Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Experimental Animals and Treatments
- Group 1: Con [normal control, saline only] (n = 7);
- Group 2: NC [negative control, scopolamine (SCP) 1 mg/kg + Saline] (n = 7);
- Group 3: Positive control [SCP 1 mg/kg + tacrine 10 mg/kg] (n = 7)
- Group 4: L1 [SCP 1 mg/kg + low dose of AH leaf 150 mg/kg] (n = 7)
- Group 5: L2 [SCP 1 mg/kg + high dose of AH leaf 300 mg/kg] (n = 7)
- Group 6: R1 [SCP 1 mg/kg + low dose of AH root 150 mg/kg] (n = 7)
- Group 7: R2 [SCP 1 mg/kg + high dose of AH root 300 mg/kg] (n = 7)
2.3. Y-Maze Test
2.4. Water Maze Test
2.5. Passive Avoidance Test
2.6. Measuring Serum ACh Concentration and AChE Acitivity
2.6.1. Acetylcholine Concentration
2.6.2. Acetylcholinesterase Activity
2.7. Measuring Serum Cytokines (IL-1β, IL-6, and IFN-γ)
2.8. Western Blot Analysis
2.9. Immunofluorescence Staining
2.10. Statistical Analysis
3. Results
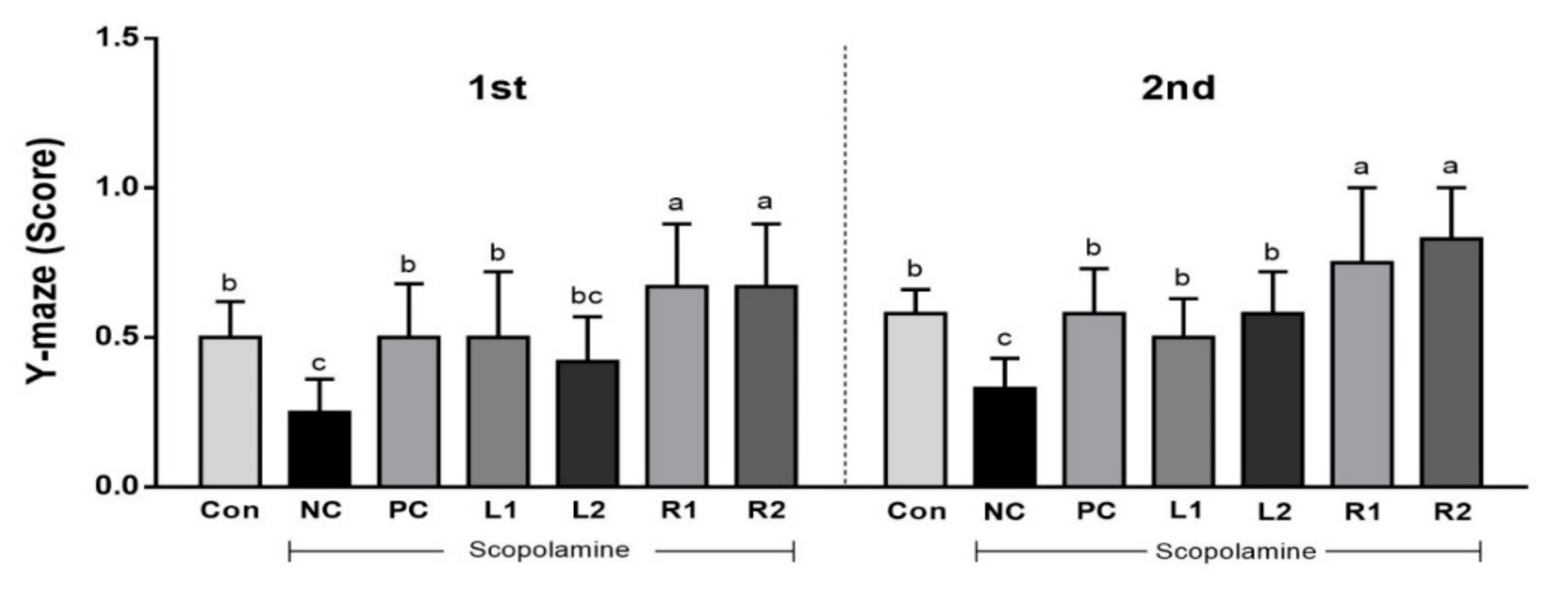
3.1. Improvement of Cognitive Function by Increasing Y-Maze Score
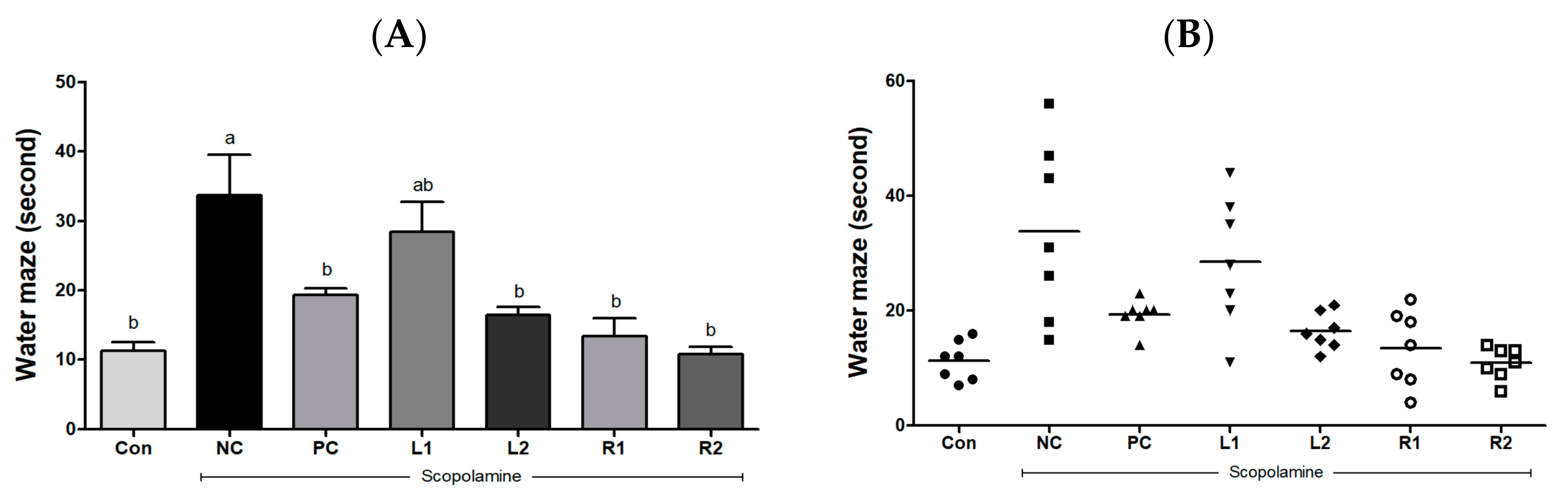
3.2. Improvement of Learning and Cognitive Function by Reducing the Escape Time from Water
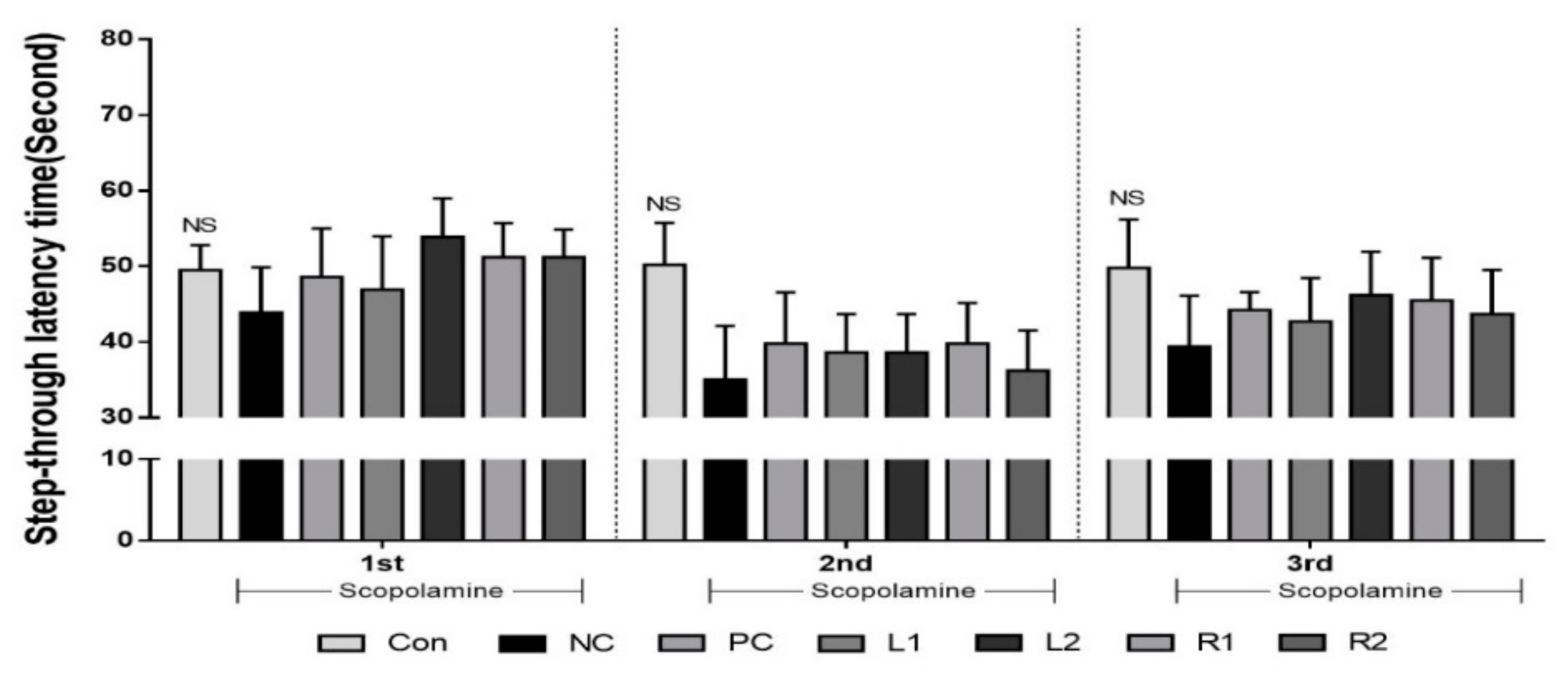
3.3. Improvement of Long-Term Memory Evaluated by the Passive Avoidance Test
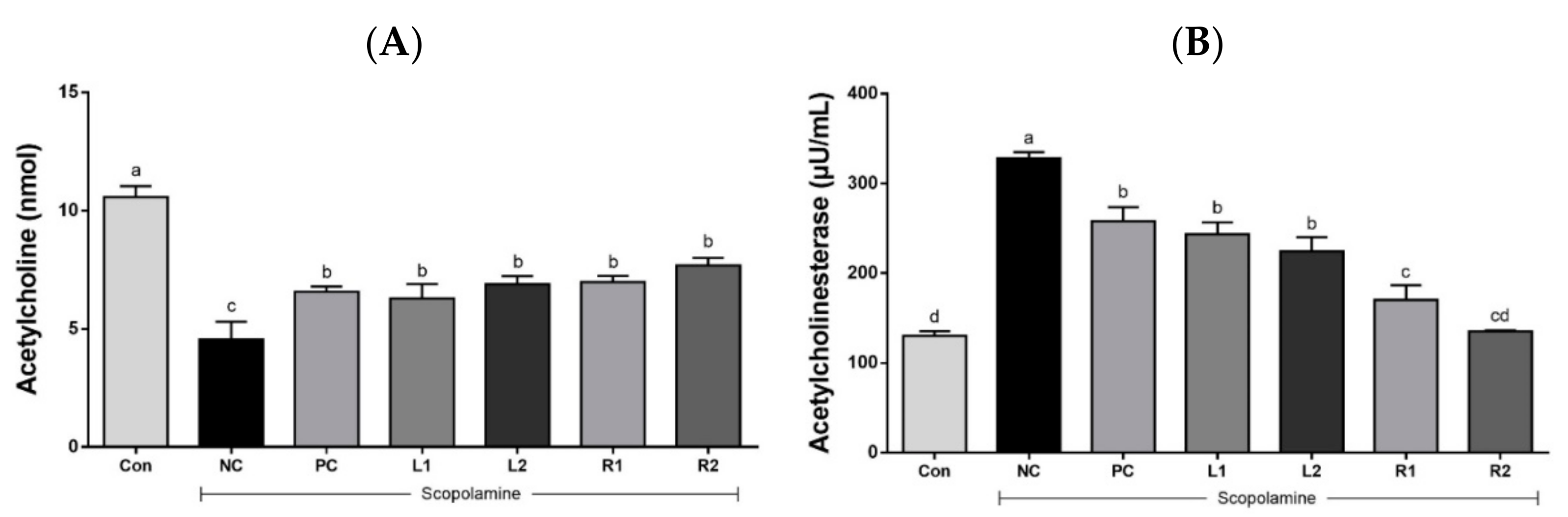
3.4. ACh Concentration and AChE Activity in Serum
3.4.1. Serum Acetylcholine Concentration
3.4.2. Acetylcholinesterase Activity
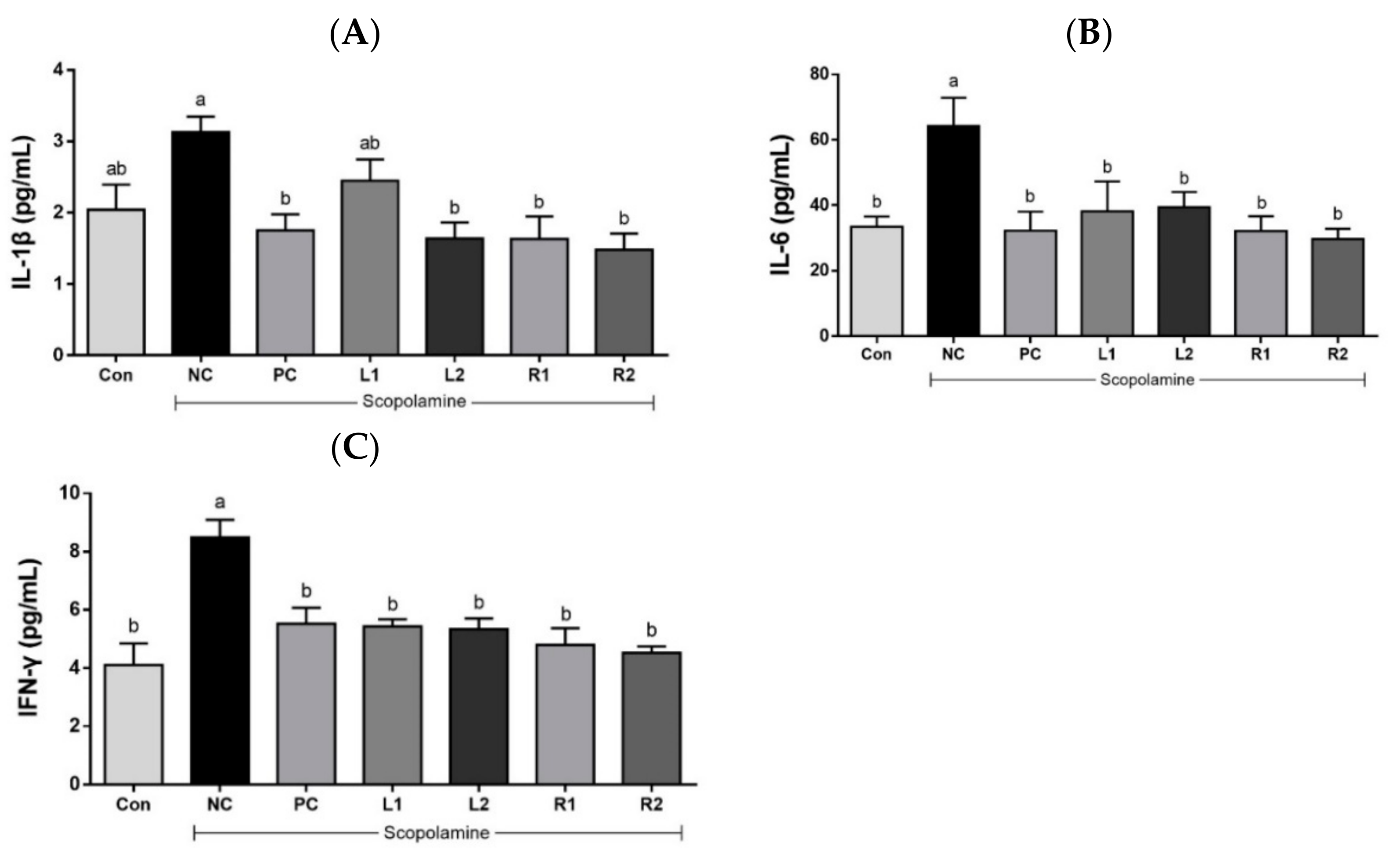
