Early Appearance of Epicardial Adipose Tissue through Human Development
Abstract
:1. Introduction
2. Materials and Methods
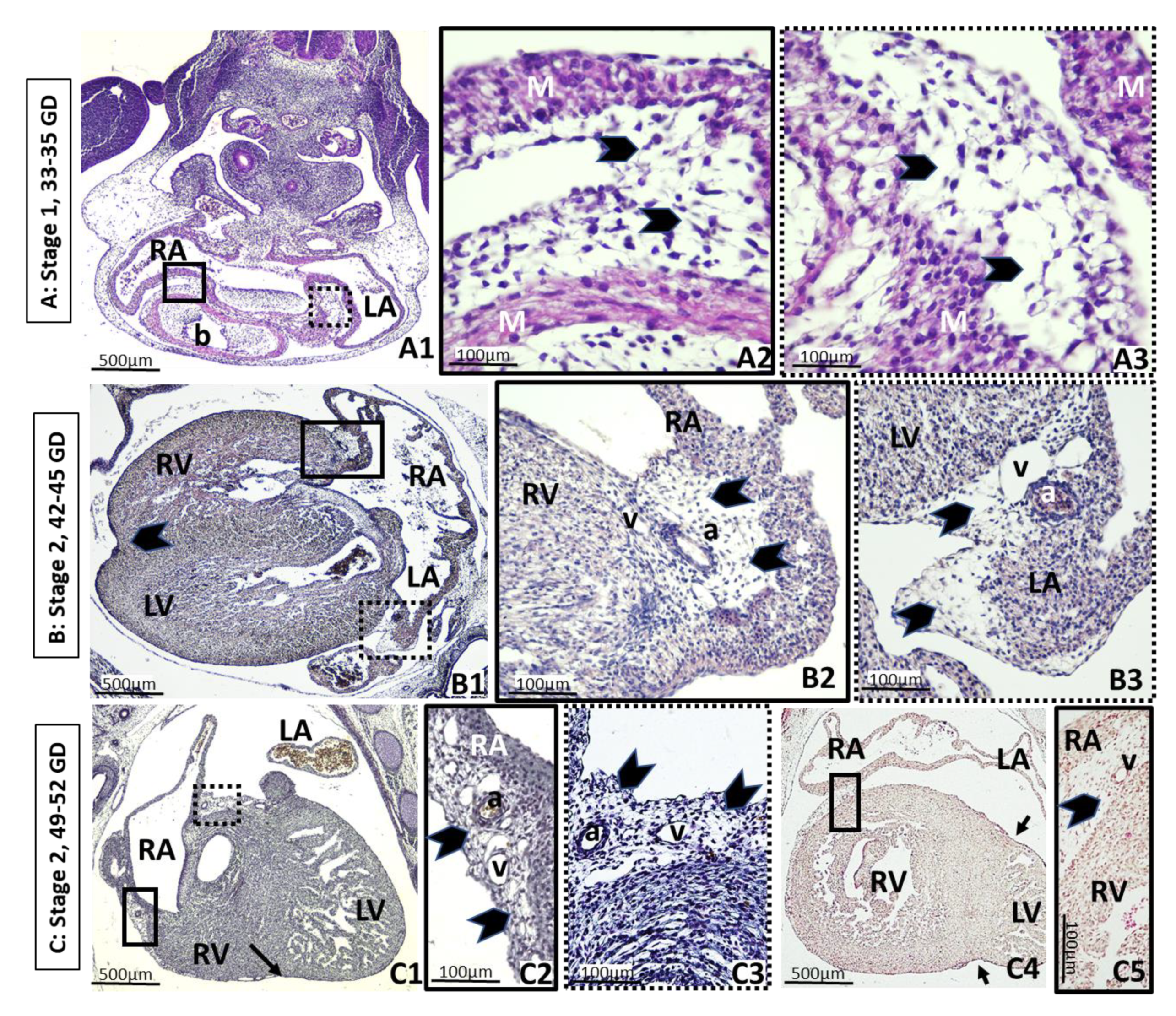
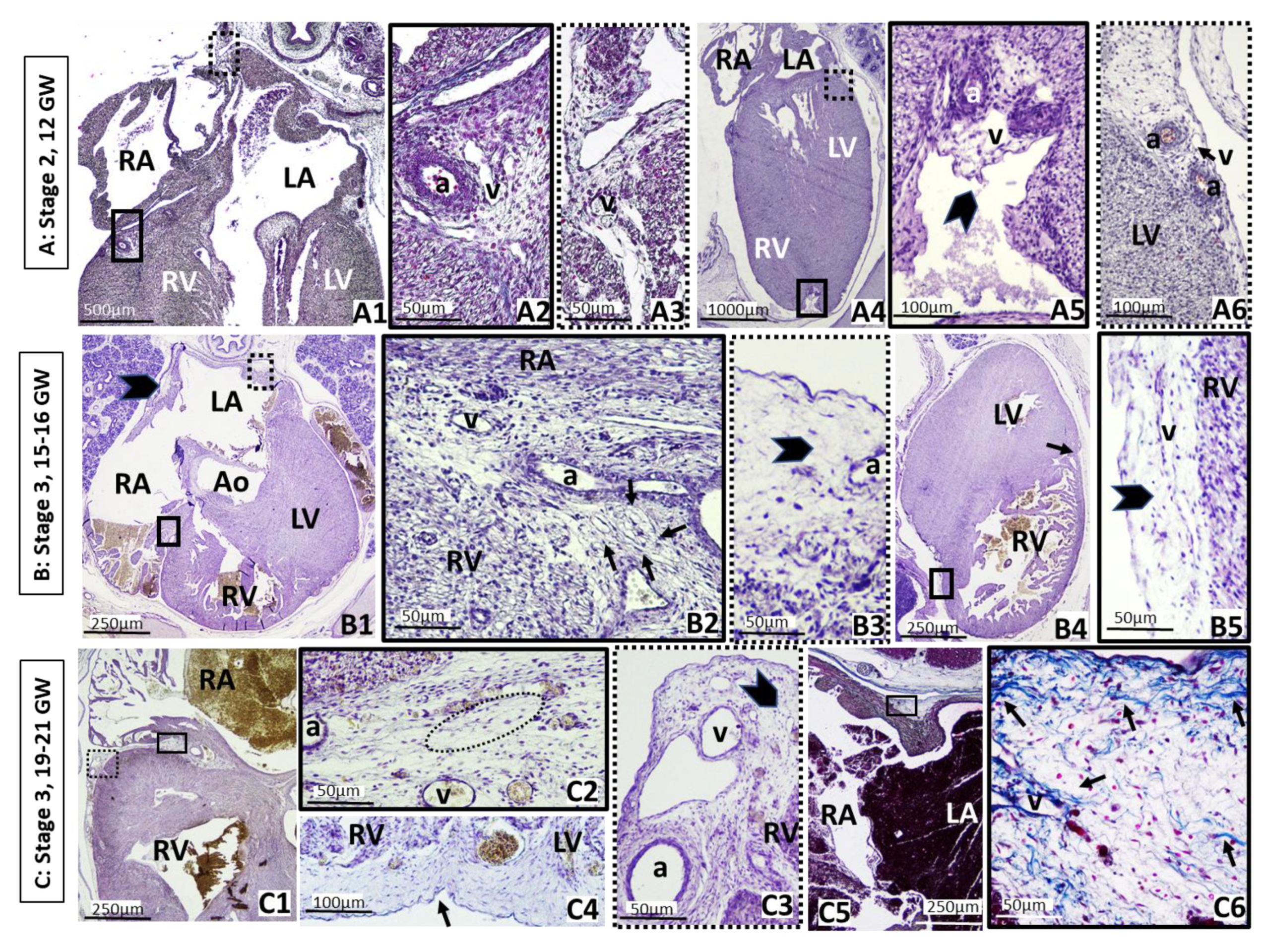
3. Results
4. Discussion
5. Strengths and Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial fat: A new cardiovascular therapeutic target. Curr. Opin. Pharmacol. 2016, 27, 13–18. [Google Scholar] [CrossRef]
- Witty, A.D.; Mihic, A.; Tam, R.Y.; Fisher, S.A.; Mikryukov, A.; Shoichet, M.S.; Li, R.-K.; Kattman, S.J.; Keller, G. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1026–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy-Marchal, C. Adipose tissue development. From animal models to clinical conditions. Endocr. Dev. 2010, 19, VII–IX. [Google Scholar] [PubMed]
- Hao, G.; Wang, X.; Treiber, F.A.; Harshfield, G.; Kapuku, G.; Su, S. Body mass index trajectories in childhood is predictive of cardiovascular risk: Results from the 23-year longitudinal Georgia Stress and Heart study. Int. J. Obes. 2018, 42, 923–925. [Google Scholar] [CrossRef] [Green Version]
- Koontz, M.B.; Gunzler, D.D.; Presley, L.; Catalano, P.M. Longitudinal changes in infant body composition: Association with childhood obesity. Pediatric Obes. 2014, 9, e141–e144. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.K.; Emmett, P.; Northstone, K.; Golding, J.; Rogers, I.; Ness, A.R.; Wells, J.C.; Dunger, D.B. Infancy weight gain predicts childhood body fat and age at menarche in girls. J. Clin. Endocrinol. Metab. 2009, 94, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Chomtho, S.; Wells, J.C.; Williams, J.E.; Davies, P.S.; Lucas, A.; Fewtrell, M.S. Infant growth and later body composition: Evidence from the 4-component model. Am. J. Clin. Nutr. 2008, 87, 1776–1784. [Google Scholar] [CrossRef]
- Admassu, B.; Ritz, C.; Wells, J.C.K.; Girma, T.; Andersen, G.S.; Belachew, T.; Owino, V.; Michaelsen, K.F.; Abera, M.; Wibaek, R.; et al. Accretion of Fat-Free Mass Rather Than Fat Mass in Infancy Is Positively Associated with Linear Growth in Childhood. J. Nutr. 2018, 148, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef]
