Clinical Condition Clearly Described? | Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | Does the Case Report Provide Takeaway Lessons? | Score Out of 8 (100%) | |
|---|---|---|---|---|---|---|---|---|---|
| Lowe et al., 2020 [60] | U | Y | Y | Y | NA | NA | N | Y | 4 (50%) |
| Lang et al., 2020 [62] | U | Y | Y | Y | NA | NA | Y | Y | 5 (62.5%) |
| Bastug et al., 2020 [63] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Chu et al., 2020 [64] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Feng et al., 2020 [65] | U | Y | Y | Y | NA | NA | N | Y | 4 (50%) |
| Perrone et al., 2020 [66] | U | N | N | Y | NA | NA | N | Y | 2 (25%) |
| Li et al., 2020 [67] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Yu et al., 2020 [68] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Tam et al., 2021 [69] | N | N | Y | Y | NA | NA | N | Y | 3 (37.5%) |
| Phadke et al., 2020 [70] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Kirtsman et al., 2020 [71] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Hinojosa el al., 2020 [72] | Y | Y | Y | Y | NA | NA | Y | Y | 6 (75%) |
| Wang et al., 2020 [73] | Y | Y | Y | Y | NA | NA | Y | Y | 6 (75%) |
| Le et al., 2020 [79] | Y | Y | Y | Y | NA | NA | N | Y | 5 (62.5%) |
| Dong et al., 2020 [82] | Y | Y | Y | Y | Y | Y | N | Y | 7 (87.5%) |
| Were There Clear Criteria for Inclusion in the Case Series? | Was the Condition Measured in a Standard, Reliable Way for All Participants Included in the Case Series? | Were Valid Methods Used for Identification of the Condition for All Participants Included in the Case Series? | Did the Case Series Have Consecutive Inclusion of Participants? | Did the Case Series Have Complete Inclusion of Participants? | Was There Clear Reporting of the Demographics of the Participants in the Study? | Was There Clear Reporting of Clinical Information of the Participants? | Were the Outcomes or Follow-up Results of Cases Clearly Reported? | Was There Clear Reporting of the Presenting Site(s)/Clinic(s) Demographic Information? | Was Statistical Analysis Appropriate? | Score Out of 10 (100%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marín et al., 2020 [54] | Y | Y | Y | Y | N | Y | Y | U | U | NA | 6 (60%) |
| Liu et al., 2020 [55] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 (100%) |
| Chen et al., 2020 [56] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 9 (90%) |
| Salvatori et al., 2020 [57] | N | Y | Y | U | U | Y | N | Y | U | NA | 4 (40%) |
| Groß et al., 2020 [58] | N | Y | Y | NA | NA | Y | Y | Y | N | NA | 5 (50%) |
| Zhu et al., 2020 [59] | N | Y | Y | NA | NA | Y | Y | Y | N | NA | 5 (50%) |
| Pereira et al., 2020 [61] | Y | Y | Y | Y | U | Y | Y | Y | Y | NA | 8 (80%) |
| Fan et al., 2021 [74] | N | Y | Y | N | N | N | Y | Y | Y | NA | 5 (62.5%) |
| Gao et al., 2020 [80] | N | Y | Y | N | NA | N | Y | Y | Y | Y | 6 (60%) |
| Were the Criteria for Inclusion in the Sample Clearly Defined? | Were the Study Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? | Score Out of 8 (100%) | |
|---|---|---|---|---|---|---|---|---|---|
| Gray et al., 2021 [45] | N | N | Y | Y | NA | NA | Y | NA | 3 (37.5%) |
| Elhalik et al., 2020 [75] | Y | Y | NA | Y | NA | NA | Y | Y | 5 (62.5%) |
| Ronchi et al., 2021 [76] | Y | Y | NA | Y | NA | NA | Y | Y | 5 (62.5%) |
| Patil et al., 2020 [77] | Y | Y | NA | Y | NA | NA | Y | Y | 5 (62.5%) |
| Bertino et al., 2020 [78] | Y | Y | NA | Y | NA | NA | Y | Y | 5 (62.5%) |
| Pace et al., 2021 [81] | Y | Y | Y | Y | NA | NA | Y | Y | 6 (75%) |
| Perl et al., 2021 [83] | N | Y | Y | Y | NA | NA | Y | Y | 5 (62.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Bermejo, M.; Peris-Ochando, B.; Murillo-Llorente, M.T. COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review. Nutrients 2021, 13, 2972. https://doi.org/10.3390/nu13092972
Pérez-Bermejo M, Peris-Ochando B, Murillo-Llorente MT. COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review. Nutrients. 2021; 13(9):2972. https://doi.org/10.3390/nu13092972
Chicago/Turabian StylePérez-Bermejo, Marcelino, Belén Peris-Ochando, and María Teresa Murillo-Llorente. 2021. "COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review" Nutrients 13, no. 9: 2972. https://doi.org/10.3390/nu13092972
APA StylePérez-Bermejo, M., Peris-Ochando, B., & Murillo-Llorente, M. T. (2021). COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review. Nutrients, 13(9), 2972. https://doi.org/10.3390/nu13092972