3.5. Effects of AH on Serum Cytokines (IL-1β, IL-6, and IFN-γ)
3.5.1. Serum IL-1β
3.5.2. Serum IL-6
3.5.3. Serum IFN-γ
3.6. Protein Expression in Liver Tissue Based on Westen Blot Analysis
3.7. Protein Expression in Brain Tissue Analyzed by Immunofluorescence Staining
3.7.1. Amyloid β-Peptide (Aβ)
3.7.2. Caspase-3 (Cas-3)
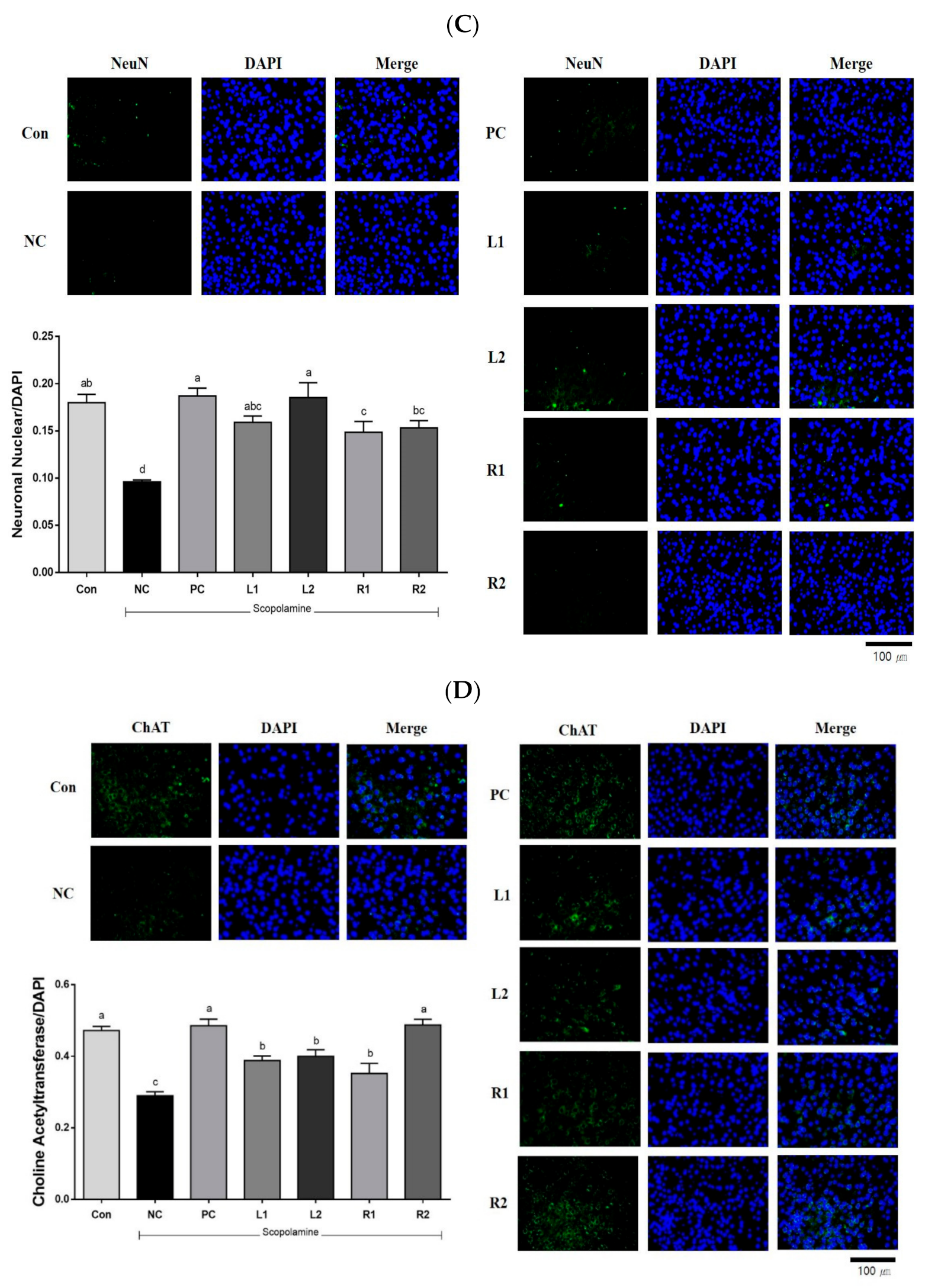
3.7.3. Neuronal Nuclear (NeuN)
3.7.4. Choline Acetyltransferase (ChAT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choe, D.J.; Ahn, H.Y.; Kim, Y.W.; Kim, T.H.; Kim, M.D.; Cho, Y.S. Improvement effect of Stachys sieboldii MIQ. according to mix ratio of calcium on memory impairment in scopolamine-induced dementia rats. J. Life Sci. 2016, 26, 812–818. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin rescue oxidative stress-mediated neuroinflamma-tion/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Ling, F.A.; Hui, D.Z.; Ji, S.M. Protective effect of recombinant human somatotropin on amyloid β-peptide induced learning and memory deficits in mice. Growth Horm. IGF Res. 2007, 17, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; He, M.T.; Kim, M.J.; Park, C.H.; Lee, J.Y.; Shin, Y.S.; Cho, E.J. Protective effects of combianation of Carthamus tinctorius L. seed and Taraxacum coreanum on scopolamine-induced memory impairment in mice. Korean J. Med. Crop. Sci. 2020, 28, 85–94. [Google Scholar] [CrossRef]