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Monti, C.B.; Codari, M.; De Cecco, C.N.; Secchi, F.; Sardanelli, F.; Stillman, A.E. Novel imaging biomarkers: Epicardial adipose tissue evaluation. Br. J. Radiol. 2020, 93, 20190770. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Borodkina, D.; Akbasheva, O.; Karetnikova, V.; Brel, N.; Alexander, K.; Barbarash, O. Relationship between epicardial and perivascular fatty tissue and adipokine-cytokine level in coronary artery disease patients. PLoS ONE 2019, 14, e0208156. [Google Scholar] [CrossRef] [Green Version]
- Mookadam, F.; Goel, R.; Alharthi, M.S.; Jiamsripong, P.; Cha, S. Epicardial fat and its association with cardiovascular risk: A cross-sectional observational study. Heart Views Off. J. Gulf Heart Assoc. 2010, 11, 103–108. [Google Scholar] [CrossRef]
- Hassan, Z.; Coelho, D.; Kokten, T.; Alberto, J.-M.; Umoret, R.; Daval, J.-L.; Guéant, J.-L.; Bossenmeyer-Pourié, C.; Pourié, G. Brain Susceptibility to Methyl Donor Deficiency: From Fetal Programming to Aging Outcome in Rats. Int. J. Mol. Sci. 2019, 20, 5692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatem, S.N.; Sanders, P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc. Res. 2014, 102, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.K.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e004378. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.-C.; Tang, W.-H.; Wang, C.-P.; Lu, L.-F.; Chung, F.-M.; Lu, Y.-C.; Hsu, C.-C.; Tsai, I.-T.; Jhuo, S.-J.; Lai, W.-T.; et al. Increased epicardial adipose tissue volume is associated with PR interval prolongation. Clin. Investig. Med. Med. Clin. Exp. 2015, 38, E45–E52. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, G.; Piedimonte, A.; Podagrosi, M.; Mercurio, R.; Mosca, A.; D’Avanzo, M.; Vania, A. Epicardial adipose tissue and signs of metabolic syndrome in children. Eat. Weight. Disord. EWD 2016, 21, 269–276. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poissonnet, C.M.; Burdi, A.R.; Bookstein, F.L. Growth and development of human adipose tissue during early gestation. Early Hum. Dev. 1983, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Burdi, A.R.; Poissonnet, C.M.; Garn, S.M.; Lavelle, M.; Sabet, M.D.; Bridges, P. Adipose tissue growth patterns during human gestation: A histometric comparison of buccal and gluteal fat depots. Int. J. Obes. 1985, 9, 247–256. [Google Scholar] [PubMed]