- Lykhmus, O.; Koval, L.; Voytenko, L.; Uspenska, K.; Komisarenko, S.; Deryabina, O.; Shuvalora, N.; Kordium, V.; Ustymenko, A.; Kyryk, V. Intravenously injected mesenchymal stem cells penetrate the brain and treat inflammation-induced brain damage and memory impairment in mice. Front. Pharm. 2019, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. The cholinergic anti-inflammatory pathway: Towards innovative treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 229. [Google Scholar] [CrossRef]
- Medeiros, R.; Figueiredo, C.P.; Pandolfo, P.; Duarte, F.S.; Prediger, R.D.; Passos, G.F.; Calixto, J.B. The role of TNF-α signaling pathway on COX-2 upregulation and cognitive decline induced by β-amyloid peptide. Behav. Brain Res. 2010, 209, 165–173. [Google Scholar] [CrossRef]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced impairment, anxiety, and depression in laboratory rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng berry aqueous extract prevents scopolamine-induced memory impairment in mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Nuerosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, M.J.; Jang, J.Y.; Lee, S.H. Allium hookeri extract inhibits adipogenesis by promoting lipolysis in high fat diet-induced obese mice. Nutrients 2019, 11, 2262. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Lee, M.J.; You, B.R.; Jin, J.S.; Lee, S.H.; Yun, Y.R.; Kim, H.J. Allium hookeri root extract exerts anti-inflammatory effects by nuclear factor-κB, down-regulation in lipopolysaccharide-induced RAW264.7 cells. BMC Complementary Altern. Med. 2017, 17, 126. [Google Scholar]