- O’Rahilly, R.; Müller, F. Developmental stages in human embryos: Revised and new measurements. Cells Tissues Organs. 2010, 192, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Aybar, L.A.; Turrero-Nogués, A.; Marantos-Gamarra, D.G. Embryonic cardiac morphometry in Carnegie stages 15–23, from the Complutense University of Madrid Institute of Embryology Human Embryo Collection. Cells Tissues Organs. 2008, 187, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Kim, J.H.; Shibata, S.; Abe, H.; Murakami, G.; Rodríguez-Vázquez, J.F. Flap valve of the heart foramen ovale revisited: Macroscopic and histologic observations of human near-term fetuses. Ann. Anat. Anat. Anz. 2019, 224, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.W.; Yamamoto, M.; Kim, J.H.; Murakami, G.; Wilting, J.; Rodríguez-Vázquez, J.F. Changes in topographical relation between the ductus arteriosus and left subclavian artery in human embryos: A study using serial sagittal sections. Folia Morphol. 2019, 78, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rahilly, R.; Müller, F. Embryonic length and cerebral landmarks in staged human embryos. Anat. Rec. 1984, 209, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sylva, M.; van den Hoff, M.J.B.; Moorman, A.F.M. Development of the human heart. Am. J. Med. Genet. A 2014, 164, 1347–1371. [Google Scholar] [CrossRef]
- López-Bermejo, A.; Prats-Puig, A.; Osiniri, I.; Martínez-Calcerrada, J.-M.; Bassols, J. Perirenal and epicardial fat and their association with carotid intima-media thickness in children. Ann. Pediatric Endocrinol. Metab. 2019, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Nat. Commun. 2019, 10, 2549. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Antoniades, C. The role of epicardial adipose tissue in cardiac biology: Classic concepts and emerging roles. J. Physiol. 2017, 595, 3907–3917. [Google Scholar] [CrossRef]
- Bale, L.K.; West, S.A.; Conover, C.A. Characterization of mouse pericardial fat: Regulation by PAPP-A. Growth Horm. IGF Res. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Cavallero, S.; Patterson, M.; Shen, H.; Xu, J.; Kumar, S.R.; Sucov, H.M. Adipogenesis and epicardial adipose tissue: A novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc. Natl. Acad. Sci. USA 2015, 112, 2070–2075. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.J.; Hausman, G.J.; Hausman, D.B. Regulation of adipose cell development in utero. Proc. Soc. Exp. Biol. Med. 1998, 219, 200–210. [Google Scholar] [CrossRef]