- Lee, S.Y.; Cho, S.S.; Li, Y.; Bae, C.S.; Park, K.M.; Park, D.H. Anti-inflammatory effect of Curcuma longa and Allium hookeri co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Rho, S.H.; You, S.; Kim, G.H.; Park, H.J. Neuroprotective effect of Allium hookeri against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress. Food Sci. Biotechnol. 2020, 29, 1519–1530. [Google Scholar] [CrossRef]
- Kim, N.S.; Choi, B.K.; Lee, S.H.; Jang, H.H.; Kim, J.B.; Kim, H.R.; Kim, D.K.; Kim, Y.S.; Yang, J.H.; Kim, H.J.; et al. Effects of Allium hookeri on glucose metabolism in type Ⅱ diabetic mice. Korean J. Pharmacogn. 2015, 46, 78–83. [Google Scholar]
- Lee, Y.; Lee, S.H.; Jeong, M.S.; Kim, J.B.; Jang, H.H.; Choe, J.S.; Kim, D.W.; Lillehoj, H.S. In vitro analysis of the immunomodulating effects of Allium hookeri on lymphocytes, macrophages, and tumor cells. J. Poult. Sci. 2016, 0160108. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Lee, S.H.; Gadde, U.D.; Oh, S.T.; Lee, S.J.; Sillehoj, H.S. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poult. Sci. 2018, 97, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yoon, K.Y. Comparison of the nutrient composition and quality of the root of Allium hookeri grown in Korean and Myanmar. Korean J. Food Sci. Technol. 2014, 46, 544–548. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Fukada, M.T.H.; Francoline-Silva, A.L.; Almedia, S.S. Early postnatal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav. Brain Res. 2002, 133, 271–277. [Google Scholar] [CrossRef]
- Alberini, C.M.; Travaglia, A. Infantile amnesia: A critical period of learning to learn and remember. J. Neurosci. 2017, 37, 5783–5795. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Woo, J.H.; Kim, A.J. The effects of Korean ginseng on memory loss in rat models. J. Korean Soc. Food Sci. Nutr. 2013, 42, 1190–1196. [Google Scholar] [CrossRef]
- Gacar, N.; Mutlu, O.; Utkan, T.; Celikyurt, I.K.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive aviodance and morris water maze test in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef]
- Shon, K.; Kim, J. Anti-dementia effects of Cornus officinalis S. et Z. extract on scopolamine induced dementia in mouse. Korean J. Pharmacogn. 2017, 11, 13. [Google Scholar]
- Yadav, S.S.; Singh, M.K.; Yadav, R.S. Organophophates induced Alzheimer’s disease: An epigenetic aspect. J. Clin. Epigenetics 2016, 2. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s diesase: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Shaftel, S.S.; Kyrkanides, S.; Olschowka, J.A.; Jen-nei, M.H.; Johnson, R.E.; O’Banion, M.K. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Investig. 2007, 117, 1595–1604. [Google Scholar] [CrossRef]
- Xu, T.; Shen, X.; Yu, H.; Sun, L.; Lin, W.; Zhang, C. Water-soluble ginseng oligosaccharide protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J. Ginseng Res. 2016, 40, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, E.R.; Wang, B.; Wan, Y.W.; Chiu, G.; Cole, A.; Yin, Z.; Proposon, N.E.; Xu, Y.; Jankowsky, J.L.; Liu, Z.; et al. Type Ⅰ interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J. Clin. Investig. 2020, 130, 1912–1930. [Google Scholar] [CrossRef]
- Iqbal, S.; Shah, F.A.; Naeem, K.; Nadeem, H.; Sarwar, S.; Ashraf, Z.; Imran, M.; Khan, T.; Anwar, T.; Li, S. Succinamide derivaties ameliorate neuroinflammation and oxidative stress in scopolamine-induced neurodegeneration. Biomolecules 2020, 10, 443. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.L.; Liu, Y.N.; Liu, L.; Wang, X.; Jiang, C.L.; Wang, Y.X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J. Neuroinflammation 2012, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease. N. Engl. J. Med. 2010, 326, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wen, P.Y.; Li, W.W.; Zhou, J. Upregulation effects of tanshione ⅡA on the expression of NeuN, Nissl body, and IκB and downregulation effects on the expressions of GFAP and NF-κB in the brain tissues of rat model of Alzheimer’s disease. Neuroreport 2015, 26, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.V.; Williams, C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, G.; Cheng, M.-H.; Xi, F.-C.; Chen, Y.; Su, T.; Li, W.-Q.; Yu, W.