- Lopomo, A.; Burgio, E.; Migliore, L. Epigenetics of Obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 151–184. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.E.; Johnston, B.M. Nutrition and fetal growth. Reprod. Fertil. Dev. 1995, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Louise, J.; Deussen, A.; Dodd, J.M. In Overweight or Obese Pregnant Women, Maternal Dietary Factors are not Associated with Fetal Growth and Adiposity. Nutrients 2018, 10, 870. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, A.; Akkurt, M.O.; Yalcin, S.; Karakoc, G.; Varol, E.; Sezik, M. Second Trimester Fetal and Maternal Epicardial Fat Thickness in Gestational Diabetic Pregnancies. Horm. Metab. Res. 2016, 48, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Akkurt, M.O.; Turan, O.M.; Crimmins, S.; Harman, C.R.; Turan, S. Increased fetal epicardial fat thickness: A novel ultrasound marker for altered fetal metabolism in diabetic pregnancies. J. Clin. Ultrasound 2018, 46, 397–402. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Li, Y.; Jing, X.; Deng, S.; Yan, Y.; She, Q. Epicardial fat tissue in patients with diabetes mellitus: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, D.; McBrien, A.; Howley, L.; Yamamoto, Y.; Sekar, P.; Motan, T.; Jain, V.; Savard, W.; Hornberger, L.K. First-Trimester Fetal Echocardiography: Identification of Cardiac Structures for Screening from 6 to 13 Weeks’ Gestational Age. J. Am. Soc. Echocardiogr. 2017, 30, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.J.; Haapala, J.L.; Foster, L.P.; Duncan, K.M.; Teague, A.M.; Kharbanda, E.O.; McGovern, P.M.; Whitaker, K.M.; Rasmussen, K.M.; Fields, D.A.; et al. Higher Maternal Diet Quality during Pregnancy and Lactation Is Associated with Lower Infant Weight-For-Length, Body Fat Percent, and Fat Mass in Early Postnatal Life. Nutrients 2019, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, E.M.; Stewart, F.M.; Ramsay, J.E.; Brown, E.A.; Meyer, B.J.; Olivecrona, G.; Griffin, B.A.; Freeman, D.J. Maternal Adipose Tissue Expansion, A Missing Link in the Prediction of Birth Weight Centile. J. Clin. Endocrinol. Metab. 2020, 105, dgz248. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Willens, H.J. Echocardiographic epicardial fat: A review of research and clinical applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Minnella, G.P.; Crupano, F.M.; Syngelaki, A.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of major heart defects by routine first-trimester ultrasound examination: Association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet. Gynecol. 2020, 55, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221 (Suppl. 1), jeb164970. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Age (Gestational Days) | Length (mm) | Carnegie Stages (CS) | n | Name of Embryo | Section Orientation | Section Thickness (µm) | Stain |
|---|---|---|---|---|---|---|---|
| 33–35 | 5 to 7 | 14 | 3 | MS-5 | Axial/Oblique | 7 | HE |
| JD-2 | Axial/Oblique | 10 | HE | ||||
| MAT-24 | Axial/Oblique | 10 | HE | ||||
| 35–37 | 7 to 9 | 15 | 3 | PV-8 | Transverse | 7 | HE |
| GI-7 | Transverse | 10 | HE | ||||
| GV-4 | Transverse | 8 | HE | ||||
| 37–40 | 9 to 11 | 16 | 6 | MS | Transverse | 8 | HE/Trichrome |
| DD-10 | Transverse | 8 | HE | ||||
| FE-1 | Transverse | 10 | HE/Trichrome | ||||
| BI-12 | Transverse | 7 | HE | ||||
| NG-2 | Transverse | 7 | HE/Trichrome | ||||
| mar-03 | Frontal | 10 | HE/Trichrome | ||||
| 39–42 | 11 to 14 | 17 | 4 | VE-4 | Transverse | 10 | HE |
| A-3 | Transverse | 7 | HE | ||||
| F-23 | Axial/Oblique | 7 | HE/Trichrome | ||||
| ES-14 | Transverse | 7 | HE | ||||
| 42–45 | 13–17 | 18 | 5 | NO | Transverse | 10 | HE |
| FO | Transverse | 7 | HE/Trichrome | ||||
| C-7 | Transverse | 7 | HE/Trichrome | ||||
| ES-15 | Transverse | 10-nov | HE | ||||
| MARC-1 | Transverse | 15–16 | HE/Trichrome | ||||
| 45–47 | 17–20 | 19 | 7 | PA | Transverse | 10 | HE |
| ES-18 | Transverse | 7 | HE/Trichrome | ||||
| ES-19 | Sagittal | 07-ago | HE/Trichrome | ||||
| C-9 | Transverse | 8 | HE/Trichrome | ||||
| MM-20 | Sagittal | 8 | HE | ||||
| ES-20 | Transverse | 8 | HE | ||||
| 47–50 | 21–23 | 20 | 4 | ES-22 | Transverse | 10 | HE |
| BI-22 | Transverse | 7 | HE/Trichrome | ||||
| F27 | Transverse | 7 | HE/Trichrome | ||||
| AC-23 | Transverse | 7 | HE/Trichrome | ||||
| 49–52 | 22–24 | 21 | 2 | GV-7 | Sagittal | 10 | HE |
| HA-24 | Sagittal | 8 | HE/Trichrome | ||||
| 52–55 | 25–27 | 22 | 4 | F-8 | Transverse | 10 | HE |
| MAL-25 | Sagittal | 8 | HE | ||||
| JP-25 | Transverse | 8 | HE | ||||
| C-27 | Sagittal | 8 | HE/Trichrome | ||||
| 50–63 | 28–31 | 23 | 3 | C11 | Transverse | 10 | HE/Trichrome |
| Mes-2 | Transverse | 10 | HE/Trichrome | ||||
| CA-4 | Transverse | 10 | HE | ||||
| Total | 41 |