-K. Changes of plasma acetylcholine and inflammatory markers in critically ill patients during early enteral nutrition: A prospective observational study. J. Crit. Care 2019, 52, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Subedi, L.; Yeo, E.J.; Kim, S.Y. Lactucopicrin ameliorated oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2014, 99, 3012–3024. [Google Scholar]
- Tracey, K.J. Physiology and immunology of cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selznick, L.A.; Zheng, T.S.; Flavell, R.A.; Rakic, P.; Roth, K.A. Amyloid beta-induced neuronal death is bax-dependent but caspase-independent. J. Neuropathol. Exp. Neurol. 2000, 59, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Mcllwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Lee, E.-B.; Jang, H.-H.; Cha, Y.-S.; Park, Y.-S.; Lee, S.-H. Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice. Nutrients 2021, 13, 2890. https://doi.org/10.3390/nu13082890
Choi J-H, Lee E-B, Jang H-H, Cha Y-S, Park Y-S, Lee S-H. Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice. Nutrients. 2021; 13(8):2890. https://doi.org/10.3390/nu13082890
Chicago/Turabian StyleChoi, Ji-Hye, Eun-Byeol Lee, Hwan-Hee Jang, Youn-Soo Cha, Yong-Soon Park, and Sung-Hyen Lee. 2021. "Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice" Nutrients 13, no. 8: 2890. https://doi.org/10.3390/nu13082890
APA StyleChoi, J.-H., Lee, E.-B., Jang, H.-H., Cha, Y.-S., Park, Y.-S., & Lee, S.-H. (2021). Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice. Nutrients, 13(8), 2890. https://doi.org/10.3390/nu13082890