| Proposed Age (Gestational Weeks) | Crown–Rump Length (mm) | Name of the Fetus | Section Orientation | Section Thickness (µm) | Stain |
|---|---|---|---|---|---|
| 9 to 11 | 38 | OY | Sagittal | 10 | HE |
| 39–40 | Faus-2 | Transverse | 10 | HE/Trichrome | |
| 45 | F45 | Sagittal | 8 | HE | |
| 45 | F1 | Transverse | 9 | HE | |
| 46 | F46 | Transverse | 8 | HE | |
| 47 | BE | Sagittal | 10 | HE/Trichrome | |
| 56 | Mall-28 | Transverse | 7 | HE | |
| 57 | MA57 | Transverse | 7 | HE | |
| 60 | F25 | Sagittal | 7 | HE/Trichrome | |
| 60 | VS | Transverse | 10 | HE | |
| 64 | X | Transverse | 10 | HE/Trichrome | |
| 12 to 15 | 74.5 | VR74 | Transverse | 10 | HE/Trichrome |
| 79 | F79 | Sagittal | 8 | HE | |
| 88 | F88 | Transverse | 7 | HE | |
| 99 | F99 | Transverse | 7 | HE | |
| 107 | NO-6 | Transverse | 10 | HE/Trichrome | |
| 113 | B-62 | Frontal | 10 | HE/Trichrome | |
| 117 | B-29 | Frontal | 10 | HE/Trichrome | |
| 119 | Bu-119 | Transverse | 10 | HE/Trichrome | |
| 16–18 | 127 | ES127 | Transverse | 7 | HE |
| 137 | Cu-2 | Transverse | 10 | HE/Trichrome | |
| 32 | 320 | EA32 | Transverse | 7 | HE/Trichrome |
| Full term | 305 | A | Transverse | 7 | HE/Trichrome |
| Total | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Miguelsanz, J.; Jiménez-Ortega, V.; Cano-Barquilla, P.; Garaulet, M.; Esquifino, A.I.; Varela-Moreiras, G.; Fernández-Mateos, P. Early Appearance of Epicardial Adipose Tissue through Human Development. Nutrients 2021, 13, 2906. https://doi.org/10.3390/nu13092906
Perez-Miguelsanz J, Jiménez-Ortega V, Cano-Barquilla P, Garaulet M, Esquifino AI, Varela-Moreiras G, Fernández-Mateos P. Early Appearance of Epicardial Adipose Tissue through Human Development. Nutrients. 2021; 13(9):2906. https://doi.org/10.3390/nu13092906
Chicago/Turabian StylePerez-Miguelsanz, Juliana, Vanesa Jiménez-Ortega, Pilar Cano-Barquilla, Marta Garaulet, Ana I. Esquifino, Gregorio Varela-Moreiras, and Pilar Fernández-Mateos. 2021. "Early Appearance of Epicardial Adipose Tissue through Human Development" Nutrients 13, no. 9: 2906. https://doi.org/10.3390/nu13092906
APA StylePerez-Miguelsanz, J., Jiménez-Ortega, V., Cano-Barquilla, P., Garaulet, M., Esquifino, A. I., Varela-Moreiras, G., & Fernández-Mateos, P. (2021). Early Appearance of Epicardial Adipose Tissue through Human Development. Nutrients, 13(9), 2906. https://doi.org/10.3390/nu13092906







